Abstract
Caffeic acid phenethyl ester (CAPE) and its derivatives exhibit considerable effects against hepatocellular carcinoma (HCC), with unquestioned safety. Here we investigated CAPE derivative 1ʹ (CAPE 1ʹ) monotherapy to HCC, compared with sorafenib. HCC Bel-7402 cells were treated with CAPE 1ʹ, the IC50 was detected using CCK-8 analysis, and acute toxicity testing (5 g/kg) was performed to evaluate safety. In vivo, tumor growth after CAPE 1ʹ treatment was evaluated using an subcutaneous tumor xenograft model. Five groups were examined, with group 1 given vehicle solution, groups 2, 3, and 4 given CAPE 1ʹ (20, 50, and 100 mg/kg/day, respectively), and group 5 given sorafenib (30 mg/kg/day). Tumor volume growth and tumor volume-to-weight ratio were calculated and statistically analyzed. An estimated IC50 was 5.6 µM. Acute toxicity tests revealed no animal death or visible adverse effects with dosage up to 5 g/kg. Compared to negative controls, CAPE 1ʹ treatment led to significantly slower increases of tumor volume and tumor volume-to-weight. CAPE 1ʹ and sorafenib exerted similar inhibitory effects on HCC tumors. CAPE 1ʹ was non-inferior to sorafenib for HCC treatment, both in vitro and in vivo. It has great potential as a promising drug for HCC, based on effectiveness and safety profile.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is among the most common malignant tumors, causing substantial numbers of tumor-related deaths worldwide1,2. Surgery is the principal method of obtaining a radical cure, including hepatectomy and liver transplantation3,4. However, most HCC patients are diagnosed when in middle or late disease stages, which are characterized by a large tumor burden, vessel invasion, extra-hepatic metastasis, and poor liver function, etc5. Therefore, most patients no longer have the opportunity for radical treatment. For these patients, systemic drug therapy plays an important role in treatment. Drug treatment can control disease progression, and prolong survival over time, especially in the present era of targeted therapy and immunotherapy6,7,8.
Systemic drug therapy includes molecular targeted drug therapy, immunotherapy, and chemotherapy, as well as drugs for the treatment of basic liver diseases, such as anti-viral treatment, liver protective treatment, and supportive treatment9,10,11. The options for drug have been rapidly advancing. Even for surgery candidates, drugs can reduce recurrence, prolong the recurrence-free survival period after surgery, and improve patients’ quality of life12,13.
Sorafenib is the first targeted drug for treatment of advanced HCC14. It is a multi-kinase inhibitor that can inhibit tumor cell proliferation, promote tumor cell apoptosis, and slow tumor angiogenesis15,16. Although sorafenib is effective, it has relatively serious adverse effects for some patients, and it is unfortunately easy for patients to develop sorafenib-resistance at an early stage17,18,19. Thus, there remains a need for new compounds having similar or better effectiveness but lower toxicity.
Natural products have great potential for application due to their low toxicity or non-toxicity and significant therapeutic effects. Many studies have supported this claim20,21,22. Among them, Caffeic acid phenethyl ester (CAPE), a phenolic compound originally derived from natural propolis, has been reported to exert notable anticancer activity23,24. It can inhibit tumor proliferation and metastasis (including HCC), and also somehow protects organ function25,26,27. Despite these benefits, the clinical therapeutic application of CAPE is limited due to its poor absorption and rapid metabolism. These limitations may be overcome by structural modification through the synthesis of CAPE-based derivatives.
A new compound was synthesized with the aid of the School of Pharmacy of Peking University. The derivative named CAPE 1ʹ overcomes the disadvantages of CAPE, while enhancing its overall efficacy and hindering drug resistance. In this study, we report the anti-HCC activity of CAPE 1ʹ compared to sorafenib, the classic therapeutic drug for HCC.
