Abstract
A novel core–shell structured magnetic cobalt oxide supported organosilica-sulfonic acid (Co3O4@SiO2/OS-SO3H) nanocomposite is prepared through a low-cost, simple, and clean method. The characterization of Co3O4@SiO2/OS-SO3H was performed by using Fourier transform infrared (FT-IR) spectroscopy, thermal gravimetric analysis (TGA), powder X-ray diffraction (PXRD), energy dispersive X-ray (EDX) spectroscopy, scanning electron microscopy (SEM), vibrating sample magnetometer (VSM), and transmission electron microscopy (TEM). The TGA and FT-IR results illustrate the high stability of the designed nanocomposite. The SEM image showed a size of about 40 nm for the Co3O4@SiO2/OS-SO3H nanoparticles. Furthermore, according to the result of VSM analysis, the saturation magnetization of this nanocomposite was about 25 emu/g. This novel material was used as an efficient nanocatalyst for the synthesis of biologically active tetrahydrobenzo[a]xanthen-11-one derivatives. These products were obtained in high to excellent yields under green conditions. The recoverability and reusability of this catalyst were also investigated under applied conditions.
Similar content being viewed by others
Introduction
The growth of magnetic nanoparticles (MNPs) from technological and scientific viewpoints has provided a new approach for medical applications, biotechnology, data storage, solid sensors, electrochromic, solar adsorbents, and catalytic applications1,2,3,4,5,6,7,8,9,10,11. Among different magnetic nanoparticles, the cobalt oxide NPs are very interesting for researchers due to their unparalleled properties such as good performance, high specific surface area, easy synthesis, high thermal and mechanical stability, and easy magnetic separation12,13,14,15,16,17,18,19,20,21,22,23. So far, different methods such as combustion, sol–gel, co-precipitation, chemical pyrolysis, and reduction have been used to synthesize magnetic cobalt oxide NPs. Between these, the reduction method has received special attention because of the low cost and time saving24,25,26,27,28,29,30,31. Since cobalt oxide NPs are chemically very active, they are easily oxidized and also self-aggregated in the environment. To solve these problems, the surface of these nanoparticles is coated with organic and inorganic materials and/or bioactive substances such as carbon, silica, polymers, peptides, etc.32,33,34,35,36,37,38,39. Among these, silica is more attractive due to its special properties such as its optical and magnetic transparency, high biocompatibility, high thermal and chemical stability, and non-toxicity. Also, silica prevents the aggregation of NPs and increases their stability. In addition, due to the presence of the hydroxyl groups on the silica surface, various catalytic functional moieties can be immobilized on it to increase the stability and performance of the final catalysts40,41,42. Some of recently reports in this matter are Co3O4@SiO2@TiO2-Ag43, Fe3O4@SiO2@GO44, Co3O4@SiO2/carbon nanocomposite45, Co3O4@SiO2-nylon637, Fe3O4@SiO2-supported IL/[Mo6O19]46 and Fe3O4@SiO2@(BuSO3H)347.
In recent years, the use of sulfonic acid groups as surface modifiers of core–shell structured nanoparticles has been considered by researchers. These have been used as strong and recoverable catalysts in organic reactions. Especially, sulfonic acid functionalized magnetic nanocomposites have been more interesting due to their easy magnetic separation. Some of reports in this matter are (Fe3O4@γFe2O3-SO3H)48, (Fe3O4@TDI@TiO2-SO3H)49, (Fe3O4@PDA-SO3H)50, (Fe3O4@D-NH-(CH2)4-SO3H)51 (Fe3O4@NS-GO)52 and (Fe3O4@OS-SO3H)53.
On the other hand, one-step multicomponent reactions that lead to the synthesis of heterocyclic compounds are one of the most practical and important organic processes. Among oxygen-containing heterocyclic compounds, xanthene derivatives have different biological applications such as antiviral, antibacterial, inhibitory, and antitumor. Therefore, in recent years, the synthesis of xanthen-11-one compounds has been investigated by using various catalysts. Some of the recently reported catalysts in this matter are p-toluenesulfonic acid (pTSA)54, trityl chloride (TrCl)55, ZnO NPs56, zwitterionic-type ionic liquid (CDIPS)57 and CoFe2O4/OCMC/Cu (BDC)58.
It is also important to note that, in recent years, the use of metal and metal oxide-based heterogeneous catalysts in organic transformations has been developed59,60,61,62. However, some of these catalytic systems suffer from problems of non-recoverability of catalyst, harsh reaction conditions, and the use of toxic organic solvents.
In view of the above and according to our experience in the preparation of magnetic nanocatalysts, herein, for the first time, a novel core–shell structured magnetic cobalt oxide supported organosilica-sulfonic acid (Co3O4@SiO2/OS-SO3H) nanocomposite is successfully prepared through a simple method. This namomaterial contains the advantages of magnetic nanoparticles such as easy separation and also the advantages of heterogeneous catalysts such as easy recoverability. The Co3O4@SiO2/OS-SO3H nanocomposite was characterized by using TGA, FT-IR, VSM, SEM, TEM, PXRD, and EDX analyses. The catalytic efficiency of this material was studied in the synthesis of biologically active xanthenes giving the desired products in high to excellent yields.
