Abstract
In the modern world, wheat, a vital global cereal and the second most consumed, is vulnerable to climate change impacts. These include erratic rainfall and extreme temperatures, endangering global food security. Research on hydrogen-rich water (HRW) has gained momentum in plant and agricultural sciences due to its diverse functions. This study examined the effects of different HRW treatment durations on wheat, revealing that the 4-h treatment had the highest germination rate, enhancing potential, vigor, and germination indexes. This treatment also boosted relative water content, root and shoot weight, and average lengths. Moreover, the 4-h HRW treatment resulted in the highest chlorophyll and soluble protein concentrations in seeds while reducing cell death. The 4-h and 5-h HRW treatments significantly increased H2O2 levels, with the highest NO detected in both root and shoot after 4-h HRW exposure. Additionally, HRW-treated seeds exhibited increased Zn and Fe concentrations, along with antioxidant enzyme activities (CAT, SOD, APX) in roots and shoots. These findings suggest that HRW treatment could enhance wheat seed germination, growth, and nutrient absorption, thereby increasing agricultural productivity. Molecular analysis indicated significant upregulation of the Dreb1 gene with a 4-h HRW treatment. Thus, it shows promise in addressing climate change effects on wheat production. Therefore, HRW treatment could be a hopeful strategy for enhancing wheat plant drought tolerance, requiring further investigation (field experiments) to validate its impact on plant growth and drought stress mitigation.
Similar content being viewed by others
Introduction
Wheat (Triticum aestivum L.) is an essential crop that plays a crucial role in ensuring food security and fueling the global economy through various value chains. Due to the growing human population worldwide and decreasing agricultural land, the need for wheat products is projected to rise by 60% by the year 20501. Therefore, meeting the global demand for wheat will require an annual increase in wheat yields of 1.6%1. Wheat contains carbohydrates (71%), protein (13%), water (13%), fat (1.5%), as well as trace amounts of phosphorus, niacin, and dietary fiber2,3. However, climate change-induced droughts can negatively affect agriculture-based systems by causing irregular rainfall patterns, requiring adjustments to crop cycles, promoting changes in diseases and insect dynamics, ultimately limiting the potential for increasing wheat production4.
In the twenty-first century, food security is facing humanity's most significant challenges, which are the constantly increasing global population and the highly unpredictable climate. The impact of climate change, including extreme temperatures and erratic rainfall patterns, will have consequences on wheat, which is the world's second most widely consumed cereal crop5. The growth and productivity of wheat are negatively affected by climate change, as it causes detrimental effects on plant physiological processes, such as crop evapotranspiration (ETc) and water use efficiency (WUE)6,7. A decline in global wheat yields of approximately 6.0 ± 2.9% is expected with every 1° increase in global temperature6. Additionally, research conducted in Iran indicates that climate change will result in a decrease in grain yield and WUE, accompanied by a higher crop water requirement5,7.
At present, predicting drought, a slowly evolving climatic phenomenon with the longest duration, is highly unlikely8. Drought can take on different forms such as meteorological, agricultural, hydrological, and socioeconomic depending on factors such as the duration of low rainfall, deficits in soil moisture, or depletion of surface and groundwater storage9. Numerous studies have reported that drought conditions have various impacts on agriculture, including a direct effect on the productivity of wheat10,11. By the end of the twenty-first century, there is a projection that agricultural regions affected by global drought will experience a significant increase12. The production of 75% of the global wheat harvested area was affected by drought, leading to an average reduction of 0.29 t ha−113,14.
The condition of drought stress is marked by insufficient water availability, causing various changes in morphology, biochemistry, physiology, and molecular structure15. Drought stress affects various aspects of plant functioning such as photosynthesis, chlorophyll production, nutrient metabolism, ion absorption and translocation, respiration, and carbohydrate metabolism15. In order to survive under drought conditions, wheat must adapt, and researchers have developed various resistant genotypes that can help maintain levels of soluble sugars, proline content, amino acids, chlorophyll content, as well as enzymatic and non-enzymatic antioxidant activities16. Drought stress caused a decline in grain production by 11–34% and a shorter grain filling period by 15–24%17. Winter wheat yield decreased by 3.09 t × ha−1 (46.8%) in semiarid conditions when compared to irrigated conditions18.
Drought stress has a significant impact on wheat cultivation in Bangladesh, a country heavily reliant on wheat as a second staple crop19. The most favorable temperature for wheat cultivation is approximately 20 °C, and it lies within the range of 17–23 °C20. In Bangladesh, where the lowest recorded temperature hovers at approximately 15 °C and the highest reaches 35 °C, the optimal temperature range for cultivating wheat is between 20 and 25 °C20. Moreover, the northwestern region is largely prone to drought conditions and encounters significantly reduced precipitation levels when compared to other areas of the country, suggesting that the temperature in this particular region is also higher than in other parts of the country21. Drought stress leads to various consequences such as diminished crop yield, stunted growth, premature ripening, reduced nutritional value, heightened vulnerability to diseases, and financial losses for farmers 19.
Plants or plant tissues can receive H2 as either a gas or a dissolved solution, which is typically created by bubbling H2 through water to create hydrogen-rich water (HRW). HRW can be administered to plants by either adding it to the soil or feed solution or by spraying it onto the foliage22. Under stressful conditions such as the presence of excessive metal ions, hydrogen-rich water (HRW) has been shown to promote root growth23. The relief of stress by H2 is believed to involve phytohormone signalling, as suggested by some studies22,24. Sun et al.25 assert that H2 is safe for human consumption and, as a result, is safe to use in plant treatments for food crops. Wu et al.26 found that cabbage showed increased levels of antioxidants when subjected to cadmium stress, while Chen et al.27 observed that the antioxidant capacity of Hypsizygus marmoreus (a type of mushroom) was elevated through the use of HRW during the postharvest period.
Despite ongoing research, the specific role of HRW in mitigating wheat's susceptibility to drought stress remains uncertain. The main aim of this manuscript was to determine the optimal duration for soaking seeds in HRW (hydrogen-rich water) and to elucidate how HRW contributes to enhancing drought resistance in wheat. This was achieved by examining the morphological and physiological transformations that took place after the seed immersion. The potential identification of increased expression of Dreb1 genes in wheat, following treatment with HRW, could contribute to enhancing drought stress tolerance. Furthermore, the study included correlation analysis to enhance and support the findings obtained during the research.