Materials and methods
Materials
CAPE 1ʹ was synthesized by the School of Pharmacy of Peking University. The HCC cell line BEL-7402, initially obtained from the American Type Culture Collection, was cryopreserved in our own lab in liquid nitrogen. Sodium carboxymethylcellulose was purchased from Sigma-Aldrich (St. Louis, MO, USA), and sorafenib from Bayer Pharmaceutical Company in Germany. The animals used in this study were male specific pathogen-free BALB/C nude mice purchased from Vital River Corp., Beijing. Kunming mice were purchased from the Academy of Military Medical Sciences. All experiments were approved by the Laboratory Animal Ethics Committee of Peking University People’s Hospital (No. 20110312). Animals enrolled in the experiment were treated in accordance with the standards set forth in the 8th Edition of Guide for the Care and Use of Laboratory Animals. All of the mice were sacrificed via exsanguination following anesthesia with 5% isoflurane. The study is reported in accordance with ARRIVE guidelines.
HCC cell inhibition analysis
HCC BEL-7402 cells were cultured in RPMI 1640 medium. Cell viability was determined by 0.4% trypan blue staining (viewed by inverted microscope), and propidium iodide (PI) staining (analyzed by flow cytometry). Viable cells were cultured at a density of 5 × 103/well in 96-well plates for 24 h. CAPE 1ʹ was dissolved in DMSO and diluted into various concentrations using 5‰ sodium carboxymethyl cellulose solution.
Six wells of HCC cells were treated with CAPE 1ʹ at concentrations of 0, 2.5, 5, 10, 20, 40, 80, and 160 µM. The culture medium volume was adjusted to 200 µL with RPMI1640, and then the HCC cells were cultured for 48 h. The CCK-8 method (Tongren Chemical Research Institute, Japan) was used to test the cell survival rate after treatment. The optical density of each well was determined at a wavelength of 450 nm. The 50% inhibitory concentration (IC50) was calculated for the HCC BEL-7402 cells.
Animal acute toxicity test
A total of 40 Kunming mice (half male and half female, 4 weeks old, weighing about 20 g) were randomly divided into an experimental group and control group, each containing 20 mice. Each mouse in the experimental group was administered the drug by gavage on the first day, while the control group was given solvent. The dose of 5 g/kg was established based on the pilot study, which used a dose of 2 g/kg. The mice were observed for toxic reactions and death, and the median lethal dose (LD50) was determined with reference to the literature28,29. After 14 days, the mice were sacrificed, and specimens of main organs (including the heart, liver, kidney, and lung) were collected to test for toxicity. The experiment was conducted following the guidelines for acute toxicity experiments of chemical drugs from the China Food and Drug Administration30.
Subcutaneous tumor model establishment
Viable HCC BEL-7402 cells were subcutaneously implanted in male 4-week-old nude mice. Four weeks later, when tumors had formed, the established tumors were seeded from the tumor-burdened mice. These tumors served as the original specimens, and were cut into pieces of 2-mm diameter. These tumor pieces were then subcutaneously implanted into the backs of nude mice. After 2 weeks, when the tumors had grown, these implanted nude mice were prepared for the subsequent experiment.
In vivo drug treatment
A total of 90 nude mice with subcutaneous tumors were randomly divided into an experimental group and control group, based on the ratio of transplanted tumor to body weight. These mice were then divided into five groups, with each group including 18 mice. One group was administered only the vehicle solution, as a negative control (group 1). Three groups were administered CAPE 1ʹ at different concentrations: 20 mg/kg/day (group 2), 50 mg/kg/day (group 3), and 100 mg/kg/day (group 4). The last group was given sorafenib (30 mg/kg/day) as a positive control (group 5).
Treatment was administered six days a week (skipping Sundays) for four weeks. Each day, we recorded the tumor size, body weight, adverse reactions, and other parameters. We calculated and analyzed the tumor volume (v = a * b2/2, where a is the long diameter in mm and b is the short diameter in mm), and the ratio of tumor volume to weight (ratio = v/w, where v is the tumor volume in mm3, and w is the body weight in g).
Statistics
Statistical analyses were performed using SPSS Statistics 20.0 software. Data are presented as mean ± standard deviation. Student's t-test test was used for comparisons between two groups (group 1 vs group 2, 3, 4, 5, respectively) of samples, and Tukey’s test in one-way ANOVA was used for comparisons between multiple groups (All 5 groups) of samples. The Dunnett statistical test was used to compare each of the treatments (different concentration of the compound) with a single control. P < 0.05 was considered statistically significant.
Ethical approval
All experiments were approved by the Laboratory Animal Ethics Committee of Peking University People’s Hospital.