Experimental
Synthesis of Co3O4@SiO2
Magnetic Co3O4 nanoparticles were firstly synthesized through a reduction procedure as follows: CoCl2.6H2O (1.32 g) was added in 25 mL absolute EtOH while stirring at room temperature (RT). Then, ethanol-dissolved pluronic P123 (0.6 g in 7 mL EtOH) was added to the above solution. After complete mixing, NaBH4 (1.47 g) was added and the resulted combination was stirred for 10 min at RT. The obtained material was magnetically separated and washed completely with warm EtOH and water to remove pluronic P123 and other impurities. The product was dried at 65 °C for 5 h and called magnetic cobalt oxide (Co3O4). For the preparation of Co3O4@SiO2, the Co3O4 NPs (1 g) were dispersed in ethanol (60 mL), while ammonia (5.3 mL, 60% wt%) was added drop-wise. Then, tetraethylorthosilicate (TEOS, 1 mL) was slowly added and the resulted mixture was stirred at RT for 16 h. Finally, the magnetic solid product was collected using a magnet, washed with water and ethanol, dried at 70 °C for 6 h, and called Co3O4@SiO2 nanocomposite.
Preparation of Co3O4@SiO2/OS-SH
To prepare the Co3O4@SiO2/OS-SH MNPs, the Co3O4@SiO2 nanomaterial (0.5 g) was added to a solution containing water (12 mL) and ethanol (50 mL). The resulting mixture was stirred at RT for 30 min. Then, ammonia (2 mL, 25% wt%) was added and it was stirred at RT for another 10 min. Next, tetraethylorthosilicate (TEOS, 1 mL) and 1,2-bis(triethoxysilyl)methane (BTEM, 1 mL) were added drop-wise, and the obtained mixture was stirred at RT for 16 h. The product was magnetically separated, washed with absolute ethanol and water, and dried at 70 °C for 6 h. After that, the resulting material (1 g) was dispersed in dried toluene (25 mL), while (3-mercaptopropyl)trimethoxysilane (0.7 mmol) was added. This mixture was refluxed for 24 h. The final product was magnetically separated, washed with absolute ethanol and water, dried at 70 °C for 6 h, and denoted as Co3O4@SiO2/OS-SH.
Preparation of Co3O4@SiO2/OS-SO3H
For this, Co3O4@SiO2/OS-SH (0.5 g) was first dispersed in methanol (20 mL). Then, hydrogen peroxide (5 mL, 35%) was added and the resulted mixture was stirred at ambient temperature for 24 h. The product was separated by using an external magnetic field. After that, this was added to a flask containing sulfuric acid solution (25 mL, 2 M) and stirred at RT for 3 h. The resulted material was separated, washed with ethanol and water, dried at 70 °C for 5 h, and denoted as Co3O4@SiO2/OS-SO3H.
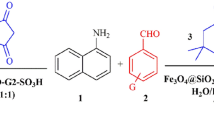
Synthesis of tetrahydrobenzo[a]xanthen-11-one derivatives
For this purpose, a mixture of benzaldehyde (1 mmol), dimedone (1 mmol), 2-naphthol (1 mmol), and Co3O4@SiO2-SO3H nanocatalyst (0.015 g) was stirred at 60 °C. The reaction progress was monitored by TLC. At the end of the reaction, the nanocatalyst was collected by a magnet and pure products were obtained after recrystallization in ethanol.
Spectral data of xanthene products
9,9-Dimethyl-12-phenyl-8,9,10,12- tetrahydrobenzo[a]xanthen-11-one
FT-IR (KBr, cm-1): 3058 (=CH, aromatic), 2932 (–CH, aliphatic), 1647 (C=O), 1616 (C=C, olefin), 1595, 1471 (C=C, aromatic), 1227 (C–O). 1H-NMR (400 MHz, DMSO, δ ppm): 0.97 (s, 3H), 1.13 (s, 3H), 2.26 (d, J = 16 Hz, 1H, COCH2), 2.31 (d, J = 16.4 Hz, 1H, COCH2), 2.58 (s, 2H), 5.72 (s, 1H), 7.07 (t, J = 7.6, 1H), 7.19 (t, J = 8, 2H), 7.33–7.47 (m, 5H), 7.76 (d, J = 8.4 Hz, 1H), 7.78 (d, J = 6.4 Hz, 1H), 8.00 (d, J = 8.4 Hz, 1H). 13C-NMR (100 MHz, DMSO, δ ppm): 28.40, 33.2, 34.0, 40.6, 51.3, 117.7, 119.7, 123.9, 124.2, 125.3, 127.3, 127.6, 128.0, 128.2, 128.9, 129.2, 130.5, 132.6, 142.2, 154.3, 164.2, 197.5.
