Abstract
Sjögren syndrome (SS) is an autoimmune disease characterized by chronic inflammatory infiltrates in the salivary and lacrimal glands. Mucosal-associated invariant T (MAIT) cells are a subset of innate-like T-cells, predominantly found in mucosal tissues with crucial role in epithelial homeostasis. Thus, MAIT cells may be implicated in mucosal alterations of SS patients. Activation markers, inflammatory and cytotoxic cytokines were examined in 23 SS patients and compared to 23 healthy controls (HC). Tissular MAIT cells in salivary gland (SG) biopsies were also analyzed. Circulating MAIT cells were decreased in SS patients with a higher expression of CD69 and a higher CD4/CD8 ratio of MAIT cells. MAIT cells showed a higher production of IFNγ, TNFα and GzB in SS compare to HC. Tissular MAIT cells were present within inflamed SG of SS patients, while they were absent in SG of HC. Overall, circulating MAIT cells are decreased in the peripheral blood of SS albeit producing higher amounts of IFNγ, TNFα, and GzB. Tissular MAIT cells are detected in salivary glands from SS with a proinflammatory tissular cytokine environment. MAIT cells with abnormal phenotype, functions and tissular homeostasis may contribute to epithelial damage in SS.
Similar content being viewed by others
Introduction
Sjogren’s syndrome (SS) is a rare autoimmune disease characterized by sicca features (dryness of the eyes and mouth), resulting from chronic inflammatory infiltrates into the salivary and lacrimal glands. The current SS diagnosis criteria include the presence of anti-SSA (Ro) antibodies or focal inflammatory infiltrates (≥ 50 mononuclear cells within 4 mm2) in labial salivary glands (LSG) of patients1. Higher focus score and the presence of germinal center-like structures in LSG of patients with SS have been correlated with severe disease2.
Mucosal associated invariant T (MAIT) cells contribute to antibacterial and antiviral immunity, and inflammatory and autoimmune diseases, with important role in tissue homeostasis and repair3,4,5. MAIT cells are an important subset of innate-like T lymphocytes with a restricted T cell receptor (TCR) usage, functionally related to NKT cells and γδT cells, and at the interface of the innate and adaptive response6,7. MAIT cells are predominantly localized in the mucosal tissue of organs such as the lung and the intestine, albeit they are also detected in blood (1–10% of T-cells). Alteration in their proportion and function are associated with various infections, inflammation, and autoimmune diseases8. MAIT cells can be identified by flow cytometry as CD3+ TCR Vα7.2+ CD161high cells3. In the peripheral blood, they display high level of chemokine receptors (i.e. CCR6, CXCR6, CXCR4, CCR5) involved in tissue trafficking, and high level of receptor for cytokines such as IL-18, IL-12 and IL-23. MAIT cells can be activated through two pathways, namely a TCR-dependent pathway involving MR1+ antigen-presenting cells, and a TCR-independent pathway mediated by IL-12 and IL-18. Upon activation, MAIT cells rapidly produce inflammatory cytokines (IFNγ, TNFα) and cytotoxic effectors (perforin, granzyme)9.
In auto-immune diseases such as multiple sclerosis10, lupus erythematous systemic11, rheumatoid arthritis12, giant cell arteritis13 and herpetiform dermatitis14, MAIT cells have been observed to infiltrate target tissues while their number in the blood were decreased in most of the studied autoimmune diseases8. The overactivation of MAIT cells in autoimmune diseases may contribute to increased apoptosis and migration of MAIT cells to inflammatory target tissues. Decreased peripheral MAIT cells, along with an activated/effector phenotype and cytotoxic function, may serve as markers of disease severity in the course of autoimmune disease such as the SS. However, the mechanism underlying MAIT cells activation and the function of these infiltrating MAIT cells in autoimmune diseases remains unknown.