Results
Morpho-physiological characteristics analysis
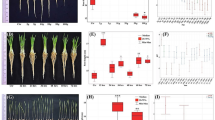
The study examined the effect of different durations of treatment with HRW on the germination rate and growth of wheat. The results showed that the 4-h treatment had the highest germination rate of 96.66% (Fig. 1a) and significantly improved germination potential (Fig. 1b), vigor index (Fig. 1c) and germination index (Fig. 1d) compared to the control group. Furthermore, the 4-h treatment led to an increase in relative water content (Fig. 2a), root and shoot dry weight (Fig. 2b) and average root and shoot length (Fig. 2c) compared to the control group. Specifically, after 10 days of treatment, the 4-h HRW treatment resulted in a relative water content in the root of 86.12% and in the shoot of 97.33% (Fig. 2a), an average root length of 22.13 cm (Fig. 2b), and average shoot height of 16.17 cm (Fig. 2b). The average root-shoot dry weight for the 4-h HRW treatment was 7.60 mg and 11.07 g, respectively (Fig. 2c). There was no discernible distinction in the number of roots (Fig. 2d) between the control and treatment groups of plants.
Germination parameters of wheat seeds; (a) illustrates germination percentage, (b) illustrates germination potential, (c) illustrates vigor index of seeds and (d) illustrates index of germination. Different letters used to indicate significant differences between the mean ± SD of replications (n = 3) at a significance level of P < 0.05.
Morphological characteristic of wheat seedlings; (a) illustrates relative water content percentage, (b) illustrates shoot and root length, (c) illustrates shoot and root dry weight and (d) illustrates average number of roots. Different letters used to indicate significant differences between the mean ± SD of replications (n = 3) at a significance level of P < 0.05.
Chlorophyll and cell death measurement
The investigation revealed that all treatments exhibited distinct concentrations of chlorophyll (chlorophyll a and b). The seeds treated with HRW for 4 h resulted in the maximum concentration of chlorophyll, measuring 11.69 mg/g (fresh weight) (Fig. 3a). In comparison to the control group, the seeds treated with HRW exhibited lower percentages of cell death. Furthermore, the root and shoot of plants treated with HRW for 4 h exhibited the lowest percentage of cell death, with values of 5.98% and 9.82% (Fig. 3b), respectively. Conversely, the control group recorded higher cell death percentages, with values of 8.02% and 12.76% (Fig. 3b) for root and shoot, respectively.
Biochemical characterization of wheat seedlings; (a) illustrates chlorophyll (a + b) content, (b) illustrates cell death percentage, (c) illustrates total soluble sugar and (d) illustrates total soluble protein. Different letters used to indicate significant differences between the mean ± SD of replications (n = 3) at a significance level of P < 0.05.
The impact on the concentration of total soluble sugar and protein
The study found that the total soluble sugar content gradually decreased in the plants treated with different durations of HRW compared to the control plants. The 6-h HRW treatment resulted in the lowest sugar concentration in both the root and shoot, with values of 21.24 mg g−1 and 23.51 mg g−1 (Fig. 3c) respectively, as compared to the control values of 31.10 mg g−1 and 32.36 mg g−1 (Fig. 3c). Conversely, the highest total soluble protein was observed in the 4-h treatment plants, with values of 1.16 mg g−1 and 1.29 mg g−1(Fig. 3d) in the root and shoot, respectively, as compared to the control values of 0.92 mg g−1 and 0.87 mg g−1(Fig. 3d).
H2O2 and NO concentration measurement
In the 4-h and 5-h treated seeds with HRW, there was a notable increase in the concentration of H2O2 as compared to the control group. The highest concentration of H2O2, measuring 6.55 μmol g−1 FW (Fig. 4a), was found in the root, while the shoot had a concentration of 18.78 μmol g−1 FW (Fig. 4a). However, the other treated seed groups showed a slight decrease in H2O2 concentration compared to the control group. The concentration of NO showed significant changes in both the root and shoot of the plants treated with HRW, as compared to the control group. The highest amount of NO was detected in both the root and shoot of the plants after being exposed to HRW treatment for 4 h, with a recorded measurement of 3.30 μmol g−1 FW (Fig. 4b) and 6.77 μmol g−1 FW (Fig. 4b), respectively, as compared to the control root and shoot, which measured 2.24 μmol g−1 FW (Fig. 4b) and 5.06 μmol g−1 FW (Fig. 4b), respectively.
Biochemical characterization of wheat seedlings; (a) illustrates total soluble H2O2, (b) illustrates NO concentration, (c) illustrates Zn concentration and (d) illustrates Fe concentration. Different letters used to indicate significant differences between the mean ± SD of replications (n = 3) at a significance level of P < 0.05.
Zn and Fe concentration measurement
Compared to the control group, the seeds treated with HRW exhibited a significant increase in Zn and Fe concentrations in both root and shoot. The root and shoot of the 4-h HRW-treated plants had the highest observed Zn concentration of 197.43 mg g−1 DW (Fig. 4c) and 66.98 mg g−1 DW (Fig. 4c), respectively, while the control group recorded 140.06 mg g−1 DW in root (Fig. 4c) and 46.23 mg g−1 DW in shoot (Fig. 4c). Similarly, the maximum Fe concentration of 109.83 mg g−1 DW in root (Fig. 4d) and 209.98 mg g−1 DW in shoot (Fig. 4d) was observed in the 4-h HRW-treated group, while the control group had 71.11 mg g−1 DW in root (Fig. 4d) and 171.56 mg g−1 DW in shoot (Fig. 4d).
