Abstract
Whether the anesthesia technique, inhalational general anesthesia (IGA) or propofol-based anesthesia (PBA), influences the long-term survival of non-metastatic breast cancer (eBC) remain unclear and controversial. We carried out a literature search on 16thJuly, 2022 for studies comparing IGA and PBA in eBC undergoing standard surgery, according to PRISMA 2020. The major endpoint in our study was overall survival (OS). Seventeen studies including four randomized clinical trials and thirteen retrospective cohort studies were included in the meta-analysis. Ten studies provided data for crude OS in unweighted eBC patients (imbalance in baseline characteristics). The summarized estimate HRs of the PBA group versus the IGA group (ten studies, N = 127,774, IGA group: 92,592, PBA group: 35,182.) was 0.83 (95%CI: 0.78–0.89). Compared with IGA, PBA was associated with both better 1-year OS (two studies, N = 104,083, IGA group: 84,074, PBA group: 20,009. Pooled HR = 0.80, 0.73–0.89) and 5-year OS (six studies, N = 121,580, IGA group: 89,472, PBA group: 32,108. HR = 0.80, 0.74–0.87). Ten studies applied PSM method to balance the baseline characteristics. In these weighted patients, PBA still showed a better OS (ten studies, N = 105,459, IGA group: 79,095, PBA group: 26,364. HR = 0.93, 0.87–1.00), a better 1-year OS (two studies, N = 83,007, IGA group: 67,609, PBA group: 15,398. HR = 0.88, 0.78–0.98) and a trend towards a better 5-year OS (nine studies, N = 121,580, IGA group: 76,797, PBA group: 24,066. HR = 0.95, 0.88–1.03). Loco-regional recurrence-free survival (LRRFS) was also better in PBA group (HR = 0.73, 0.61–0.86). The present study is the first comprehensive meta-analysis to demonstrate that propofol-based anesthesia could significantly improve OS and LRRFS in non-metastatic breast cancer patients, compared with inhalational anesthesia.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most common malignancy and is the leading cause of cancer mortality in women1. Mortality of early-stage/non-metastatic breast cancer (eBC) is usually attributable to cancer relapse including loco-regional recurrence and distant organ metastasis despite standard surgical resection, either mastectomy or breast conserving surgery. Surgery is still the primary and most effective treatment for eBC, but residual disease in the form of scattered micro-metastases and tumour cells is usually unavoidable. A series of effective treatments have been developed to improve the prognosis, including chemotherapy, endocrine therapy, target therapy, radiotherapy and immune therapy, etc.2,3. However, there is still a long way to go to improve the cure rate of eBC.
Anesthesia is a fundamental technique for breast surgery, and it could be generally divided into two methods nowadays: inhalational general anesthesia (IGA) with anesthetic gases (majorly sevoflurane), and propofol-based anesthesia (PBA)4. The relationship between the anesthetic technique and breast cancer prognosis has not yet been clarified5. Surgical stress, opioids, and inhalation anesthesia have been reported to cause perioperative immunosuppression and thus increase cancer recurrence and decrease survival6,7,8,9. As the most widely used inhalation anesthetics, sevoflurane (SEVO) could suppress cell-mediated immunity (CMI) and promote tumor cell proliferation and angiogenesis through inducing the apoptosis of T lymphocytes and upregulating the expression of HIF-1a10,11. While propofol as an intravenous anesthetics does not suppress CMI, and instead it could increase cytotoxic T lymphocyte (CTL) activity, decrease inflammatory cytokine levels and suppresse COX-2 and PGE2 functions.Moreover, propofol could maintain the host innate immunity and immune defense via NK cells11,12,13,14,15,16,17,18, and thus propofol-based anesthesia has been supposed to increase the survival of cancer patients with surgery.
Although retrospective cohort studies have shown that the anesthetic technique may affect long-term survival after cancer surgery, whether PBA has an advantage over IGA in the prognosis of breast cancer patients is still controversial and inconclusive19. Several retrospective cohort studies have been published to address that PBA is generally associated with a better overall survival than IGA after breast cancer surgery. However, some recent retrospective studies concerning breast cancer showed that there were no differences between the two anesthesia methods in survival. Meanwhile, RCTs have not yet provided sufficient evidence that the anesthetic technique is associated with the recurrence rate or long-term outcomes in patients undergoing breast cancer surgery, possibly due to their limited sample size or short follow-up time20,21,22.