Results
IC50 analysis
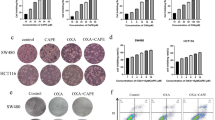
HCC BEL-7402 cells were cultured and seeded as planned. Cell viability was estimated to be over 90% by 0.4% trypan blue staining and above 95% based on PI staining (Fig. 1A). Several CAPE derivatives were tested by in vitro analysis. CAPE 1ʹ was the most effective derivative, with the lowest IC50 (5.6 µM). Figure 1B presents the inhibition curve. At the higher tested concentrations, about 80% of cells were suppressed.
Acute toxicity test
We conducted a preliminary experiment in which four mice were administered a dose of 2 g/kg by gavage. No mice died during 7 days of observation. Therefore, according to the Guiding Principles for Acute Toxicity Experiment of Chemical Drugs, we proceeded to conduct the formal experiment with a dose of 5 g/kg. One mouse in the experimental group died on the second day, and was found to have intrathoracic perfusion fluid after dissection. This death was considered to be caused by improper intragastric perfusion on the first day. The other mice showed a 100% survival rate, and no toxic reaction was observed. All the data was summarized in Table 1. Since the drug dosage used in this experiment was up to 5 g/kg, and no animal death or visible side effects were observed, the LD50 cannot be calculated. CAPE-1′ can be considered a non-toxic or low-toxic drug, without risk of serious acute poisoning to vital organs (Fig. 2).
In vivo treatment
After the mice were divided into groups, their baseline characteristics were analyzed. Single-factor analysis of variance revealed a P value of 0.973 for baseline tumor volume, and a P value of 0.934 for the baseline ratio of tumor volume to weight. This indicates that the groups did not significantly differ in tumor volume or ratio of tumor volume to weight at baseline.
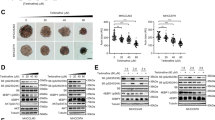
At end of the experiment, each group's survivorship after the four weeks was 15/18, 16/18, 15/18, 15/18, and 14/18, respectively. We calculated the tumor volume growth and ratio of tumor volume to weight in the mice of each group. To assess the anti-tumor effect reflected by tumor growth, these two parameters were compared between groups (negative control vs all experiment groups respectively). For tumor volume growth, the P values were as follows: P(1,2) = 0.014, P(1,3) = 0.042, P(1,4) = 0.313, and P(1,5) = 0.022. This analysis revealed that each experimental group exhibited significantly slower tumor volume growth compared to the negative control group. For the ratio of tumor volume to weight, the P values were as follows: P(1,2) = 0.004, P(1,3) = 0.007, and P(1,4) = 0.105, P(1,5) = 0.008.
Compared to sorafenib treatment, CAPE 1ʹ treatment had a remarkable inhibitory effect on the HCC tumor—with the CAPE 1ʹ groups and the sorafenib group showing similar anti-HCC effects. For tumor volume growth, the P values were as follows: P(2,5) = 0.998, P(3,5) = 0991, and P(4,5) = 0.326. For the ratio of tumor volume to weight, the P values were as follows: P(2,5) = 0.986, P(3,5) = 0.999, and P(4,5) = 0.450. Even at the lower dosage of 20 mg/kg, CAPE 1ʹ had an obvious inhibitory effect on HCC, non-inferior to sorafenib. These findings are displayed in Figs. 3, 4.
Discussion
The results of the present study demonstrated that CAPE 1ʹ was one of most effective inhibitory agents against HCC, based on both in vitro and in vivo analysis. In fact, its effects were almost non-inferior to those of the traditional drug sorafenib. Notably, CAPE 1ʹ exhibited no obvious toxicity, making it potentially one of most promising therapeutic drugs for HCC.
There are plenty of research prospects for natural products 31,32,33. Notably, CAPE is a natural compound derived from propolis, which shows various biological activities—with the most important and promising being its anti-tumor properties34,35. To overcome the physical and chemical inertness of CAPE, and to enhance its anti-tumor effect, a series of derivatives were synthesized and tested. Many experiments have been performed to test these derivatives’ effects on a variety of tumors36,37. Initial experiments show that these agents have considerable anti-tumor activity, but there remains a lack of in vivo experiments, especially clinical investigations.