The impact of antioxidant enzymes (SOD, APX, CAT) on young plants
The levels of antioxidant activity were notably higher in the 3-h, 4-h, and 5-h HRW treatments, but only slightly increased in the other treatments compared to the control. The maximum CAT activity was observed in the root and shoot during the 4-h HRW treatment, with a value of 10.07 nmol min−1(mg protein−1) (Fig. 5a), and with a value of 11.32 nmol min−1 (mg protein−1) (Fig. 5a), respectively. The HRW-treated seeds demonstrated increased APX activities in both the shoot and root compared to the control. The 4-h HRW treatment resulted in the highest APX activity levels in the shoot and root, with values of 9.84 nmol min−1 (mg protein−1) (Fig. 5b) and 2.89 nmol min−1 (mg protein−1) (Fig. 5b), respectively. Similar results were also observed in the SOD activities, with the maximum activity levels in the shoot and root measured at 9.75 nmol min−1 (mg protein−1) (Fig. 5c) and 8.92 nmol min−1 (mg protein−1) (Fig. 5c), respectively.
Gene expression analysis
According to the real-time PCR analysis, the expression of the Dreb1 gene was relatively consistent across the control, 2 h, 3 h, 4 h, and 5 h HRW treatment groups at the onset of drought induction (0 h). However, after 24 h post-induction of drought, the HRW treated group exhibited a significant increase in Dreb1 gene expression compared to the control group (Fig. 6). The HRW treatment for 3 h and 4 h demonstrated significantly elevated expression of the Dreb1 gene compared to both the control group and other treatment groups. Notably, the 4-h HRW treatment exhibited the highest expression of the Dreb1 gene (Fig. 6), indicating an approximate 90% increase compared to the control group.
Correlation analysis
The heat-map (Fig. 7) illustrated the significant Pearson correlation among 34 plants’ morphological, biochemical, and enzymatic parameters after HRW treatment at different time intervals. The yellow color in the heat-map indicated a highly significant R value (R = 1), while the purple color represents a non-significant R value (R = 0). The correlation analysis revealed that the 4-h HRW treatment exhibited a highly significant relationship compared to both the control group and other treatment groups (Fig. 7). However, four parameters, namely root PCD, shoot PCD, root TSS, and shoot TSS, showed non-significance at the 4-h treatment group, which also holds importance for wheat drought tolerance activity. The findings from the Global analysis of similarities (ANOSIM) revealed considerable dissimilarities between the wheat seedlings treated with HRW at different time intervals and the control group (Table S1). The statistical significance (P = 0.001) indicated that these differences are highly significant from a statistical standpoint. During the PCO plot analysis, PCO1 accounted for 47.8% of the total variation, while PCO2 contributed to 27.2% of the total variation (Fig. S1).
Heat map depicts the significant Pearson correlation among wheat seedlings morphological, biochemical and enzymatically 34 parameters after HRW treated seeds at different time intervals. The yellow color key indicates higher R value, while blue indicates lower value. The seedling parameters S. DW (Shoot dry weight), R. RWC (root relative water content), Gr (germination rate), R. length (root length), S. RWC (shoot relative water content), S. DW (shoot dry weight), Iv (vigor index), R. TSP (total soluble protein of root), S. Zn (Zn concentration of shoot), R. Fe (Fe concentration of root), S. Fe (Fe concentration of shoot), R. NO (NO concentration of root), Gp (germination potential), S. NO (NO concentration of shoot), R. Zn (Zn concentration of root), Chlorophyll (chlorophyll a and b), S. SOD (superoxide dismutase of shoot), Ig (Index of germination), S. CAT (catalase activity of shoot), R. CAT (catalase activity of root), R. SOD (superoxide dismutase activity of root), S. length (shoot length), S. TSP (total soluble protein of shoot), R. H2O2 (H2O2 concentration of root), S. APX (ascorbate peroxidase activity of shoot), R. APX (ascorbate peroxidase activity of root), Dreb1 24 h (Dreb1 gene expression after 24 h of treatment), Dreb1 0 h (Dreb1 gene expression after 0 h of treatment), R. TSS (total soluble sugar of root), S. TSS (total soluble sugar of root), R. No. (average root number), S. PCD (program cell death of shoot) and R. PCD (program cell death of root) are mentioned here. In the figure, 4 h treated (T3) wheat seed showed highest significant R value (R = 1) compare to rest of the treated seed. The root dry weight was also increased when seeds were treated at 5 h and 6 h likely 4 h treated seeds.
Discussion
Drought is a significant abiotic factor that hinders plant growth and development, which negatively affects agricultural need28,29. Half a century ago, researchers found out that introducing external H2 gas significantly enhances the process of seed germination30. Our experiment revealed that the 4-h treatment of seeds with HRW was significantly more effective than both the control group and other treatment groups in promoting morphological growth, as evidenced by increased germination rate, germination potential, vigor index, root-shoot length and number, as well as fresh and dry weight of root and shoot. After the implementation of HRW treatment to T. aestivum L. seeds, notable refinements were picked out in their biochemical profiles, functions, and physical structure. The substantial functional heterogeneity between the seeds subjected to HRW treatment and the control group were evident throughout the entire experimental period. Previous studies have noted a comparable morphological advancement in barley where subjecting HRW to drought circumstances resulted in an increase in the rate of seed germination and a reduction in the rate of inhibition of seedling growth31. Furthermore, the treatment of rice plants with HRW resulted in an increase in both plant height and dry weight32. Wheat seedlings, altering their physical traits to manage drought stress, frequently employ diverse strategies to confront limited water availability33,34,35. Yet, hydrogen-rich water might have the potential to aid in drought resilience by enabling these alterations in form and structure.
In our present investigation, it was discovered that plants derived from seeds treated with HRW exhibited a significant increase in the accumulation of both RWC and chlorophyll content, both of which are crucial for plant growth, with RWC playing a crucial role in maintaining water status and stress tolerance29, while chlorophyll is an essential component for photosynthesis and energy production36. Prior several studies have indicated that administering HRW can mitigate the reduction in chlorophyll content caused by salt stress in both cucumber seedlings37 and Arabidopsis38. In comparison to the distilled water control group, the application of HRW resulted in a gradual reduction of soluble sugar content in both root and shoot of the plant. Specifically, the total soluble sugar content showed a significant decrease in the group treated with HRW for 6 h, in contrast to other treatment groups. A previous study reported that decreasing the level of soluble sugar in plants enhances their ability to withstand environmental stressors like drought and heat39. Conversely, a significant rise in soluble protein content was noticed, with seeds treated with HRW for 4 h exhibiting maximum values of 1.15 mg/g and 1.29 mg/g, in contrast to the control, for the four respective treatment condition. Previous research has found that HRW enhances total soluble protein levels in cucumber seedlings to mitigate salt tolerance 37 and in alfalfa to alleviate cadmium toxicity40. Research exploring the effects of hydrogen-rich water on wheat seedlings’ physiology suggests its potential to mitigate the effects of drought stress by modulating these physiological parameters.