With increasing and conflicting results reported by different studies, we conducted the present meta-analysis to evaluate the controversial value on the survival of eBC for the two anesthetic techniques.
Methods and materials
Searching strategy and publication selection
We conducted a systemic literature search on16thJuly, 2022 of online databases, including EMbase, Pubmed, Scopus and Cochrane library (including Cochrane Central Register of Controlled Trials) for all studies concerning on propofol-based anesthesia and inhalational general anesthesia in early-stage BC patients by two reviewers (YJZ and PY) independently. The searching strategy isto search the following words in the title/abstract: (“breast cancer” OR breast) AND (“inhalation anaesthesia” OR isoflurane OR sevoflurane OR desflurane OR “nitrous oxide” OR “intravenous anaesthesia” OR “total intravenous anaesthesia” OR TIVA OR propofol). Only studies in English language were included.
Cross-referenced articles, and the potential relevant unpublished studies on important international conference websites, i.e. ESMO (European Society for Medical Oncology), ASCO (American Society of Clinical Oncology), and SABCS (San Antonio Breast Cancer Symposium), have also been gone through.
This study has been registered at PROSPERO2022 (ID:CRD42022334904. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022334904).
Inclusion and exclusion criteria
The inclusion criteria for eligible trials were as follows: (1) prospective/retrospective studies recruiting early-stage invasive BC patients (stage I–III); (2) patients should have undergone standard breast surgery, either mastectomy or breast conserving surgery under anesthesia; (3) studies should have included comparisons of long-term survival between IGA and PBA group; (4) detailed survival data, including overall survival (OS), recurrence-free survival (RFS), loco-regional recurrence-free survival (LRRFS) and distant metastasis-free survival (DMFS), etc., should have been presented in the studies. Definitions of survival data has been described in these studies; (4) In the case of studies derived from duplicate data sources, only the latest and the most comprehensive publication was included.
Studies meeting all the above criteria were included; otherwise, they were excluded.
Data extraction and study objectives
Two independent investigators (YJZ and LB) performed the data extraction. The following information was extracted if available: author name, publication year, journal name, type of study, data source, enrolment period and clinical stage of patients, age range/median age, race, breast surgery type (mastectomy or breast conservation), number (No.) of patients in each group, detailed anesthetic techniques and drugs, median follow-up years, balanced or not in baseline characteristics of the two groups (with weighting methods). Survival data were extracted if available,otherwise, we extracted and transformed them from the survival curve. Hazard ratios (HRs) and the corresponding 95% confidence intervals (95% CIs) of three studies (RCTs as Yan 2018, Yan 2019 and Cho 2017) could also be found in the three previously published meta-analysis21,22,23.
The primary objective of the present meta-analysis was to evaluate the survival benefits of propofol-based anesthesia in eBC patients, compared with inhalational general anesthesia. The major index was OS, RFS, LRRFS and DMFS.
Statistical analysis
HRs and the corresponding 95%CIs for PBA v.s. IGA on survival rate were extracted or calculated from each publication. Pooled HRs and 95% CIs for endpoints presented in forest plots were estimated using the Mantel–Haenszel method, using either fixed- or random-effects models. Statistical heterogeneity was evaluated using I2 statistics. When between-estimate heterogeneity was indicated (I2 > 25%), a random-effects model was applied.
The quality of the eligible cohort studies was assessed using the Newcastle–Ottawa Scale (NOS). The results demonstrated that the quality of all the included studies was generally high (score 8–9).
We also applied funnel plots and Egger’s test (indicated by p < 0.05) to determine the publication bias. All statistical analysis was performed with Stata 12.0 software. All tests were two-sided, and statistical significance was set at P < 0.05.
Ethics approval and consent to participate
Ethics approval and trial registration numbers were not applicable to the present meta-analysis.