9,9-Dimethyl-12-(4-nitrophenyl)-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one
FT-IR (KBr, cm-1): 3060 (=CH, aromatic), 2930 (–CH, aliphatic), 1650 (C=O), 1618 (C=C, olefin), 1594, 1475 (C=C, aromatic), 1515, 1341 (NO2), 1223 (C–O). 1H-NMR (400 MHz, DMSO, δ ppm): 0.98 (s, 3H), 1.00 (s, 3H), 2.24 (d, J = 16.5 Hz, 1H, COCH2), 2.35 (d, J = 16 Hz, 1H, COCH2), 2.60 (s, 2H), 5.69 (s,1H), 7.34 (d, J = 9.2, 1H), 7.38–7.48 (m, 2H), 7.51 (d, J = 8.8 Hz, 2H), 7.82–7.85 (m, 3H), 8.06 (d, J = 8.5 Hz, 2H). 13C-NMR (100 MHz, DMSO, δ ppm): 28.2, 33.2, 33.9, 40.5, 51.4, 117.8, 119.8, 123.8, 124.1, 125.5, 127.6, 128.0, 129.1, 129.2, 130.5, 132.6, 147.1, 147.3, 154.2, 164.1, 197.4.
Results and discussion
The Co3O4 NPs were synthesized through the reduction of CoCl2.6H2O in the presence of NaBH4. Next, Co3O4@SiO2 nanocomposite was prepared via a sol–gel process. In the following, the surface of Co3O4@SiO2 was modified with a layer of organosilica through co-condensation of BTEM and TEOS to give Co3O4@SiO2/OS nanomaterial. In fact, the SiO2/OS shell was prepared to protect Co3O4 NPs against oxidation and destruction by acid. Moreover, the organosilica (OS) layer also increases the surface lipophilicity of the material improving the performance of the designed catalyst in organic reactions. After that, Co3O4@SiO2/OS was modified with MPTMS groups to deliver Co3O4@SiO2/OS-SH nanocomposite. Finally, to obtain the Co3O4@SiO2/OS-SO3H nanocatalyst, the SH moieties of the Co3O4@SiO2/OS-SH nanocomposite were oxidized to SO3H in the presence of H2O2 (Fig. 1).
Figure 2 shows the FT-IR spectra of Co3O4, Co3O4@SiO2, Co3O4@SiO2/OS, and Co3O4@SiO2/OS-SO3H. The characteristic peaks at 3400 and 620 cm−1, for all materials, are related to the O–H and Co–O bonds, respectively (Figs. 2a–d). The intense absorption peaks at 1081 and 928 cm−1 are, respectively, related to unsymmetrical and symmetrical vibrations of the Si–O–Si bonds (Figs. 2b–d). Also, the peaks observed at 2825–2961 cm−1 can be assigned to the vibrations of aliphatic C–H bonds (Figs. 2c,d). The peak observed at 1107 cm−1 is related to S=O bond, which is partially overlapped with the silica peaks, confirming the successful oxidation of SH–SO3H (Fig. 2d).
Figure 3 illustrates the PXRD pattern of Co3O4@SiO2/OS-SO3H nanocomposite. As shown, the diffraction peaks of Co3O4 NPs are appeared at 2θ = 23.2°, 30.1°, 35.5°, 41.2°, 47.5°, 60° and 71.6° proving the high stability of crystalline structure of the Co3O4 NPs during the synthesis of Co3O4@SiO2/OS-SO3H nanocomposite.
The EDX analysis was used to investigate the presence of O, C, Co, Si, and S in the structure of the Co3O4@SiO2/OS-SO3H nanocomposite. As shown in Fig. 4, the signals of C, O, Si, S and Co elements are clearly seen in weight% of 18.9, 42.62, 15.85, 0.93, 21.7 and 21.7, respectively. This confirms the successful incorporation/immobilization of cobalt oxide and silicasulfonic acid moieties into/onto the framework of the designed nanocomposite.
Also, the VSM analysis of the Co3O4@SiO2/OS-SO3H nanocomposite showed a saturation magnetization of about 25 emu/g (Fig. 5). This result proves the good magnetic properties of the prepared nanocomposite which is a very important characteristic in the catalytic processes.
The SEM image of the Co3O4@SiO2/OS-SO3H nanocomposite is demonstrated in Fig. 6. As shown, sponge-like particles with spherical morphology and an average size of 40 nm are observed for this material.
TEM image of Co3O4@SiO2/OS-SO3H nanocomposite confirmed that the structure of the designed nanocomposite is almost spherical. Also, the image demonstrates a dark core (Co3O4) enclosed by a gray silica/organosilica layer confirming the core shell structure of this nanocomposite (Fig. 7).
Thermal stability of the prepared nanocomposite was also investigated by thermal gravimetric analysis (TGA). The first weight loss below 150 °C is corresponded to the removal of adsorbed water and alcoholic solvents. The second weight loss at 151–220 °C is related to elimination of supported propansulfonic acid moieties. The main weight loss observed at 225–600 °C is corresponded to decomposition and removal of organic groups in the shell framework. These results confirm high thermal stability of the designed catalyst (Fig. 8).