Our research has demonstrated that HRW treatment for a duration of 4 h significantly increased the levels of H2O2 and NO in both the root and shoot of wheat seeds, as compared to the control group. This finding is noteworthy as H2O2 and NO are pivotal signaling molecules that participate in a wide range of developmental and physiological processes in plants, including germination, growth, root organogenesis, pollen tube growth, flowering, and responses to biotic and abiotic stimuli31,41. Our findings also suggest that the application of HRW treatment to wheat seeds, particularly for 4 h, can markedly increase the concentrations of Zn and Fe in both the root and shoot, thus indicating the promising potential of HRW treatment for enhancing the levels of these nutrients in plants. Several studies have reported that the provision of sufficient quantities of Zn and Fe nanoparticles can alter the drought tolerance of crops such as wheat, sunflower, tomato, and red cabbage 41,42. H2O2, NO, Fe, and Zn are vital for boosting wheat plants' resilience to drought as well as maintaining their balanced levels is crucial for optimal plant function42. Regulating these elements through hydrogen-rich water treatment could greatly enhance wheat seedlings' ability to withstand drought stress31.
In arid environment, plant cells generate operational reactive oxygen species (ROS), while their antioxidant enzymes function as inhibitors of ROS generation and reactivity to environmental stresses. These enzymes, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT), are crucial in mitigating oxidative damage and reducing ROS production in plants. Among them, CAT is particularly noteworthy in scavenging H2O2 to impede ROS formation32,37,43. Our study revealed that the application of HRW to plants led to enhanced levels of CAT, SOD, and APX activities relative to the control group. In this study wheat seedlings that were exposed to HRW for 4–5 h exhibited a noteworthy boost in the activities of these enzymes relative to other treatment groups. Furthermore, the enzymes ascorbate peroxidase (APX), catalase (CAT), and superoxide dismutase (SOD) play critical role in enhancing drought tolerance in wheat plants33,44.
A recent study found that certain genes, such as DREB145, TaMYB30-B46, TaASR147, AtWRKY3048 and HVA149 greatly enhance wheat's resistance to drought stress. To demonstrate the expression of wheat genes related to drought stress, we examined the expression of DREB1 genes following drought induction. Our results indicated that HRW has a notable ability to naturally induce the expression of the DREB1 gene. Remarkably, the 4-h treatment of HRW showed the highest expression of the DREB1 gene, signifying an approximate 90% increase compared to the control group. This finding strongly suggests that HRW has the potential to enhance the drought tolerance of wheat.
The application of HRW treatment on wheat seeds led to substantial enhancements in growth factors and the accumulation of essential components in wheat plants. It elevated nutrient levels and boosted antioxidant enzyme activity, thereby mitigating oxidative damage and reducing ROS production. HRW exhibited potential in devising novel approaches to combat the detrimental impacts of climate change on wheat production, including the activation of drought tolerance genes.
Methods
Preparation of hydrogen-rich water (HRW)
In this experiment, utilized a hydrogen water generator to produce hydrogen-rich water (HRW), which is water that contains dissolved molecular hydrogen gas. The generator employed an electrolysis process that split the water molecules into hydrogen gas and oxygen gas. The hydrogen gas was then dissolved into the water, creating hydrogen-rich water. The hydrogen water generator also included a mechanism to separate the hydrogen and oxygen gases, ensuring that only hydrogen gas was dissolved into the water. The hydrogen water generator utilized in the experiment has a cylindrical shape and features an output dispenser for dispensing the hydrogen-rich water. The generator's body is constructed using e glass or metal, while the electrodes are composed of platinum and titanium. This particular hydrogen machine has a capacity of 380 mL, and through the process of electrolysis, it can produce high hydrogen concentrations of up to 1500 ppb.
Seed treatment with HRW
HRW was prepared by hydrogen water generator. Then wheat seeds (BARI-33) were washed thoroughly and immersed in distilled water for 30 min that are collected from Regional Wheat Research Institution, Rajshahi. We followed established protocols for collecting plant specimen, ensuring that our actions do not endanger the survival of plant species. The plant materials and data were used in a responsible and ethical manner. Distilled water was used for control and HRW was used for treatment seeds. Randomly selected wheat seeds were submersed in six different petri dishes as control, 2-h, 3-h, 4-h, 5-h and 6 h. After the treatment the control and treated wheat seeds were placed into each 90 mm petri dish containing 2 layers moistened of tissue papers at the bottom for germination. A total of 720 (3 replicates × 6 groups × 40 seeds) wheat seeds were selected randomly and divided into six groups as per instruction of ISTA 2018 [International Seed Testing Association (ISTA)]. Then the petri dishes were taken at growth chamber at 23 ± 2 °C temperature and 50% humidity for germination parameters observation.
Morpho-physiological characteristics
The germination parameters and plant growth were evaluated according to the procedure described by Zhou et al.50. The germination rate (GR), germination potential (GP), Index of Germination (IG), and Vigor Index (VI) were calculated using the same method as previously reported. After 10 days in the petri dishes, the plant growth was assessed. The plants were carefully removed from the tissue paper, and their roots were washed with tap water. The lengths of the roots and shoots were measured, and the number of roots was recorded.
Assessing the relative water content (RWC)
The method for determining the relative water content of plant roots and shoots, as described by González and González-Vilar51, involved taking five plants from petri dishes, washing the roots and removing any excess water, and measuring the fresh weight (FW) of the root and shoot separately. The roots and shoots were immersed in petri dishes containing distilled water for a duration of 2 h, and their turgid weight (TW) was recorded. Next, the dry weights (DW) of plant roots and shoots were determined following the method described by Arshadullar and Zaidi S52. The plant shoots were cut at their base and allowed to dry for 4 days at a temperature of 75 °C to eliminate moisture. Once completely dried, the weight of the shoots was measured using an electronic scale. Likewise, the roots from the same plants underwent a 4-days incubation at 75 °C for moisture removal and were subsequently weighed using an electronic scale. The RWC was then calculated using a formula by WEATHERLEY53.