Current advances in the understanding of MAIT cell functions suggests dual functions of MAIT cells in in immune-mediated tissue injury and repair in a context of autoimmune disease15. MAIT cells could exacerbate inflammation and tissue injury by producing pro-inflammatory cytokines and cytotoxic molecules or mediate tissue protection in restoration of tissue integrity after acute injury by regulating other immune cell subsets and secreting tissue repair factors16,17,18.
Despite this knowledge, there is limited evidence regarding the role of innate immune system and innate-like T cell dynamics in SS. Our hypothesis is that abnormal frequencies, phenotypes, and functions of MAIT cells in peripheral blood and targeted-tissues of SS patients, may be implicated in epithelial damage of SS. We therefore aimed to characterize the proportion and activation phenotype of circulating MAIT cells in SS patients as compared to age- and sex-matched healthy controls (HC). Furthermore, we analyzed the inflammatory and cytotoxic functions of circulating MAIT cells upon stimulation, and whether MAIT cells were detected in labial salivary gland biopsies (LSGB) of SS patients and healthy controls (HC) as well as their tissue cytokine environment.
Results
Population characteristics
Demographic and laboratory characteristics of the 23 SS patients and 23 HC (22 women and one man in each group) are summarized in Table 1. SS patients presented with sicca symptoms (all patients), joint pain (6/23, 26%), neurological disorders (6/23, 26%), parotid swelling (3/23, 13%), skin rash (1/23, 4%) and pulmonary involvement (1/23, 4%). Anti-SSA antibodies found to be positive in 16/23 (70%) SS patients, and LSGB was pathological in 13 (57%) of them. Twelve SS patients, 12 (52%) had no treatment, nine (39%) were taking hydroxychloroquine, six (26%) were taking prednisone and one (4%) was treated with methotrexate. Additionally, six SS patients (26%) presented with another concomitant connective tissue disorder.
Reduced numbers, higher CD4/CD8 ratio, higher basal CD69 and mortality of circulating MAIT cells from SS patients
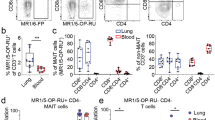
The gating strategy used to identify MAIT cells is depicted in Fig. 1A. MAIT cells were defined as CD3+ , TCRVα7.2+ , CD161high. The flow cytometry analysis focused on their expression of CD4/ CD8, the CD69 activation marker and the 7-AAD viability marker (Fig. 1B–E). The percentage of MAIT cells among CD3+ T cells were significantly decreased in SS patients (n = 23) compared to controls (n = 23): 0.87% [0.33–1.7] versus 3,3% [1.6–5] (p < 0.0001) (Fig. 1B). MAIT cells are known to be subdivided into CD4+ , CD8+ , and double-negative (DN) subsets with a predominance of CD8 + MAIT cells, some DN MAIT cells, and very rare CD4+ MAIT cells. In SS patients, expression of CD4 and CD8 in MAIT cells showed an increased CD4+ /CD8+ MAIT cell ratio compared to HC: 9.7% [3.25–13.5] versus 2.9% [0.7–2.4] (p < 0.01) (Fig. 1C). The percentage of CD69 + cells among MAIT cells was higher in SS patients as compared to HC: 19% [10–31.25] and 5.85% [5–17.25] respectively (p < 0.02) (Fig. 1D). 7-AAD staining in MAIT cells was increased in SS patients compared to HC: 9% [2–18] vs 2% [0.5–8.5], respectively (p < 0.02) (Fig. 1E).