CAPE derivatives have shown considerable anti-tumor activity in liver cancer. Liu et al. 38. designed a series of derivatives, authenticated their antineoplastic activity in vitro, and calculated their IC50. At same time, preliminary animal experiments were conducted. Compared with these previous compounds, our presently tested compound (CAPE 1ʹ) had a lower IC50 and substantially better effect, emphasizing the importance of in vivo analysis. However, all of these CAPE derivatives are mostly synthesized in the laboratory, with low yields37,39,40. They generally cannot be obtained commercially, which limits the ability to perform relevant experimental comparisons. It is also difficult to produce uniform derivatives. Future studies are needed to examine these issues.
The anti-tumor effects of CAPE have mainly been demonstrated in animal experiments. No previous study has performed a non-inferior effect comparison between CAPE and the most commonly used drugs in clinical settings. For advanced liver cancer, sorafenib is a standard first-line treatment method that has been widely used in clinical practice for almost two decades41. Thus, here we compared our derivative CAPE 1′ with sorafenib, and found that they showed comparable efficacy under the same experimental conditions. Although most often tolerable, sorafenib can have some severe adverse effects42,43. Importantly, CAPE 1' showed much better safety, with seemingly no obvious toxicity observed.
Investigations on CAPE's anti-tumor activity demonstrate that it produces cell cycle arrest, limits growth and transcription factor expression, and inhibits invasion, metastasis, and angiogenesis. It can also relieve the negative effects of anti-cancer medications, prevent and reverse the carcinogenic effects of some viruses and chemicals, and boost tumor cell apoptosis44. Many signal transduction pathways, including PI3K/Akt, p38MAPK, JNK, and non-classical Wnt pathways, are closely associated with its molecular mechanism45,46. For CAPE 1’, the mechanism may be somehow similar. It should, nevertheless, be confirmed in the next lab communications. Therefore, more research to clarify the molecular processes will be organized.
Many studies show that CAPE has cytotoxicity towards a variety of tumor cells and does not harm normal cells27,47. It also somehow protects organ function. Our present study demonstrated that the derivative CAPE 1ʹ showed significant time- and dose-dependent effects against HCC, suggesting great potential for HCC treatment. Increasing the dose of CAPE 1ʹ seemed to yield much better tumor suppression. Considering its good safety profile and antineoplastic effect, application in clinical trials is very realistic. In the present era of comprehensive therapy for HCC, monotherapy is not preferred48,49. CAPE, especially its improved derivative CAPE 1ʹ, can be used for auxiliary application in combination with other clinically approved drugs, such as sorafenib. In this regimen, CAPE 1ʹ could provide organ protection along with anti-tumor effects. A collaborative clinical trial will be carried out in the future following the elucidation of a more definitive molecular mechanism.
Conclusion
In conclusion, the derivative CAPE 1ʹ exhibited considerable anti-HCC effect, comparable to that of sorafenib. The anti-tumor effect was improved by using an increased dosage. Importantly, in vivo animal experiments also revealed that CAPE 1ʹ had a favorable safety profile. CAPE and its derivatives could potentially function through many molecular mechanisms involving tyrosine kinases and anti-angiogenesis, and have also shown some ability to protect organ function. Therefore, in the clinical setting, these agents might be considered as a therapeutic choice for patients who have contraindication or substantial risks preventing the use of tyrosine kinase inhibitors and antiangiogenic drugs. The present findings warrant further study of CAPE 1ʹ for HCC treatment.
Data availability
The data presented in this study are available on request from the corresponding authors.
Abbreviations
- CAPE:
-
Caffeic acid phenethyl ester
- HCC:
-
Hepatocellular carcinoma
- PI:
-
Propidium iodide
- IC50:
-
50% Inhibitory concentration
- LD50:
-
Median lethal dose
References
Kulik, L. & El-Serag, H. B. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 156, 477–491 (2019).
Chidambaranathan-Reghupaty, S., Fisher, P. B. & Sarkar, D. Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification. Adv. Cancer Res. 149, 1–61 (2021).
Machairas, N., Tsilimigras, D. I. & Pawlik, T. M. State-of-the-art surgery for hepatocellular carcinoma. Langenbecks Arch. Surg. 406, 2151–2162 (2021).