Biochemical changes analysis
Chlorophyll (a + b) determination
To determine the chlorophyll concentration, fresh young shoots were ground with a mortar and pestle in 90% methanol. The mixture was then centrifuged, and the supernatant was collected in tubes. The optical density of the supernatant was measured at 662 nm for chlorophyll a and 653 nm for chlorophyll b using a spectrometer (Analytic Gena, Germany). The total chlorophyll concentration was calculated using a standard procedure54.
Assessing the concentration of total soluble sugar
To determine the total soluble sugar concentration in the roots and shoots, the method described by Dubois et al.55 was used. The roots and shoots were homogenized in aqueous ethanol (v/v 80%) at 12,000 rpm for 5 min, and the resulting supernatants were collected in tubes. Next, 0.2% anthrone reagent was mixed with the clear supernatant. The sample mixtures were incubated in a boiling water bath for 8 min and immediately placed on ice. In order to ascertain the overall concentration of soluble sugars, the ice-cold samples' optical density was assessed at 620 nm. Subsequently, the total soluble sugar content was quantified by comparing the optical density data at 620 nm with a standard glucose curve.
Assessing the concentration of total soluble protein
The amount of soluble protein in both roots and shoots was determined using a spectrophotometric method56. To isolate the protein, the roots and shoots were thoroughly washed, weighed, and finely ground using a chilled mortar and pestle in a buffer solution comprising 2 mM EDTA, 50 mM Tris–HCl, pH 7.5, and 0.04% (v/v) 2-mercaptoethanol. The resulting homogenates were subsequently subjected to centrifugation at 12,000 rpm for 10 min at 25 °C, and the clear liquid (supernatant) was then transferred into quartz cuvettes. The clear liquid obtained after centrifugation was combined with 1 mL of Coomassie Brilliant Blue (CBB), and its optical density was measured at 595 nm using a spectrophotometer (Analytic Gena, Germany). The total soluble protein content was determined by comparing the optical density data at 595 nm with a standard curve of BSA (bovine serum albumin)56.
Assessing the concentration of nitric oxide (NO)
The level of NO in seedlings’ roots, and shoots was measured by monitoring the changes in the absorption of hemoglobin, which results in the conversion of oxyhemoglobin (HbO2) to methemoglobin (metHb) in the presence of NO57. The samples were homogenized in a chilled NO buffer (1 mL) and then centrifuged at 10,000 rpm for 10 min. The resulting supernatants were combined with a 5 mM HbO2 solution and left to incubate for 7 min at room temperature. To assess the conversion rate of HbO2 to methemoglobin (metHb), which serves as an indicator of the presence of NO, the optical density was measured at 401 nm using spectrophotometer (Analytic Gena, Germany).
Assessing the concentration of H2O2
The study by Alexieva et al.58, involved measuring H2O2 levels in fresh roots and shoots. The procedure comprised of rinsing the samples with distilled water, blending them in 0.1% TCA, and then subjecting the homogenate to centrifugation. The resulting supernatant was combined with 1 M KI and 10 mM phosphate buffer, left in darkness for an hour, and afterward, the optical density of the obtained extract was measured at 390 nm using a spectrophotometer.
Assessing the cell death
To analyze cell death in both the roots and shoots, the Evans blue method59 was used with some modifications. Initially, the separated root and shoot samples were immersed in a 0.25% Evans blue solution at room temperature for 15 min. Next, the solution was replaced with 1.0 mL of 80% ethyl alcohol and incubated for 10 min. The samples were then placed in a water bath at 50 °C for 15 min, followed by centrifugation at 12,000 rpm for 10 min. The clear supernatant was subjected to spectrophotometric analysis at 600 nm, and the occurrence of cell death was evaluated by comparing it to the absorbance of untreated fresh root and shoot samples.
Assessing the Zn and Fe concentration
In accordance with Kabir et al.60, the concentration of Zn an Fe were determined. Initially, plant tissues (both root and shoot) were collected and washed with 1 mM CaSO4 for 5 min followed by a thorough rinse with distilled water. The cleaned samples were then dried in an incubator at 80 °C for 4 days. After the drying process was completed, the plant tissues were mixed with 3 mL HNO3 and 1 mL H2O2 in a test tube and heated at 75 °C for 12 min. The concentrations of Zn and Fe were examined through Flame Atomic Absorption Spectroscopy, employing an ASC-6100 auto sampler and an air-acetylene atomization gas mixture system (Model No. AA-6800, Shimadzu). Standard solutions of Zn and Fe were individually prepared to establish their respective concentrations.
Determining the concentration of antioxidant enzymes (CAT, SOD and APX)
The CAT, SOD and APX enzymes activity of the plant root and shoot were measured by following the method. To measure all antioxidant activities, 0.3 g of leaf and root samples were separately crushed in phosphate buffer (10 mL, 100 mM, pH 7.0) and then the resulting homogenate samples were centrifuged at 12,000 rpm for 12 min to separate the supernatant. For CAT activity, a reaction mixture of 2 mL was prepared with potassium phosphate buffer (1.5 mL, 100 mM, pH 7.0), H2O2 (400 μL of 6%), and 100 μL of leaf extract. The reduction in absorbance was assessed at 240 nm using a UV–Vis spectrophotometer (T60 UV Visible Spectrophotometer), and the measurement of CAT activities was carried out following the method described by Verma and Dubey61. For SOD, reaction components were prepared by mixing sodium bicarbonate or carbonate buffer (1.3 mL, 50 mM, pH 9.8), EDTA (100 µL, 0.1 mM), and Epinephrine (500 µL, 0.6 mM). Then, adrenochrome formation was measured at 475 nm (T60 UV Visible Spectrophotometer). Subsequently, the SOD activity of the samples is determined by computing the SOD activity based on the acquired absorbance values and then comparing these values to the standard curve created using the known SOD enzyme, as outlined by Sun and Zigman62. In the case of APX, reaction mixtures were prepared using potassium phosphate buffer (1 mL of 100 mM, pH 7.0), ascorbic acid (500 μL of 0.2 mM), EDTA (100 μL of 0.2 mM), H2O2 (300 μL of 6%), and 100 μL of plant extract. The decrease in absorbance was recorded at 290 nm using a UV–Vis spectrophotometer (T60 UV Visible Spectrophotometer) at 10-s intervals up to 1 min and the measurement of APX activities was carried out following the method described by Verma and Dubey61 The specific activity of the enzyme was expressed as μmol min−1 [(mg protein)−1].