Comparison of MAIT circulating blood cells frequency and phenotypes from SS patients and HC subjects. (A) Representative gating strategy and flow cytometry plots of MAIT cells in PBMC from an SS patient and a control as determined by flow cytometry. (B) Representative flow cytometry plots of peripheral blood mononuclear cells (PBMCs) from an SS patient and a HC, displayed from left to right: total MAIT cells as a percentage of CD3+ cells, CD4+ and CD8+ cell percentages among MAIT cells, CD69+ cell percentage among MAIT cells, and 7-AAD+ cell percentage among MAIT cells without initially excluding apoptotic cells. (C) Representative dot plot of MAIT cell subsets defined by TCRVα7.2 expression in combination with CD161high, CD3+ are shown as percentages of the CD3+ T cell population in SS and HC. (D) Representative dot plot of CD4/CD8 staining of all MAIT cells in SS and HC. (E) Representative dot plot of activation marker CD69+ staining of all MAIT cells in SS and HC. (F) Representative dot plot of apoptotic marker 7-AAD+ staining of all MAIT cells in SS and HC without elimination of 7AAD+ cells during the gating strategy. Bar plots show all individuals and median [interquartile range]. HC healthy control, SS Sjögren’s syndrome patients. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (Mann–Whitney test).
Higher production of TNFα, IFNγ and granzyme B in MAIT cells from SS patients
To examine cytokine production in MAIT cells, we incubated PBMCs from 20 SS and 20 HC for 5 h in the presence of PMA and Ionomycine. The expression of IFNγ, TNFα, GzB in the MAIT cell population was examined at the single-cell level by intracellular flow cytometry (Fig. 2A).
Comparison of MAIT cell cytokine and granzyme B production in peripheral blood between SS patients and healthy control (HC) subjects. (A) Representative flow cytometry plots showing from left to right: IFNγ-producing MAIT cells after PMA and ionomycin stimulation, TNFα-producing MAIT cells after PMA and ionomycin stimulation, granzyme B (GzB)-producing MAIT cells before stimulation, and GzB-producing MAIT cells after PMA and ionomycin stimulation in one SS patient's PBMC and one HCs PBMC. (B) “Representative dot plot showing IFNγ and TNFα staining of all MAIT cells after PMA and ionomycine stimulation in SS patients and HC”. (C) Representative dot plot showing GzB staining of all MAIT cells in SS patients and HC. Bar plots show all individuals and median [interquartile range]. HC healthy control, SS Sjögren’s syndrome patients. *p < 0.05, **p < 0.01 (Mann–Whitney test).
After PMA and ionomycin stimulation, a higher proportion of MAIT cells from SS patients (n = 20) were found to produced proinflammatory cytokines when compared to HC MAIT cells (n = 20): IFNγ (59% [49.25–66] versus 45% [32.5–49.35]; p < 0.001) and TNFα (65.5% [50.75–73.75] versus 52% [34.25–59.75]; p < 0.001) (Fig. 2B). Additionally, the frequency of GzB-producing MAIT cells was increased in SS patients compared to HCs, both before (5% [2–15] versus 1.5% [0.63–2.8]; p < 0.05) and after (17.5% [6.1–32] versus 5.05% [1.8–9.8]; p < 0.01) stimulation with PMA and ionomycine (Fig. 2C).
MAIT cells identification in LSGB from patients
To investigate the possibility that the observed decreased in MAIT cell frequency in PBMC might be associated to possible MAIT cell migration into inflamed target tissues during SS, we examined whether MAIT cells infiltrated the LSG of SS patients. LSGB samples from two SS patients with grade 4 sialadenitis were assessed using confocal microscopy to identify MAIT cells based on the expression of CD3 (white), TCRVα7.2 (red) and IL18Rα (green). MAIT cells (CD3+ Vα7.2TCR+ IL-18Rα+ cells) were detected in the SS LSGB (n = 2) while none were observed in normal LSGB samples from HC (n = 2) (Fig. 3A).
Labial salivary gland MAIT cell frequency in SS patients and HC subjects. (A) Confocal microscopy analysis of MAIT cells in labial salivary gland biopsy (LGSB) sample from SS patient with sialadenitis grade IV according to Chisholm and Mason classification. CD3 is shown in white, TCRVα7.2 in red, IL-18Rα in green, and DAPI depicts nuclei of LSGB cells in blue. The images are magnified at ×40. A higher magnification view (×80) shows the co-localization of the three MAIT-specific markers used. The tertiary panel (HC) shows the results of a staining with the same primary and secondary antibodies on a normal labial salivary gland biopsy. (B) Representative gating strategy and flow cytometry plots of MAIT cells in labial salivary gland mononuclear cells (LSGMC) from an SS patient and a HC, as determined by flow cytometry.