After the characterization of the designed nanocomposite, its catalytic activity was studied in the synthesis of tetrahydrobenzo[a]xanthen-11-ones. To optimization of the conditions, the condensation between benzaldehyde, dimedone, and 2-naphthol was selected as a test model. The study showed that the presence of the catalyst is necessary for the progress of the reaction and by using 0.015 g of Co3O4@SiO2/OS-SO3H, the highest yield was resulted (Table 1, entries 1–4). The study also showed that among EtOH, toluene, CH2Cl2, and CH3CN solvents, in EtOH the best result is obtained (Table 1, entry 3 vs. entries 5–7). Also, the study of the temperature effect illustrated that the best temperature for this process is 60 °C (Table 1, entry 3 vs. entries 8–11). Accordingly, the use of 0.015 g catalyst, ethanol solvent, and 60 °C were selected as optimum conditions (Table 1, entry 3).
Next, the substrate scope of this catalytic system was studied under optimal conditions. This demonstrated that all aldehyde substrates containing both electron-donor and electron-acceptor substituents successfully react with dimedone and 2-naphthol in the presence of Co3O4@SiO2/OS-SO3H nanocatalyst to give corresponding tetrahydrobenzo[a]xanthen-11-one in high yields (Table 2).
Then, the recoverability and reusability of the Co3O4@SiO2/OS-SO3H nanocatalyst were investigated. For this, the reaction between benzaldehyde, dimedone, and 2-naphthol by using Co3O4@SiO2/OS-SO3H nanocatalyst under optimal conditions was selected as a test model. After completion of the process, Co3O4@SiO2/OS-SO3H was separated and reused in another reaction under the same conditions as the first run. These steps were repeated and the results displayed that Co3O4@SiO2/OS-SO3H can be recovered and reused for at least seven runs with no important decrease in its activity (Table 3).
To prove the stability of the catalyst structure during the reaction, the recovered catalyst, after the fifth run, was washed several times with ethanol and characterized by using EDX and PXRD analyses. The PXRD analysis of the recovered catalyst showed a pattern with seven peaks at 2θ = 23.2°, 30.1°, 35.5°, 41.2°, 47.5°, 60° and 71.6° (SI, Fig. 1S). This result is in good agreement with the PXRD pattern of the fresh nanocatalyst confirming the high stability of the crystalline structure of magnetic Co3O4@SiO2/OS-SO3H NPs under applied conditions.
The EDX analysis of the recovered catalyst, after the fifth run, also showed the presence of expected C, O, Si, S, and Co elements in a wt% of 18.65, 42.18, 15.71, 0.9, and 22.56, respectively (SI, Fig. 2S). These results are approximately the same as those of fresh catalyst confirming the high stability of the composition of the designed catalyst under applied conditions.
The activity of Co3O4@SiO2/OS-SO3H nanocatalyst was compared with a number of catalysts reported in the synthesis of tetrahydrobenzo[a]xanthen-11-ones (Table 4). The result showed that our catalyst is better than others in terms of recovery times, reaction temperature, and yield of product. These findings can be ascribed to high stability, good lipophilicity, and the magnetic properties of the Co3O4@SiO2/OS-SO3H nanocatalyst.
A plausible mechanism for the synthesis of tetrahydrobenzo[a]xanthen-11-ones in the presence of Co3O4@SiO2/OS-SO3H is shown in Fig. 9. Firstly, the aldehyde is activated by the catalyst to give intermediate [I]. Next, the activated aldehyde and 2-naphthol react with each other via a Knoevenagel condensation to deliver intermediate [II]. In the next step, the nucleophilic attack of dimedone to the intermediate [II], through a Michael-type addition, gives intermediate [IV]. Finally, an intramolecular cyclization is performed to give the desired product.
Conclusion
In summary, a novel core–shell structured magnetic cobalt oxide supported organosilica-sulfonic acid nanocomposite was synthesized and called Co3O4@SiO2/OS-SO3H. The high thermal and chemical stability of the designed nanocomposite were confirmed by using EDX, TGA and FT-IR techniques. The SEM and TEM images illustrated a spherical morphology for this material. The good magnetic property of this material was confirmed by VSM. The Co3O4@SiO2/OS-SO3H nanocomposite was used as an effective catalyst in the synthesis of tetrahydrobenzo[a]xanthen-11-ones under mild reaction conditions. The desired xanthene products were obtained in high to excellent yield and selectivity at a relatively short reaction time. The catalyst was also recovered and reused several times with no significant decrease in its efficiency. Some applications of Co3O4@SiO2/OS-SO3H in other chemical processes are underway in our laboratory.
Data availability
All data and materials are included in the manuscript.
References
Shen, X.-P., Miao, H.-J., Zhao, H. & Xu, Z. Synthesis, characterization and magnetic properties of Co3O4 nanotubes. Appl. Phys. A. 91, 47–51 (2008).