Drought mitigating activity
Morphological stress observation
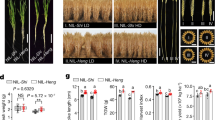
To evaluate plant response to drought stress, ten plants were chosen for each treatment, replicated three times. Watering was withheld for 10 days to simulate stress conditions in potted plants. Following stress induction, discussed below, plants were rehydrated and allowed a recovery period of 5 days. Surviving plants were recorded as rescued individuals.
PEG treatment
Polyethylene glycol (PEG), with a molecular weight of 6000, was utilized to artificially impose drought stress on wheat plants63,64. Both control and treatment wheat seedlings were subjected to a 15% concentration of PEG, inducing severe drought effects after 10 days of germination. Each Petri dish was supplied with 100 mL of PEG solution and the duration of drought stress endured for 12 days in each treatment. The initial measurements were taken immediately after administering the PEG treatment, with the final readings being collected after 24 h of exposure to PEG.
RNA isolation and gene expression analysis
In this study, the methodology described by Rahman et al.65 was employed to analyze RNA isolation and gene expression. Specifically, quantitative reverse transcription PCR techniques were used to investigate the expression of Dreb1 in leaves. To begin, 30 mg of leaves were ground in liquid nitrogen with a mortar and pestle, and total RNA was extracted using the Promega SV total RNA isolation system. Following DNase treatment, 1 μL of extracted RNA from select samples was evaluated for RNA degradation by running it in a 1% agarose gel. Next, first-strand cDNA was generated using the Promega Reverse Transcription systems, and RNA extraction was confirmed by observing it in an agarose gel electrophoresis and quantified by Nano drop. RNase was then utilized to purify the cDNA, and real-time PCR analysis was conducted using the CFX Opus 96 real-time PCR system, with a program consisting of a 3-min incubation at 95 °C, 40 cycles of 30 s at 94 °C, 15 s at 56 °C, and 30 s at 72 °C. Finally, actin was utilized as an internal control to normalize the gene expression levels.
Statistical analysis
The figures in this study were created using Graph Pad Prism 9.5.1.733. The experimental design involved a one-factor completely randomized approach with three replicates, and data variability and result validity were assessed through two-way analysis of variance using Graph Pad Prism 9.5.1.733 as well. The relationship between parameters was evaluated using correlation analysis and principal component analysis. To compare the means of the various treatments, Turkey's multiple range test was employed with a significance level of 0.05. The data were normalize using square root transformation before statistical analysis. The ANOSIM (Analysis of Similarity) were conducted to identify the significant differences of characters with controls in Primer E (Version 7). The Principal Coordinates Analysis (PCO) showed the ANOSIM based ordination of controls and treatments (2 h, 3 h, 4 h, 5 h and 6 h). Later the pair-wise comparisons were also conducted using Draftsman plots. Finally, Heat map analysis was performed in R (version 4.1.3) using package heatmaply to visualize the Pearson correlation among the 34 parameters. The data were also normalized before analysis in R.
Statement on experimental research and field studies on plants or plant parts
We acknowledge the intrinsic value of plant life and recognize the importance of preserving genetic diversity and ecological functions. In conducting experimental research on plants or plant parts, we strived to minimize harm to plants and minimize our impact on plant populations and ecosystems. We followed established protocols for collecting plant specimen, ensuring that our actions do not endanger the survival of plant species. The plant materials and data were used in a responsible and ethical manner.
Statement regarding seed collection
We hereby confirm that the collection of wheat seeds from the Regional Wheat Research Institution in Rajshahi was conducted in strict accordance with the institution's guidelines and regulations. Prior to collecting the seeds, all necessary permissions were obtained from the appropriate authorities at the institution. The collection process adhered to the stipulated protocols to ensure the preservation of the institution's research resources and the maintenance of ethical standards. We express our gratitude to the Regional Wheat Research Institution for granting us the opportunity to access and collect these valuable seeds for our research purposes.
Conclusion
The future impact of climate change on wheat production is predicted to worsen due to increasing occurrences of severe droughts, heat stress, and other environmental stresses. Nevertheless, our research has indicated that the application of HRW to wheat seeds can significantly improve various growth factors such as germination rate, germination potential, vigor index, root-shoot length, number, and dry weight. It also enhances the accumulation of crucial components like RWC, chlorophyll content, and soluble proteins. Additionally, the use of HRW can increase the levels of essential nutrients such as Zn and Fe and boost the activity of antioxidant enzymes like CAT, SOD, and APX. This can mitigate oxidative damage and reduce ROS production in plants. The encouraging potential of HRW treatment in increasing nutrient and antioxidant levels in plants can lead to the development of new methodologies that can help adapt to the negative impacts of climate change on wheat production. Advanced molecular evidence indicates that HRW has a robust capacity to trigger the expression of the Dreb1 gene, which is crucial in conferring drought tolerance to wheat plants. Still, more research is necessary to gain a deeper understanding of the topic, and to clarify the methodology for protecting against potential negative impacts.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Bapela, T., Shimelis, H., Tsilo, T. J. & Mathew, I. Genetic improvement of wheat for drought tolerance: Progress. Challenges and Opportunities. Plants 11, 25 (2022).
Shewry, P. R. & Hey, S. J. The contribution of wheat to human diet and health. Food Energy Secur. 4, 178–202 (2015).
Day, L., Augustin, M. A., Batey, I. L. & Wrigley, C. W. Wheat-gluten uses and industry needs. Trends Food Sci. Technol. 17, 82–90 (2006).