To validate this observation, we performed flow cytometry analysis on cell pellets obtained from mechanically homogenized LSGB samples. We confirmed the presence of CD3+ , TCRVα7.2+ , CD161high cells in LSGB samples from SS patients (n = 4), with the same panel and gating strategy as employed for PBMC analysis. In contrast, no MAIT cells were detected in LSGB samples from HC (n = 8). The frequency of MAITs among LSGB T cells was 2.36% [0.55–19.59] for SS patients and 0% [0–0] for HC (Fig. 3B). The phenotypes of these cells were predominantly CD8+ , with a subset exhibiting a double-negative phenotype (Table 2). Notably, we did not observe any CD4+ MAIT cells in this tissue.
Presence of IL-17, IFNγ, and IFNα producing cells in LSGB of SS patients
To characterize the cytokine environment in inflamed target tissue, we conducted immunohistochemistry on LSGB samples obtained from SS patients (n = 5) (Fig. 4A) and from HC (n = 5) (Fig. 4E). In all SS samples, we observed the presence of cells producing IFNα, IFNγ and IL-17 (Fig. 4B–D, respectively). Conversely, among HC, only one LSGB sample exhibited cells producing IFNγ, while no IL-17 and IFNα producing cells were detected (Fig. 4F–H, respectively).
Labial salivary gland (LSG) from SS patient (grade IV according to Chisholm and Mason classification) and HC (normal LSG): Detection of IFNα, IFNy, IL-17 producing cells in labial salivary gland (LSG) tissue from SS patient (A–D) grade IV according to Chisholm and Mason classification; and HC (E–H) normal LSG. (A,E) Representative images of hematoxylin and eosin (HES) stain of LSG paraffin sections from (A) one SS and (E) one HC. (B–D, F–H) Representative immunoperoxidase stains of serial sections. Primary antibodies as indicated: (B,F) mouse anti-human IFNα on (B) SS and (F) HC; (C,G) mouse anti-human IFNy on (C) SS and (G) HC; (D,H) mouse anti-human IL-17 on (D) SS and (H) HC. Arrows indicate positive stained cells. The scale is noted in right higher corner of the figure. Data shown are representative of single staining from n = 5 SS and n = 5 HC.
Discussion
Although MAIT cells are recognized to play a crucial role in epithelial homeostasis and are implicated in several autoimmune diseases19, their accurate functions in SS, characterized by an autoimmune epithelitis with lymphocytic infiltrates that invade the epithelial structures of affected organs, remain to be elucidated.
According to our data obtained on blood and tissues compartments, we show that the decrease of MAIT cells in Sjögren syndrome could be linked to an increased cell death and/or an enhanced migration into inflamed tissues (Fig. 5). In peripheral blood, we demonstrated that circulating MAIT levels are decreased in SS patients. Mirroring this reduction of MAIT cells frequency in periphery, we observed many immune dysregulations in SS patients compare to matched HC, with enrichment of activated, pro-apoptosis, cytotoxic and pro-inflammatory phenotypes of circulating MAIT cells. Main results in peripheral blood of SS patients were a higher expression of the activation marker CD69 and higher apoptosis (7-AAD staining) on MAIT cells, a higher capacity to produce inflammatory cytokines (IFNγ and TNFα) by MAIT cells after PMA-iono stimulation and a higher capacity to produce cytotoxic cytokine (GzB) by MAIT cells at baseline and after PMA-iono stimulation. In inflamed tissues of labial salivary glands from SS patients, we observed the presence of tissular MAIT cells in inflammatory microenvironment characterized by the presence of abundant IFNα, IFNγ and IL-17. Thus, our results suggest that the reduction of MAIT cells in the peripheral blood of SS might be due, at least in part, to activation-induced cell death, and possibly to the migration of MAIT cells in inflamed tissues of SS patients. Whether MAIT cells might contributed to epithelial damage or conversely to tissue repair and protection in SS needs to be explored in future studies.