Results
Eligible studies
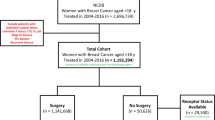
A systematic search of databases and international conferences yielded two thousand six hundred and twenty-one (2621) publications. One thousand and fifty -two articles (1052) remained after excluding duplicates. After title and abstract screening, nine hundred and two (902) more articles were excluded. During the full-text review, a further one hundred and thirty-three (133) articles were excluded for the following reasons: Studies including Other cancer type (n = 27), Reviews (n = 8), Studies from duplicated data source (n = 3), No data for survival (n = 70), No intervention/control (n = 25). Ultimately, seventeen (17) publications meeting the inclusion criteria were recruited24,25,26,27,28,29,30,31,32,33,34,35,36,37. The PRISMA flow diagram is shown in Fig. 1.
Characteristics of the enrolled studies
Among the seventeen eligible studies, thirteen25,26,27,28,29,30,31,33,36,37,38,39,40 were retrospective cohort studies, four24,32,34,35 were prospective randomized clinical trials (RCTs). All eligible studies published full-text articles. The characteristics and the corresponding extracted survival data of the eligible studies are presented in Tables 1–4, respectively.
As shown in Table 1, two methods of propofol-based anesthesia (PBA) were reported in these publications: propofol-based total intravenous anesthesia (TIVA), and paravertebral blocks (PVB) with propofol sedation. For inhalational general anesthesia (IGA), most studies used sevoflurane as the inhalation anesthetics, while only one study used desflurane.
To note, Dr. Zhang et.al. published three articles and the patients were derived from Taiwan Cancer Registry Database (TCRD) during 2009/01/01 to 2018/12/31. However, the surgery method or PBA method for breast cancer patients were different in these three publications. Dr. Enlund et.al. also published three articles in Swedish population, but the inclusion period for patients and the derived database were not duplicated in the three articles. Thus we included all these publications in analysis.
As listed in Tables 2–4, HRs and their corresponding 95%CI for PBA versus IGA have been provided in most articles, while these information in three RCTs (Yan 2018, Yan 2019 and Cho 2017) with small sample size could be extracted from survival curve or from previous meta-analysis20,21.
Efficacy of propofol-based anesthesia
Crude overall survival in unweighted patients
We treated the publications with unbalanced baseline characteristics (especially for T, N, M stage and ER, PR, HER-2 expressions) as unweighted eBC patients. The available overall survival (OS) data in such publications were curde OS. Ten studies provided data for crude OS in unweighted eBC patients (Table 2). Among them, three publications were prospective RCTs (Yan 2018, Yan 2019 and Cho 2017), but the recruited patients were unbalanced in baseline N stage and molecular subtypes. For crude OS, the separate and pooled HRs and 95% CIs were shown in Fig. 2A. No between-study heterogeneity was noted (p = 0.141, I-square = 33.4%). The summarized estimate HR of the PBA group versus the IGA group (ten studies, N = 127774, IGA group: 92592, PBA group: 35182.) was 0.83 (95% CI: 0.78–0.89).
Efficacy of propofol-based anesthesia in unweighted patients. The summarized estimate HR of the PBA group versus the IGA group in unweighted patients (ten studies, N = 127,774, IGA group: 92,592, PBA group: 35,182.) was 0.83 (95% CI: 0.78-0.89. Figure 2A). Compared with IGA, PBA was associated with both better1-year OS (two studies, N = 104,083, IGA group: 84,074, PBA group: 20,009. PooledHR = 0.80, 0.73–0.89. Figure 2B) and 5-year OS (six studies, N = 121,580, IGA group: 89,472, PBA group: 32,108. HR = 0.80, 0.74-0.87. Figure 2C).
However, the crude OS data provided in these ten publications had bias due to the different median follow-uptime: some were one-year OS data, while some were 5-year. To decrease the bias, we classified the data and conducted subgroup analysis. The summarized HRs for 1-year and 5-year OS were shown in Fig. 2B,C. Compared with IGA, PBA was associated with both better 1-year OS (two studies, N = 104083, IGA group: 84074, PBA group: 20009. Pooled HR = 0.80, 0.73–0.89) and 5-year OS (six studies, N = 121580, IGA group: 89472, PBA group: 32108. HR = 0.80, 0.74–0.87). The 2-year OS was similar in three publications but with a small sample size (three studies, N = 210, IGA group: 103, PBA group: 107. Pooled HR = 1.12, 95% CI: 0.90–1.40, forrest plot not shown).