Seidov, Z., Açıkgöz, M., Kazan, S. & Mikailzade, F. Magnetic properties of Co3O4 polycrystal powder. Ceram. Int. 42, 12928–12931 (2016).
Yin, K. et al. Magnetic properties of Co3O4 nanoparticles on graphene substrate. J. Alloys Compd. 720, 345–351 (2017).
Abid, H. N., Nayef, U. M. & Mutlak, F. A. Preparation and characterization Co3O4 nanoparticles on porous silicon for humidity sensor by photoluminescence. Optik 178, 379–383 (2019).
Vijayanandan, A. S. & Balakrishnan, R. M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manage. 218, 442–450 (2018).
Venkatesh, R. et al. Tailoring the physical properties and electrochromic performance of nebulizer spray coated Co3O4 films through copper doping. Solid State Ion. 334, 5–13 (2019).
Ahmad, S. et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomedicine. 14, 5087 (2019).
Iravani, S. & Varma, R. S. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 22, 2643–2661 (2020).
Memon, S. A. et al. Plant material protected cobalt oxide nanoparticles: Sensitive electro-catalyst for tramadol detection. Microchem. J. 159, 105480 (2020).
Abdallah, A. & Awad, R. Sm and Er partial alternatives of Co in Co3O4 nanoparticles: Probing the physical properties. Phys. B: Condens. Matter. 608, 412898 (2021).
Mahmoudi-Gom Yek, S., Azarifar, D., Khaleghi-Abbasabadi, M., Keypour, H. & Mahmoudabadi, M. Heterogenized magnetic graphene oxide-supported N6-Schiff base Cu(II) complex as an exclusive nanocatalyst for synthesis of new pyrido [2, 3-d] pyrimidine-7-carbonitrile derivatives. Appl. Organomet. Chem. 34, e5989 (2020).
Makhlouf, S. A. Magnetic properties of Co3O4 nanoparticles. Magn. Magn. Mater. 246, 184–190 (2002).
Jia, C.-J. et al. Co3O4–SiO2 nanocomposite: A very active catalyst for CO oxidation with unusual catalytic behavior. J. Am. Chem. Soc. 133, 11279–11288 (2011).
Ali, G. A., Fouad, O. A. & Makhlouf, S. A. Structural, optical and electrical properties of sol–gel prepared mesoporous Co3O4/SiO2 nanocomposites. J. Alloys Compd. 579, 606–611 (2013).
Ali, G. A., Fouad, O. A., Makhlouf, S. A., Yusoff, M. M. & Chong, K. F. Co3O4/SiO2 nanocomposites for supercapacitor application. J. Solid State Electrochem. 18, 2505–2512 (2014).
Lin, C.-C., Guo, Y. & Vela, J. Microstructure effects on the water oxidation activity of Co3O4/porous silica nanocomposites. ACS Catal. 5, 1037–1044 (2015).
Ghasemzadeh, M. A., Mirhosseini-Eshkevari, B. & Abdollahi-Basir, M. H. Rapid and efficient one-pot synthesis of 3, 4-dihydroquinoxalin-2-amine derivatives catalyzed by Co3O4@ SiO2 core-shell nanoparticles under ultrasound irradiation. Comb. Chem. High Throughput Screen. 19, 592–601 (2016).
Ibrahim, E. et al. Electric, thermoelectric and magnetic characterization of γ-Fe2O3 and Co3O4nanoparticles synthesized by facile thermal decomposition of metal-Schiff base complexes. Mater. Res. Bull. 99, 103–108 (2018).
Packiaraj, R., Devendran, P., Venkatesh, K., Manikandan, A. & Nallamuthu, N. Electrochemical investigations of magnetic Co3O4 nanoparticles as an active electrode for supercapacitor applications. J. Supercond. Nov. Magn. 32, 2427–2436 (2019).
Geetha, V., Induja, S. & Puthilibai, G. Effects on the crystal structure, magnetic and optical properties Nd-doped Co3O4 nanoparticles prepared by microwave synthesis. J. Supercond. Nov. Magn. 33, 1405–1411 (2020).
Gulati, A., Malik, J. & Kakkar, R. Peanut shell biotemplate to fabricate porous magnetic Co3O4 coral reef and its catalytic properties for p-nitrophenol reduction and oxidative dye degradation. Colloids Surf. A Physicochem. Eng. Asp. 604, 125328 (2020).
UmaSudharshini, A., Bououdina, M., Venkateshwarlu, M., Manoharan, C. & Dhamodharan, P. Low temperature solvothermal synthesis of pristine Co3O4 nanoparticles as potential supercapacitor. Surf. Interfaces 19, 100535 (2020).
Khaleghi Abbasabadi, M., Azarifar, D. & Esmaili Zand, H. R. Sulfonic acid-functionalized Fe3O4-supported magnetized graphene oxide quantum dots: A novel organic-inorganic nanocomposite as an efficient and recyclable nanocatalyst for the synthesis of dihydropyrano [2, 3-c] pyrazole and 4H-chromene derivatives. Appl. Organomet. Chem. 34, e6004 (2020).