Daryanto, S., Wang, L. & Jacinthe, P. A. Global synthesis of drought effects on maize and wheat production. PLoS One 11, 25 (2016).
Ishaque, W. et al. Quantifying the impacts of climate change on wheat phenology, yield, and evapotranspiration under irrigated and rainfed conditions. Agric. Water Manage. 275, 25 (2023).
Zhao, C. et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 114, 9326–9331 (2017).
Paymard, P., Yaghoubi, F., Nouri, M. & Bannayan, M. Projecting climate change impacts on rainfed wheat yield, water demand, and water use efficiency in northeast Iran. Theor. Appl. Climatol. 138, 1361–1373 (2019).
Karatayev, M., Clarke, M., Salnikov, V., Bekseitova, R. & Nizamova, M. Monitoring climate change, drought conditions and wheat production in Eurasia: The case study of Kazakhstan. Heliyon 8, 14 (2022).
Naumann, G. et al. Global Changes in Drought Conditions Under Different Levels of Warming. Geophys. Res. Lett. 45, 3285–3296 (2018).
Arora, N. K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2, 95–96 (2019).
Javadinejad, S., Dara, R. & Jafary, F. Analysis and prioritization the effective factors on increasing farmers resilience under climate change and drought. Agric. Res. 10, 497–513 (2021).
Huang, J., Haipeng, Yu., Guan, X., Wang, G. & Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 25, 25 (2015).
Yue, Y., Yang, W. & Wang, L. Assessment of drought risk for winter wheat on the Huanghuaihai Plain under climate change using an EPIC model-based approach. Int. J. Digit. Earth 15, 690–711 (2022).
Kim, W., Iizumi, T. & Nishimori, M. Global patterns of crop production losses associated with droughts from 1983 to 2009. J. Appl. Meteorol. Climatol. 58, 1233–1244 (2019).
Sallam, A., Alqudah, A. M., Dawood, M. F. A., Baenziger, P. S. & Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 20, 25 (2019).
Abid, M. et al. Nitrogen nutrition improves the potential of wheat (Triticum aestivum L.) to alleviate the effects of drought stress during vegetative growth periods. Front. Plant Sci. 7, 25 (2016).
Islam, A. et al. Evaluation of the tolerance ability of wheat genotypes to drought stress: Dissection through culm-reserves contribution and grain filling physiology. Agronomy 11, 25 (2021).
Nehe, A. S. et al. Root and canopy traits and adaptability genes explain drought tolerance responses in winter wheat. PLoS One 16, (2021).
Rahman, M. M. & Miah, G. M. Wheat production in North West region of Bangladesh. Bangl. J. Adm. Manage. 20, 15–27 (2017).
Tasnim, Z., Saha, S. M., Hossain, M. E. & Khan, M. A. Perception of and adaptation to climate change: The case of wheat farmers in northwest Bangladesh. Environ. Sci. Pollut. Res. 30, 32839–32853 (2023).
Habiba, U., Shaw, R. & Takeuchi, Y. Farmer’s perception and adaptation practices to cope with drought: Perspectives from Northwestern Bangladesh. Int. J. Disaster Risk Reduct. 1, 72–84 (2012).
Hancock, J. T. Editorial for special issue: “Production and role of molecular hydrogen in plants”. Plants 11, 25 (2022).
Wu, Q. et al. Hydrogen-rich water promotes elongation of hypocotyls and roots in plants through mediating the level of endogenous gibberellin and auxin. Funct. Plant Biol. 47, 771–778 (2020).
Zeng, J., Zhang, M. & Sun, X. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS One 8, 25 (2013).
Sun, Q., Han, W. & Nakao, A. Biological safety of hydrogen. Hydrog. Mol. Biol. Med. 20, 35–48. https://doi.org/10.1007/978-94-017-9691-0_3 (2015).
Wu, Q., Su, N., Cai, J., Shen, Z. & Cui, J. Hydrogen-rich water enhances cadmium tolerance in Chinese cabbage by reducing cadmium uptake and increasing antioxidant capacities. J. Plant Physiol. 175, 174–182 (2015).
Chen, H. et al. Hydrogen-rich water increases postharvest quality by enhancing antioxidant capacity in Hypsizygus marmoreus. AMB Express 7, 25 (2017).
Zhu, J. K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273 (2002).
Ahmad, A. et al. Screening of wheat (Triticum aestivum L.) genotypes for drought tolerance through agronomic and physiological response. Agronomy 12, 25 (2022).
Renwick, G. M., Giumarro, C. & Siegel, S. M. Hydrogen metabolism in higher plants. Plant Physiol. 39, 303–306 (1964).
Song, R. et al. Exogenous hydrogen promotes germination and seedling establishment of barley under drought stress by mediating the ASA-GSH cycle and sugar metabolism. J. Plant Growth Regul. https://doi.org/10.1007/s00344-022-10742-x (2022).
Fu, X. et al. Hydrogen rich water (HRW) induces plant growth and physiological attributes in fragrant rice varieties under salt stress. Res. Sq. 20, 1–28 (2020).
Ahmad, Z. et al. Enhancing drought tolerance in wheat through improving morphophysiological and antioxidants activities of plants by the supplementation of foliar silicon. Phyton (B. Aires) 89, 529–539 (2020).
Raza, M. A. S. et al. Morpho-physiological and biochemical response of wheat to various treatments of silicon nano-particles under drought stress conditions. Sci. Rep. 13, 25 (2023).
Abeed, A. H. A., Eissa, M. A. & Abdel-Wahab, D. A. Effect of exogenously applied jasmonic acid and kinetin on drought tolerance of wheat cultivars based on morpho-physiological evaluation. J. Soil Sci. Plant Nutr. 21, 131–144 (2021).
Alharbi, K. et al. Alleviate the drought stress on Triticum aestivum L. Using the algal extracts of sargassum latifolium and corallina elongate versus the commercial algal products. Life 12, 25 (2022).
Yu, Y. et al. Regulation of growth and salt resistance in cucumber seedlings by hydrogen-rich water. J. Plant Growth Regul. 42, 134–153 (2023).