Overall survival in weighted patients
Although the above results demonstrated at the first time that PBA had a better OS than IGA in eBC patients, the limitation was obvious: the baseline characteristics in the two groups were extremely unbalanced. Thus, we could not apply the results in clinical practice directly. Fortunately, we figured out that many studies had also recognized it and used propensity score matching (PSM) method to decrease the bias. We treated these OS data derived from well-balanced patients after PSM as weighted OS.
Ten studies provided weighted OS data in these well-matched eBC patients (Table 3). They were all retrospective cohort studies. For these weighted OS, the separate and pooled HRs and 95%CIs were shown in Fig. 3A. No between-study heterogeneity was noted (p = 0.946, I-square = 0.00%). The summarized estimate HR of the PBA group versus IGA group (ten studies, N = 105459, IGA group: 79095, PBA group: 26364.) was 0.93 (95% CI: 0.87–1.00).
Efficacy of propofol-based anesthesia in weighted patients. The summarized estimate HR of the PBA group versus the IGA group in weighted patients (ten studies, N = 105,459, IGA group: 79,095, PBA group: 26,364.) was 0.93 (95% CI: 0.87-1.00. Figure 3A). Compared with IGA, PBA was associated with both better1-year OS (two studies, N = 83,007, IGA group: 67,609, PBA group: 15,398. PooledHR = 0.88, 0.78–0.98. Figure 3B) and a trend towards better 5-year OS (nine studies, N = 121,580, IGA group: 76,797, PBA group: 24,066. HR = 0.95, 0.88-1.03. Figure 3C).
We further classified the data according to the follow-up time and conducted subgroup analysis. The summarized HR for 1-year and 5-year OS was shown in Fig. 3B,C. Compared with IGA, PBA was associated with a better1-year OS (two studies, N = 83007, IGA group: 67609, PBA group: 15398. Pooled HR = 0.88, 0.78–0.98) and a trend towards better 5-year OS (nine studies, N = 121580, IGA group: 76797, PBA group: 24066. HR = 0.95, 0.88–1.03).
The summarized HRs for the weighted eBC patients indicated that PBA still had a significantly superior OS, when compared with IGA.
Other survival data
We also conducted analysis in other survival data, including RFS, LRRFS and DMFS (Tables 3,4).
For RFS, six studies provided crude data (N = 3230, IGA group: 2682, PBA group: 548.). The summarized HRs for crude RFS in PBA group was 0.93 (0.75, 1.14), see Fig. 4A. Five studies provided weighted RFS data after PSM in patients (N = 6602, IGA group: 3424, PBA group: 3178.). The summarized HRs for weighted RFS in PBA group was 0.96 (0.80, 1.16), see Fig. 4B.
Impact of propofol-based anesthesia on RFS. For RFS, six studies provided crude data (N = 3230, IGA group: 2682, PBA group: 548.). The summarized HRs for crude RFS in PBA group was 0.93 (0.75, 1.14), see Fig. 4A. Five studies provided weighted RFS data after PSM in patients (N = 6602, IGA group: 3424, PBA group: 3178.). The summarized HRs for weighted RFS in PBA group was 0.96 (0.80, 1.16), seeFig. 4B.
Three publications from Dr. Zhang et.al. provided weighted LRRFS and DMFS data (Table 4) in well-balanced patients (N = 8072, IGA group: 4036, PBA group: 4036) derived from Taiwan Cancer Registry Database, China. The summarized HRs for LRRFS in PBA group was 0.73 (0.61, 0.86), while the summarized HRs for DMFS was 0.86 (0.74, 1.00), see Fig. 5A,B.
Impact of propofol-based anesthesia on LRRFS and DMFS. Three publications provided weighted LRRFS and DMFS data in well-balanced patients (N = 8072, IGA group: 4036, PBA group: 4036). The summarized HRs for LRRFS in PBA group was 0.73 (0.61, 0.86), while the summarized HRs for DMFS was 0.86 (0.74, 1.00), see Fig. 5A,B.