Ozkaya, T., Baykal, A., Koseoğlu, Y. & Kavas, H. Synthesis of Co3O4 nanoparticles by oxidation-reduction method and its magnetic characterization. Open Chem. 7, 410–414 (2009).
Farhadi, S., Safabakhsh, J. & Zaringhadam, P. Synthesis, characterization, and investigation of optical and magnetic properties of cobalt oxide (Co3O4) nanoparticles. J. nanostructure chem. 3, 1–9 (2013).
Shatrova, N. et al. Elaboration, characterization and magnetic properties of cobalt nanoparticles synthesized by ultrasonic spray pyrolysis followed by hydrogen reduction. Mater. Res. Bull. 86, 80–87 (2017).
Hitkari, G., Sandhya, S., Gajanan, P., Shrivash, M. & Deepak, K. Synthesis of chromium doped cobalt oxide (Cr: Co3O4) nanoparticles by co-precipitation method and enhanced photocatalytic properties in the visible region. J. Mater. Sci. Eng. 7, 2169–2222 (2018).
Abdallah, A. & Awad, R. Study of the structural and physical properties of Co3O4 nanoparticles synthesized by Co-precipitation method. J. Supercond. Nov. Magn. 33, 1395–1404 (2020).
Reena, R. S., Aslinjensipriya, A., Jose, M. & Das, S. J. Investigation on structural, optical and electrical nature of pure and Cr-incorporated cobalt oxide nanoparticles prepared via co-precipitation method for photocatalytic activity of methylene blue dye. J. Mater. Sci. Mater Electron. 31, 22057–22074 (2020).
Mohanta, J., Dey, B. & Dey, S. Magnetic cobalt oxide nanoparticles: sucrose-assisted self-sustained combustion synthesis, characterization, and efficient removal of malachite green from water. J. Chem. Eng. Data. 65, 2819–2829 (2020).
Mohammadi, S. Z., Lashkari, B. & Khosravan, A. Green synthesis of Co3O4 nanoparticles by using walnut green skin extract as a reducing agent by using response surface methodology. Surf. Interfaces 23, 100970 (2021).
Ali, G. A., Fouad, O. A., Makhlouf, S. A., Yusoff, M. M. & Chong, K. F. in Advanced Materials Research. 447–451 Trans Tech Publ
Safari, J., Tavakoli, M. & Ghasemzadeh, M. A. H3PMo12O40-immobilized chitosan/Co3O4: A novel and recyclable nanocomposite for the synthesis of pyrimidinedione derivatives. Appl. Organomet. Chem. 33, e4748 (2019).
Zhou, L. et al. Double-shelled Co3O4/C nanocages enabling polysulfides adsorption for high-performance lithium–sulfur batteries. ACS Appl. Energy Mater. 2, 8153–8162 (2019).
Guo, J., Zhang, Y., He, Y.-C. & Shan, J. Photocatalytic performance of Co3O4/C based on ZIF-67/C composite materials. Polyhedron 175, 114215 (2020).
Qin, G. et al. Dispersed MoS2 nanosheets in core shell Co3O4@ C nanocubes for superior potassium ion storage. Appl. Surf. Sci. 514, 145946 (2020).
Mohammadi, S. Z., Safari, Z. & Madady, N. A novel Co3O4@ SiO2 magnetic nanoparticle-nylon 6 for high efficient elimination of Pb(II) ions from wastewater. Appl. Surf. Sci. 514, 145873 (2020).
Chen, X.-L. et al. Highly dispersed and stabilized Co3O4/C anchored on porous biochar for bisphenol a degradation by sulfate radical advanced oxidation process. Sci. Total Environ. 777, 145794 (2021).
Khaleghi Abbasabadi, M. & Azarifar, D. β-Alanine-functionalized magnetic graphene oxide quantum dots: an efficient and recyclable heterogeneous basic catalyst for the synthesis of 1H-pyrazolo [1, 2-b] phthalazine-5,10-dione and 2,3-dihydroquinazolin-4 (1H)-one derivatives. Appl. Organomet. Chem. 34, e5872 (2020).
Vaidya, S., Thaplyal, P., Ramanujachary, K., Lofland, S. & Ganguli, A. K. Synthesis of core–shell nanostructures of Co3O4@ SiO2 with controlled shell thickness (5–20 nm) and hollow shells of silica. J. Nanosci. Nanotechnol. 11, 3405–3413 (2011).
Abdel Ghafar, H. H., Ali, G. A., Fouad, O. A. & Makhlouf, S. A. Enhancement of adsorption efficiency of methylene blue on Co3O4/SiO2 nanocomposite. Desalin. Water Treat. 53, 2980–2989 (2015).
Shi, X., Quan, S., Yang, L., Shi, G. & Shi, F. Facile synthesis of magnetic Co3O4/BFO nanocomposite for effective reduction of nitrophenol isomers. Chemosphere 219, 914–922 (2019).