Moeckli, B., Majno, P., Orci, L. A., Peloso, A. & Toso, C. Liver transplantation selection and allocation criteria for hepatocellular carcinoma: a european perspective. Semin. Liver Dis. 41, 172–181 (2021).
Couri, T. & Pillai, A. Goals and targets for personalized therapy for HCC. Hepatol. Int. 13, 125–137 (2019).
Baxter, M. A., Glen, H. & Evans, T. R. Lenvatinib and its use in the treatment of unresectable hepatocellular carcinoma. Future Oncol. 14, 2021–2029 (2018).
D’Alessio, A. et al. Atezolizumab plus bevacizumab for unresectable or metastatic hepatocellular carcinoma. Exp. Rev. Anticancer Ther. 21, 927–939 (2021).
Rizzo, A., Ricci, A. D., Gadaleta-Caldarola, G. & Brandi, G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Exp. Rev. Gastroenterol. Hepatol. 15, 1245–1251 (2021).
the role of viral hepatitis in hepatocellular carcinoma. hlomai A., de Jong Y.P., Rice C.M. Virus associated malignancies. Semin Cancer Biol. 26, 78–88 (2014).
Llovet, J. M., Montal, R., Sia, D. & Finn, R. S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 15(10), 599–616 (2018).
Llovet, J. M. et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 3, 386–401 (2022).
Akateh, C. et al. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J. Gastroenterol. 25, 3704–3721 (2019).
Zhang, W., Zhang, B. & Chen, X. P. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front. Med. 15, 155–169 (2021).
Huang, A., Yang, X. R., Chung, W. Y., Dennison, A. R. & Zhou, J. Targeted therapy for hepatocellular carcinoma. Signal Transduct. Target Ther. 5, 146 (2020).
Marisi, G. et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers?. World J. Gastroenterol. 24, 4152–4163 (2018).
Garten, A. et al. Sorafenib-induced apoptosis in hepatocellular carcinoma is reversed by SIRT. Int. J. Mol. Sci. 20, 4048 (2019).
Tang, W. et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct. Target Ther. 5, 87 (2020).
Xia, S., Pan, Y., Liang, Y., Xu, J. & Cai, X. The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 51, 102610 (2020).
Wang, C. et al. CDK12 inhibition mediates DNA damage and is synergistic with sorafenib treatment in hepatocellular carcinoma. Gut 69, 727–736 (2020).
Mathew, B. T. et al. Halotolerant marine rhizosphere-competent actinobacteria promote salicornia bigelovii growth and seed production using seawater irrigation. Front. Microbiol. 11, 552 (2020).
Murali, C. et al. Camel whey protein hydrolysates induced G2/M cell cycle arrest in human colorectal carcinoma. Sci. Rep. 11, 7062 (2021).
Xie, Y. et al. Network pharmacology and experimental investigation of Rhizoma polygonati extract targeted kinase with herbzyme activity for potent drug delivery. Drug Deliv. 28, 2187–2197 (2021).
Ozturk, G. et al. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): review of melanomas, lung and prostate cancers. Eur. Rev. Med. Pharmacol. Sci. 16, 2064–2068 (2012).
Firat, F., Özgül, M., Türköz, U. E. & Inan, S. Effects of caffeic acid phenethyl ester (CAPE) on angiogenesis, apoptosis and oxidative stress in various cancer cell lines. Biotech. Histochem. 94, 491–497 (2019).
Kart, A., Cigremis, Y., Karaman, M. & Ozen, H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp. Toxicol. Pathol. 62, 45–52 (2010).
Akyol, S. et al. The potential usage of caffeic acid phenethyl ester (CAPE) against chemotherapy-induced and radiotherapy-induced toxicity. Cell Biochem. Funct. 30, 438–443 (2012).
Balaha, M., De Filippis, B., Cataldi, A. & di Giacomo, V. CAPE and neuroprotection: a review. Biomolecules 11, 176 (2021).
Boente-Juncal, A. et al. Reevaluation of the acute toxicity of palytoxin in mice: determination of lethal dose 50(LD50) and No-observed-adverse-effect level (NOAEL). Toxicon 177, 16–24 (2020).
Mishra, A., Dewangan, G., Mandal, T. K. & Chkraborty, J. LD50 dose fixation of nanohybrid-layered double hydroxide-methotrexate. Indian J. Pharmacol. 54(5), 379–380 (2022).