Publication bias
Egger’s test and funnel plots were used to detect and describe publication bias. No significant publication bias was identified in the data pooling (data not shown).
Discussion
Whether the anesthesia technique and drugs influences the long-term outcomes of early stage breast cancer has been always an interesting question for oncologists and anesthesiologists. Propofol has been proved to be more favorable in breast cancer surgery than volatile general anesthetics (sevoflurane) in biomarker studies: a better inhibition on VEGF-C and TGF-β, more activated natural killer cells and higher cancer cell apoptosis.
However, the differences in long-term outcomes between propofol and volatile general anesthetics were not so apparent. The previous studies were few and they reported various survival results. The most important thing to note, most studies recruited limited and unbalanced patients with various follow-up time. Thus the bias in the these studies were inherent and concrete. Several meta-analysis have been published concentrating on this question, but due to the limited sample size and unweighted data, they were not convincing to end this argument.
For example, four published systemic reviews/meta-analysis concerning on our topic19,21,22,23. These four related previous reviews and meta-analysis have their disadvantages. Publications of Chang CY, et.al. and Yap A, et.al. included several kinds of cancers and did not concentrate on anesthetic technique and and prognosis breast cancer. Thus the number of eligible studies were very limited (4 included studies). Publication of Pang QY, et.al.concentrate on the side effects of anesthetic technique, thus the number of eligible studies on survival was also very limited (3 included studies). Only Lv R, et.al. include 7 studies. Moreover, the most important issue of all the four studies is, the baseline characteristics in the include studies are unbalanced, which brings the major bias.
In the present meta-analysis, we included many latest studies with relative large sample sizes (17 studies). More importantly, many studies applied propensity score matching methods (PSM) to balance the baseline characteristics between propofol group and inhalation anesthetics group. PSM is a method to minimize selection bias between interventional groups when estimating causal intervention effects in uneven population. The treatment groups (propofol- or inhalation- anesthesia) were matched on a propensity score. The propensity score is the probability of intervention assignment conditional on the current baseline characteristics. For breast cancer patients, we treated the TNM stage and molecule subtypes (ER, PR, HER-2 expressions) as the most important characteristics. Patients should be balanced in these index after PSM. Further, we have conducted analysis according to the crude or weighted data. Thus the bias in our study has been kept to an absolute minimum, when compared with previous studies.
The most important message of present meta-analysis is that propofol-based anesthesia is associated with a better overall survival in early-stage breast cancer patients, when compared with inhalation-maintained anesthesia. It has been a long-expected but unproven clinical answer so far. The benefits could be found in both unweighted and weighted patients. Thus we think that these results could bring important impacts to the anesthesia choice for breast cancer surgery: propofol-based anesthesia should be preferred over inhalation anesthesia. We are also looking forward to the results of more well-designed undergoing RCTs, including the “CAN Study”41, to further confirm this question.
However, we should recognize that anesthesia period during surgery is short and once-only use for most breast cancer patients, and thus its function is hard to present. We also acknowledge that most anesthesia today are mixed, with both propofol and volatile anesthetics applied. In our cancer center, propofol are popularly used in the anesthesia induction, while volatile anesthetics are used for anesthesia maintaining. Thus, a non-propofol or a non-inhalation anesthesia are not popular nowadays, which could bring difficulties in analyze the clinical outcomes between propofol and volatile anesthetics.
Another important influencing factor is the status of eBC patients receiving surgery: whether they are newly diagnosed, or treated after neoadjuvant therapies (chemotherapy, endocrine therapy, anti-HER2 therapy, radiotherapy). In theory, the benefits of propofol mainly contribute to its function on the immune system and disseminated tumor cells during surgery. Then its efficacy may be greatly weakened in those patients with a complete or good partial remission after neoadjuvant therapies. In recent years, the ratio of neoadjuvant therapies has been increased significantly in eBC patients, which would also influence whether the patients benefit from the anesthesia technology during surgery.