Ghasemzadeh, M. A., Elyasi, Z. & Monfared, M. R. Enhanced removal of methyl violet dye from aqueous solution by a novel Co3O4@ SiO2@ TiO2-Ag heterogeneous semiconductor. Comb. Chem. High Throughput Screen. 25, 883–894 (2022).
Wanjeri, V., Sheppard, C., Prinsloo, A., Ngila, J. & Ndungu, P. Isotherm and kinetic investigations on the adsorption of organophosphorus pesticides on graphene oxide based silica coated magnetic nanoparticles functionalized with 2-phenylethylamine. J. Environ. Chem. Eng. 6, 1333–1346 (2018).
Dien, L. X. et al. Facile synthesis of Co3O4@ SiO2/carbon nanocomposite catalysts from rice husk for low-temperature CO Oxidation. Mol. Catal. 518, 112053 (2022).
Kargar, S., Elhamifar, D. & Zarnegaryan, A. Core–shell structured Fe3O4@ SiO2-supported IL/[Mo6O19]: A novel and magnetically recoverable nanocatalyst for the preparation of biologically active dihydropyrimidinones. J. Phys. Chem. 146, 109601 (2020).
Jamasbi, N. et al. Silica-coated modified magnetic nanoparticles (Fe3O4@ SiO2@(BuSO3H) 3) as an efficient adsorbent for Pd2+ removal. Chemosphere 307, 135622 (2022).
Mahmoudi, H., Jafari, A. A., Saeedi, S. & Firouzabadi, H. Sulfonic acid-functionalized magnetic nanoparticles as a recyclable and eco-friendly catalyst for atom economical Michael addition reactions and bis indolyl methane synthesis. RSC Adv. 5, 3023–3030 (2015).
Tabrizian, E. & Amoozadeh, A. A new type of SO3 H-functionalized magnetic-titania as a robust magnetically-recoverable solid acid nanocatalyst for multi-component reactions. RSC Adv. 6, 96606–96615 (2016).
Taheri, S., Veisi, H. & Hekmati, M. Application of polydopamine sulfamic acid-functionalized magnetic Fe3O4 nanoparticles (Fe3O4@ PDA-SO 3 H) as a heterogeneous and recyclable nanocatalyst for the formylation of alcohols and amines under solvent-free conditions. New J. Chem. 41, 5075–5081 (2017).
Maleki, B., Reiser, O., Esmaeilnezhad, E. & Choi, H. J. SO3H-dendrimer functionalized magnetic nanoparticles (Fe3O4@ DNH (CH2) 4SO3H): Synthesis, characterization and its application as a novel and heterogeneous catalyst for the one-pot synthesis of polyfunctionalized pyrans and polyhydroquinolines. Polyhedron 162, 129–141 (2019).
Khaleghi-Abbasabadi, M. & Azarifar, D. Magnetic Fe3O4-supported sulfonic acid-functionalized graphene oxide (Fe3O4@ GO-naphthalene-SO 3 H): A novel and recyclable nanocatalyst for green one-pot synthesis of 5-oxo-dihydropyrano [3, 2-c] chromenes and 2-amino-3-cyano-1,4,5,6-tetrahydropyrano [3, 2-c] quinolin-5-ones. Res. Chem. Intermed. 45, 2095–2118 (2019).
Shaker, M. & Elhamifar, D. Sulfonic acid supported on magnetic methylene-based organosilica as an efficient and recyclable nanocatalyst for biodiesel synthesis via esterification. Front. Energy Res. 8, 78 (2020).
Khurana, J. M. & Magoo, D. pTSA-catalyzed one-pot synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a] xanthen-11-ones in ionic liquid and neat conditions. Tetrahedron Lett. 50, 4777–4780 (2009).
Khazaei, A. et al. Organocatalyst trityl chloride efficiently promoted the solvent-free synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones by in situ formation of carbocationic system in neutral media. Catal. Commun. 20, 54–57 (2012).
Safaei-Ghomi, J. & Ghasemzadeh, M. A. A simple and efficient synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a] xanthen-11-ones by ZnO nanoparticles catalyzed three component coupling reaction of aldehydes, 2-naphthol and dimedone. S. Afr. J. Chem. 67, 27–32 (2014).
Rafiee, E. & Shahebrahimi, S. Effect of heteropoly acids on structure, electrochemical behavior, acidic properties and catalytic activity of zwitterionic-type ionic liquid. Inorg. Chim. Acta 498, 119086 (2019).
Ghasemzadeh, M. A. & Ghaffarian, F. Preparation of core/shell/shell CoFe2O4/OCMC/Cu (BDC) nanostructure as a magnetically heterogeneous catalyst for the synthesis of substituted xanthenes, quinazolines and acridines under ultrasonic irradiation. Appl. Organomet. Chem. 34, e5580 (2020).
Rezvani, M. A., Ardeshiri, H. H. & Aghasadeghi, Z. Extractive-oxidative desulfurization of real and model gasoline using (gly) 3H [SiW12O40]⊂ CoFe2O4 as a recoverable and efficient nanocatalyst. Energy Fuels 37(3), 2245–2254 (2023).