Zou, Y. X. et al. Preclinical safety assessment of antipyrine combined with lidocaine hydrochloride as ear drops. Regul Toxicol. Pharmacol. 103, 34–40 (2019).
Alam, M. et al. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. 12, 860508 (2022).
Bouabdallah, S., Al-Maktoum, A. & Amin, A. Steroidal saponins: naturally occurring compounds as inhibitors of the hallmarks of cancer. Cancers 15, 3900 (2023).
Awad, B., Hamza, A. A., Al-Maktoum, A., Al-Salam, S. & Amin, A. Combining crocin and sorafenib improves their tumor-inhibiting effects in a rat model of diethylnitrosamine-induced cirrhotic-hepatocellular carcinoma. Cancers 15, 4063 (2023).
Bhargava, P. et al. Experimental evidence for therapeutic potentials of propolis. Nutrients 13, 2528 (2021).
Murtaza, G. et al. Caffeic acid phenethyl ester and therapeutic potentials. Biomed. Res. Int. 2014, 145342 (2014).
Beauregard, A. P. et al. CAPE analogs induce growth arrest and apoptosis in breast cancer cells. Molecules 20, 12576–12589 (2015).
Hossain, R. et al. Propolis: an update on its chemistry and pharmacological applications. Chin. Med. 17, 100 (2022).
Liu, X. et al. Discovery of CAPE derivatives as dual EGFR and CSK inhibitors with anticancer activity in a murine model of hepatocellular carcinoma. Bioorg. Chem. 107, 104536 (2021).
Zhang, P., Tang, Y., Li, N. G., Zhu, Y. & Duan, J. A. Bioactivity and chemical synthesis of caffeic acid phenethyl ester and its derivatives. Molecules 19, 16458–16476 (2014).
Gießel, J. M., Loesche, A., Csuk, R. & Serbian, I. Caffeic acid phenethyl ester (CAPE)-derivatives act as selective inhibitors of acetylcholinesterase. Eur. J. Med. Chem. 177, 259–268 (2019).
Abdelgalil, A. A., Alkahtani, H. M. & Al-Jenoobi, F. I. Sorafenib. Profiles Drug Subst. Excip. Relat. Methodol. 44, 239–266 (2019).
Bracarda, S. et al. Early detection, prevention and management of cutaneous adverse events due to sorafenib: recommendations from the Sorafenib Working Group. Critic. Rev. Oncol./Hematol. 82(3), 378–386 (2012).
Tovoli, F. et al. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J. Hepatol. 71, 1175–1183 (2019).
Olgierd, B., Kamila, Ż, Anna, B. & Emilia, M. The pluripotent activities of caffeic acid phenethyl ester. Molecules 26, 1335 (2021).
Chan, G. C., Cheung, K. W. & Sze, D. M. The immunomodulatory and anticancer properties of propolis. Clin. Rev. Allergy Immunol. 2013, 262–273 (2013).
Wan, Q. et al. Protection of CAPE-pNO2 against chronic myocardial ischemia by the TGF-Β1/Galectin-3 pathway in vivo and in vitro. Inflammation 45, 1039–1058 (2022).
Akyol, S. et al. In vivo and in vitro antıneoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr. Cancer. 65, 515–526 (2013).
Forner, A., Reig, M. & Bruix, J. Hepatocellular carcinoma. Lancet 391, 1301–1314 (2018).
Gilles, H., Garbutt, T. & Landrum, J. Hepatocellular carcinoma. Crit. Care Nurs. Clin. North Am. 34, 289–301 (2022).
Author information
Authors and Affiliations
Contributions
LG and WW contributed to the conception of the study. FY and ZD performed the experiment. NL contributed to analysis and manuscript preparation. LG and XZ performed the data analysis and wrote the manuscript. JC and JP helped perform the analysis with constructive discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gong, L., Wang, W., Yu, F. et al. Caffeic acid phenethyl ester derivative exerts remarkable anti-hepatocellular carcinoma effect, non-inferior to sorafenib, in vivo analysis. Sci Rep 14, 14546 (2024). https://doi.org/10.1038/s41598-024-65496-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-65496-1