After all, if we decided to apply the propofol-based anesthesia, then the next question is which is the optimal technique: propofol-based total intravenous anesthesia or paravertebral blocks with propofol sedation. In the two technology, the propofol dose and drug concentration in blood are different. More clinical trials could be conducted to further elucidate which propofol-based anesthesia could be better.
Conclusions
The present study is the first comprehensive meta-analysis to demonstrate that propofol-based anesthesia could significantly improve OS and LRRFS in non-metastatic breast cancer patients, compared with inhalational anesthesia. More prospective RCTs were needed to further clarify the target population of PBA and the optimal anesthesia usage for propofol.
Data availability
The data used to support the findings of this study are included within the article.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Burstein, H. J. et al. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol 32, 1216–1235 (2021).
Caparica, R., Brandao, M. & Piccart, M. Systemic treatment of patients with early breast cancer: recent updates and state of the art. Breast 48(Suppl 1), S7–S20 (2019).
Buddeberg, B. S. & Seeberger, M. D. Anesthesia and Oncology: Friend or Foe?. Front. Oncol. 12, 802210 (2022).
Kim, R., Kawai, A., Wakisaka, M. & Kin, T. Current Status and Prospects of Anesthesia and Breast Cancer: Does Anesthetic Technique Affect Recurrence and Survival Rates in Breast Cancer Surgery?. Front. Oncol. 12, 795864 (2022).
Hiller, J. G., Perry, N. J., Poulogiannis, G., Riedel, B. & Sloan, E. K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 15, 205–218 (2018).
Roham Salek, M. D. Seyed Ahmad Mohajeri, Ali Talaei, Azar Fanipakdel, Seyed Alireza Javadinia: Amelioration of anxiety, depression, and chemotherapy related toxicity after crocin administration during chemotherapy of breast cancer: A double blind, randomized clinical trial. Phytother. Res. 35, 5143–5153 (2021).
Sedighi Pashaki, A. M. K. et al. A Randomized, Controlled, Parallel-Group, Trial on the Effects of Melatonin on Fatigue Associated with Breast Cancer and Its Adjuvant Treatments. Integr. Cancer Ther. https://doi.org/10.1177/1534735420988343 (2021).
Sedighi Pashaki, A. S. F. et al. A Randomized, Controlled, Parallel-Group, Trial on the Long-term Effects of Melatonin on Fatigue Associated With Breast Cancer and Its Adjuvant Treatments. Integr. Cancer Ther. https://doi.org/10.1177/15347354231168624 (2023).
Loop, T. et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology 102, 1147–1157 (2005).
Oh, C. S. et al. Effect of Equipotent Doses of Propofol versus Sevoflurane Anesthesia on Regulatory T Cells after Breast Cancer Surgery. Anesthesiology 129, 921–931 (2018).
Kushida, A., Inada, T. & Shingu, K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol Immunotoxicol 29, 477–486 (2007).
Freeman, J. et al. Effect of Perioperative Lidocaine, Propofol and Steroids on Pulmonary Metastasis in a Murine Model of Breast Cancer Surgery. Cancers (Basel) 11, 613 (2019).
Hovaguimian, F. et al. Anesthesia and Circulating Tumor Cells in Primary Breast Cancer Patients: A randomized controlled trial. Anesthesiology 133, 548–558 (2020).
Iwasaki, M. et al. The differential cancer growth associated with anaesthetics in a cancer xenograft model of mice: Mechanisms and implications of postoperative cancer recurrence. Cell. Biol. Toxicol. https://doi.org/10.1007/s10565-022-09747-9 (2022).
Desmond, F., McCormack, J., Mulligan, N., Stokes, M. & Buggy, D. J. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 35, 1311–1319 (2015).
Ni Eochagain, A., Burns, D., Riedel, B., Sessler, D. I. & Buggy, D. J. The effect of anaesthetic technique during primary breast cancer surgery on neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and return to intended oncological therapy. Anaesthesia 73, 603–611 (2018).
Tavare, A. N., Perry, N. J., Benzonana, L. L., Takata, M. & Ma, D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int. J. Cancer 130, 1237–1250 (2012).