Rezvani, M. A., Ardeshiri, H. H., Ghafuri, H. & Hosseini, S. Highly oxidative desulfurization of thiophenic model fuels and real gasoline by Keggin-type heteropolyanion immobilized on polyaniline and chitosan as an efficient organic–inorganic nanohybrid catalyst. J. Appl. Polym. Sci. 140, e53950 (2023).
Rezvani, M. A., Aghmasheh, M., Hassani, A. & Hassani Ardeshiri, H. Synthesis and characterization of a new hybrid nanocomposite based on di-substituted heteropolyanion-quantum dots as a high-performance nanocatalyst for organic dye removal from wastewater. J. Coordi. Chem. 75, 507–523 (2022).
Dalvand, S. et al. Graphene oxide@ Fe3o4@ Tungstate modified ionic liquid as a novel electrode material for high-performance supercapacitor. Int. J. Hydrogen Energy 48, 10098–10107 (2023).
Kulangiappar, K., Anbukulandainathan, M. & Raju, T. Synthetic communications: An international journal for rapid communication of synthetic organic chemistry. Synth. Commun. 1, 2494–2502 (2014).
Rohaniyan, M., Davoodnia, A., Beyramabadi, S. A. & Khojastehnezhad, A. Phosphomolybdic acid supported on Schiff base functionalized graphene oxide nanosheets: Preparation, characterization, and first catalytic application in the multi-component synthesis of tetrahydrobenzo [a] xanthene-11-ones. Appl. Organomet. Chem. 33, e4881 (2019).
Heydari, R. & Shahrekipour, F. One-pot synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a] xanthen-11-ones by using of neutral and efficient organocatalysts under solvent-free conditions. Res. Chem. Intermed. 41, 4581–4586 (2015).
Zang, H. et al. An efficient and green one-pot synthesis of 12-Aryl-8, 9, 10, 12-tetrahydrobenzo[a]xanthen-11-one derivatives promoted by sulfamic acid in [BMIM] BF4 Ionic Liquid. Chin. J. Chem. 30, 362–366 (2012).
Sharifi Sharif Abad, S., Mirjalili, B. B. F. & Bamoniri, A. Fe3O4@ nano-walnut Shell/BIII as a new natural based catalyst for synthesis of tetrahydrobenzo[a]Xanthene-11-One derivatives. Polycycl. Aromat. Compd. https://doi.org/10.1080/10406638.2022.2144907 (2022).
Maleki, A., Aghaei, M. & Ghamari, N. Facile synthesis of tetrahydrobenzoxanthenones via a one-pot three-component reaction using an eco-friendly and magnetized biopolymer chitosan-based heterogeneous nanocatalyst. Appl. Organomet. Chem. 30, 939–942 (2016).
Sonei, S., Gholizadeh, M. & Taghavi, F. Cu (II) anchored on modified magnetic nanoparticles: as a green and efficient recyclable nano catalyst for one pot synthesis of 12-Aryl-8,9,10,12tetrahydrobenzo[a]xanthene-11-oneʺ. Polycycl. Aromat. Compd. https://doi.org/10.1080/10406638.2018.1531431 (2019).
Balou, J., Khalilzadeh, M. A. & Zareyee, D. An efficient and reusable nano catalyst for the synthesis of benzoxanthene and chromene derivatives. Sci. Rep. 9, 1–9 (2019).
Acknowledgements
The authors thank the Yasouj University and the Iran National Science Foundation (INSF) for supporting this work.
Funding
There is no funding to declare.
Author information
Authors and Affiliations
Contributions
H.A.: writing—original draft, investigation, resources, formal analysis. D.E.: Conceptualization, writing—review and editing, supervision, visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ardeshirfard, H., Elhamifar, D. Magnetic cobalt oxide supported organosilica-sulfonic acid as a powerful nanocatalyst for the synthesis of tetrahydrobenzo[a]xanthen-11-ones. Sci Rep 13, 14134 (2023). https://doi.org/10.1038/s41598-023-41234-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-41234-x
This article is cited by
-
Modified magnetic chitosan with mono(6-amino-6-deoxy)-β-cyclodextrin as a novel catalyst toward the synthesis of pyrazolopyranopyrimidines and pyrano[2,3-c]pyrazole-3-carboxylates
Scientific Reports (2025)
-
Co3O4@mSiO2 nanocomposite supported Pd/ionic liquid as an efficient and magnetically recoverable nanocatalyst
Scientific Reports (2025)
-
Synthesis and characterization of FeCuAlO4 as a reusable heterogeneous acidic nanocatalyst for preparation of 2-amino-1,3,4-thiadiazole derivatives in water
Scientific Reports (2025)
-
Fe3O4@SiO2@NTMPThio-Cu: a sustainable and eco-friendly approach for the synthesis of heterocycle derivatives using a novel dendrimer template nanocatalyst
Scientific Reports (2024)