Xie, Y., Mao, Y., Lai, D., Zhang, W. & Shen, W. H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS One 7, 25 (2012).
De Pascali, M. et al. Xylella fastidiosa and drought stress in olive trees: A complex relationship mediated by soluble sugars. Biology (Basel) 11, 25 (2022).
Cui, W., Gao, C., Fang, P., Lin, G. & Shen, W. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J. Hazard. Mater. 260, 715–724 (2013).
Paul, G. K. et al. Volatile compounds of Bacillus pseudomycoides induce growth and drought tolerance in wheat (Triticum aestivum L.). Sci. Rep. 12, 25 (2022).
Rizwan, M. et al. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214, 269–277 (2019).
Taran, N. et al. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanosc. Res. Lett. 12, 25 (2017).
Sedaghat, M., Hazrati, S. & Omrani, M. Use of zeolite and salicylic acid as an adaptation strategy against drought in wheat plants. South Afr. J. Bot. 146, 111–117 (2022).
Zhou, Y. et al. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field. J. Exp. Bot. 71, 1842–1857 (2020).
Zhang, L. et al. A wheat R2R3-MYB gene, TaMYB30-B, improves drought stress tolerance in transgenic Arabidopsis. J. Exp. Bot. 63, 5873–5885 (2012).
Hu, W. et al. TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ. 36, 1449–1464 (2013).
El-Esawi, M. A., Al-Ghamdi, A. A., Ali, H. M. & Ahmad, M. Overexpression of atWRKY30 transcription factor enhances heat and drought stress tolerance in wheat (Triticum aestivum L.). Genes (Basel) 10, 24 (2019).
Habib, I. et al. Dehydrin responsive HVA1 driven inducible gene expression enhanced salt and drought tolerance in wheat. Plant Physiol. Biochem. 180, 124–133 (2022).
Zhou, R. et al. Effects of atmospheric-pressure N2, He, air, and O2 Microplasmas on mung bean seed germination and seedling growth. Sci. Rep. 6, 25 (2016).
González, L. & González-Vilar, M. Determination of relative water content. Handb. Plant Ecophysiol. Tech. 20, 207–212 (2001).
Arshadullar, M. & Zaidi, S. Role of total plant dry weight in the assesment of variation for salinity tolerance in Gossypium hirsutum. Sarhad J. Agric. 23, 857 (2007).
Weatherley, P. E. Studies in the water relations of the cotton plant: I the field measurement of water deficits in leaves. New Phytol. 49, 81–97 (1950).
Lichtenthaler, H. K. & Wellburn, A. R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 11, 591–592 (1983).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Guy, C., Haskell, D., Neven, L., Klein, P. & Smelser, C. Hydration-state-responsive proteins link cold and drought stress in spinach. Planta 188, 265–270 (1992).
Orozco-Cárdenas, M. L. & Ryan, C. A. Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol. 130, 487–493 (2002).
Alexieva, V., Sergiev, I., Mapelli, S. & Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell Environ. 24, 1337–1344 (2001).
JacynBaker, C. & Mock, N. M. An improved method for monitoring cell death in cell suspension and leaf disc assays using evans blue. Plant Cell. Tissue Organ Cult. 39, 7–12 (1994).
Kabir, A. H., Hossain, M. M., Khatun, M. A., Sarkar, M. R. & Haider, S. A. Biochemical and molecular mechanisms associated with zn deficiency tolerance and signaling in rice (Oryza sativa L.). J. Plant Interact. 12, 447–456 (2017).
Verma, S. & Dubey, R. S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 164, 645–655 (2003).
Sun, M. & Zigman, S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal. Biochem. 90, 81–89 (1978).
Mahpara, S. et al. The impact of PEG-induced drought stress on seed germination and seedling growth of different bread wheat (Triticum aestivum L.) genotypes. PLoS One 17, 14 (2022).
Batool, M. et al. Rapeseed morpho-physio-biochemical responses to drought stress induced by PEG-6000. Agronomy 12, 25 (2022).
Rahman, M. M. et al. Mechanisms and signaling associated with LPDBD plasma mediated growth improvement in wheat. Sci. Rep. 8, 25 (2018).
Acknowledgements
The authors are grateful to Osaka University, Japan for their support in the research. The authors also extend their appreciation to the Researchers Supporting Project Number (RSP2024R367), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.A.I., M.S.I. and M.A.S.; methodology: M.A.I., M.N.A.S., S.M.I., S.B., J.B., S.N.I. and A.K.D.; software: M.S.U., M.A.E.E., S.Z., M.S.I., and M.A.S.; validation: M.S.U., M.A.E.E., S.Z., M.S.I., and M.A.S.; formal analysis: M.A.I., M.N.A.S., S.M.I., S.B., J.B., A.S., L.S.W., S.N.I. and A.K.D.; investigation, M.A.I., M.N.A.S., S.M.I., S.B., J.B., A.S., L.S.W., and A.K.D.; resources: M.A.S., M.A.E.E., M.S.U., and S.Z.; data curation: M.A.I., M.N.A.S., S.M.I., S.B., J.B., A.S., L.S.W., and A.K.D.; writing—original draft preparation: M.A.I., M.N.A.S., S.M.I.,S.B.; writing—review and editing, M.A.S., M.S.I., M.S.U., and S.Z.; visualization: M.A.S.; supervision: M.A.S. and M.S.I.; project administration: M.A.S., M.A.E.E., M.S.U., and S.Z. All authors have read and agreed to the published this version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Islam, M.A., Shorna, M.N.A., Islam, S. et al. Hydrogen-rich water: a key player in boosting wheat (Triticum aestivum L.) seedling growth and drought resilience. Sci Rep 13, 22521 (2023). https://doi.org/10.1038/s41598-023-49973-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-49973-7
This article is cited by
-
Nitrogen use efficiency in wheat through integrated fertilizer management in Eastern Amhara, Ethiopia
BMC Plant Biology (2025)
-
Enhancing cadmium stress resilience in rice seedlings via Bacillus pseudomycoides: modulation of heavy metal transporters and physiological responses for sustainable agriculture
Environmental Science and Pollution Research (2025)