Chang, C. Y. et al. Anesthesia and Long-term Oncological Outcomes: A Systematic Review and Meta-analysis. Anesth. Analg 132, 623–634 (2021).
Lv, R., Zhang, C., Huang, Y. & Xiao, P. Effect of Different General Anesthesia Methods on the Prognosis of Patients with Breast Cancer after Resection: A Systematic Review and Meta-analysis. Comput. Math. Methods Med. 2022, 6846079 (2022).
Pang, Q. Y., Duan, L. P., Jiang, Y. & Liu, H. L. Comparison of Outcomes After Breast Cancer Surgery Between Inhalational and Propofol-Based Intravenous Anaesthesia: A Systematic Review and Meta-Analysis. J. Pain Res. 14, 2165–2177 (2021).
Yap, A., Lopez-Olivo, M. A., Dubowitz, J., Hiller, J. & Riedel, B. Anesthetic technique and cancer outcomes: A meta-analysis of total intravenous versus volatile anesthesia. Can. J. Anaesth. 66, 546–561 (2019).
Rui Lv, C. Z., Yuyuan, H. & Peng, X. Effect of Different general anesthesia methods on the prognosis of patients with breast cancer after resection: A systematic review and meta-analysis. Comput. Math. Methods Med. 2022, 6846079 (2022).
Cho, J. S. et al. The Effects of Perioperative Anesthesia and Analgesia on Immune Function in Patients Undergoing Breast Cancer Resection: A Prospective Randomized Study. Int J Med Sci 14, 970–976 (2017).
Enlund, M. et al. Survival after primary breast cancer surgery following propofol or sevoflurane general anesthesia-A retrospective, multicenter, database analysis of 6305 Swedish patients. Acta Anaesthesiol/ Scand. 64, 1048–1054 (2020).
Enlund, M. et al. The choice of anaesthetic–sevoflurane or propofol–and outcome from cancer surgery: A retrospective analysis. Ups. J. Med. Sci. 119, 251–261 (2014).
Enlund, M., Berglund, A., Enlund, A. & Bergkvist, L. Volatile versus propofol general anesthesia and long-term survival after breast cancer surgery: A national registry retrospective cohort study. Anesthesiology https://doi.org/10.1097/ALN.0000000000004309 (2022).
Hong, B. et al. Anesthetics and long-term survival after cancer surgery-total intravenous versus volatile anesthesia: a retrospective study. BMC Anesthesiol. 19, 233 (2019).
Huang, Y. H. et al. Propofol-based total intravenous anesthesia did not improve survival compared to desflurane anesthesia in breast cancer surgery. PLoS One 14, e0224728 (2019).
Kim, M. H. et al. Does the type of anesthesia really affect the recurrence-free survival after breast cancer surgery?. Oncotarget 8, 90477–90487 (2017).
Lee, J. H., Kang, S. H., Kim, Y., Kim, H. A. & Kim, B. S. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: A retrospective study. Korean J. Anesthesiol. 69, 126–132 (2016).
Sessler, D. I. et al. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 394, 1807–1815 (2019).
Shiono, S. et al. Comparison of 1-year recurrence-free survival between sevoflurane and propofol use for general anesthesia management in primary breast cancer surgery. J. Anesth. 34, 694–701 (2020).
Yan, T. et al. Effects of anesthetic technique and surgery on myeloid-derived suppressor cells and prognosis in women who underwent breast cancer surgery: A prospective study. Cancer Manag. Res. 11, 5513–5522 (2019).
Yan, T., Zhang, G. H., Wang, B. N., Sun, L. & Zheng, H. Effects of propofol/remifentanil-based total intravenous anesthesia versus sevoflurane-based inhalational anesthesia on the release of VEGF-C and TGF-beta and prognosis after breast cancer surgery: a prospective, randomized and controlled study. BMC Anesthesiol. 18, 131 (2018).
Yoo, S. et al. Total Intravenous Anesthesia versus Inhalation Anesthesia for Breast Cancer Surgery: A Retrospective Cohort Study. Anesthesiology 130, 31–40 (2019).
Yoon, S. et al. Impact of Propofol-based Total Intravenous Anesthesia Versus Inhalation Anesthesia on Long-term Survival after Cancer Surgery in a Nationwide Cohort. Ann. Surg. https://doi.org/10.1097/SLA.0000000000005568 (2022).
Zhang, J., Chang, C. L., Lu, C. Y., Chen, H. M. & Wu, S. Y. Long-term oncologic outcomes of breast conserving surgery with propofol-based total intravenous anesthesia or volatile inhalational general anesthesia without propofol: A propensity score-matched, population-based cohort study. Am. J. Cancer Res. 11, 4966–4980 (2021).
Zhang, J., Chang, C. L., Lu, C. Y., Chen, H. M. & Wu, S. Y. Paravertebral block in regional anesthesia with propofol sedation reduces locoregional recurrence in patients with breast cancer receiving breast conservative surgery compared with volatile inhalational without propofol in general anesthesia. Biomed. Pharmacother. 142, 111991 (2021).
Zhang, J., Chang, C. L., Lu, C. Y., Chen, H. M. & Wu, S. Y. Anesthesia With Propofol Sedation Reduces Locoregional Recurrence in Patients With Breast Cancer Receiving Total Mastectomy Compared With Non-Propofol Anesthesia. Front. Oncol. 12, 708632 (2022).
Enlund, M., Enlund, A., Berglund, A. & Bergkvist, L. Rationale and Design of the CAN Study: an RCT of Survival after Propofol- or Sevoflurane-based Anesthesia for Cancer Surgery. Curr. Pharm. Des. 25, 3028–3033 (2019).
Author information
Authors and Affiliations
Contributions
Yingjun Zhang, Ping Yu, Feng Ye and Na Li conceived the study and wrote the manuscript. Yingjun Zhang, Ping Yu, Lei Bian took charge of database searching, data interpretation and language editting. Feng Ye, Lei Bian, Wanwei Huang and Na Li preformed all data analysis. All the authors were all involved in approval of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Yu, P., Bian, L. et al. Survival benefits of propofol-based versus inhalational anesthesia in non-metastatic breast cancer patients: a comprehensive meta-analysis. Sci Rep 14, 16354 (2024). https://doi.org/10.1038/s41598-024-67291-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-67291-4








Mats Enlund
In a new meta-analysis in Scientific Reports concerning the choice of anesthetic for anesthesia maintenance and the possible impact on long-term survival, the authors, Y. Zhang and coworkers, found that propofol-based anesthesia provided better long-term survival than did inhalational-based anesthesia [1].
In Discussion, the authors state that they are awaiting the results of the CAN study. As I am the initiator and head-PI of this study, I just want to inform that the breast cancer part of the CAN study was published last year [2]. There was no difference in long-term survival between patients randomized to propofol or sevoflurane in this RCT with a median follow-up time of 76.7 months (i.e., 6.4 years). “With 1,650 patients in the breast cancer cohort, 80% power was obtained to detect a survival difference of five percentage points with a P-value of less than 0.05. Adding an additional 114 patients (7%) provided a reasonable safety margin for patient loss or technical failures.” Other factors, such as co-morbidity and aggravating tumor characteristics, were of importance for mortality. Younger age, normal BMI, and non-smoking were protective factors, however limited.
A scientific question of relevance is whether the truth can be reliably found in 1,764 randomized patients as opposed to 127,774 patients in a meta-analysis of a mixture of retrospective studies and a few small RCTs?
Mats Enlund
Center for Clinical Research, Uppsala University
Vastmanland Hospital
Entrance 29
SE-721 89 Vasteras
Sweden
References
1.Zhang Y, Yu P, Bian L, Huang W, Li N, Ye F. Survival benefits of propofol-based versus inhalational anesthesia in non-metastatic breast cancer patients: a comprehensive meta-analysis. Sci Rep 2024; 14: 16354.
2.Enlund M, Berglund A, Enlund A, et al. Impact of general anaesthesia on breast cancer survival: a 5-year follow up of a pragmatic, randomised, controlled trial, the CAN-study, comparing propofol and sevoflurane. EClinicalMedicine 2023; 60: 102037.