Our data suggest that changes in the abundance and phenotype of MAIT cells within the peripheral blood cell population, with significantly reduced MAIT levels but elevated activation, apoptosis, and expression of cytotoxic and pro-inflammatory molecules indicative of a dysfunctional and exhausted profile, characterize patients with Sjögren syndrome. Significant decreases in circulating MAIT cells could suggest either an increased cell death and/or an enhanced MAIT cells migration into inflamed tissues.
The results obtained in our study confirm a decrease in the frequency of circulating MAIT cells in the peripheral blood cells of patients with SS and are consistent with the existing literature20,21,22. The reduction of MAIT cells in peripheral blood has been demonstrated in many cases of auto-immune or inflammatory diseases with the hypothesis of MAIT cells migration into the inflamed tissue-targeted (i.e. colon inflamed mucosa in ulcerative colitis, bronchoalveolar lavage and airway mucosa in asthma and the lungs of sarcoidosis) since MAIT cells constitutively express chemokine receptors such as CCR67,23,24,25,26. The ligands, CCL20 and β-defensin, are produced mainly in inflamed sites and attract CCR6+ cells by inducing migration27.
As previously reported, MAIT cells are predominantly CD8+ and sometimes double negative CD4− CD8−28. In our cohort of SS patients, we observed a significant difference favoring a higher CD4+ /CD8+ ratio among MAIT cells compared to controls, similar to what has been observed previously in SS21,29,30 and in SLE11.
We found that CD69 expression of MAIT cells from SS patients was spontaneously increased as compared to controls, unfortunately we did not study any other activation marker. Such overactivation was previously observed in patients with active SLE31. Thus, activation status of circulating MAIT cells could be associated with disease activity. We also observed higher apoptosis (7-AAD staining) in circulating MAIT cells of SS patients. In SLE, the reduced number of MAIT cells was also partially due to the enhanced cell death of MAIT cells, possibly by activation-induced cell death31. The CD69 expression levels on MAIT cells in SLE correlated with disease activity31. Altogether, these results suggest that activation-induced cell death might be one of the mechanisms to explain the reduction of circulating MAIT cells in peripheral blood of SS patients.
Moreover, we showed that MAIT cells from SS patients produced more pro-inflammatory cytokines (IFNγ and TNFα) than MAIT cells from matched-HC. MAIT cells belong to the innate-like T-cell family of cells with a memory phenotype, which allows them to rapidly release IFNγ, TNFα, and in some circumstances IL17, exerting an immunomodulatory role on the ensuing immune response32. In a context of auto-immune disease such as SLE, high level of inflammatory cytokines correlated with the activated state of MAIT31. These inflammatory cytokines production and inflammatory environment could contribute to the cytokine-mediated activation of MAIT cells. In SS, our results differ from those reported in the publication by Wang and al.22.
The increased production of granzyme B by MAIT cells of SS patients at baseline and after stimulation with PMA and ionomycine suggests higher degranulation and cellular cytotoxic potential of MAIT cells during SS. High levels of CD69 and GzB expressed by circulating MAIT cells in SS patients compare to HC, might reflected an already activated state of MAIT cells in vivo in SS patients. Taken together, our results demonstrated that circulating MAIT cells in SS exhibit an abnormal phenotype with sustained activation, degranulation, and increased apoptosis, suggesting a mechanism of activation-induced cell death as possible reason for the lower proportion of MAIT cells in peripheral blood of SS.
Two main hypotheses (or a combination of both) may explain the phenomenon of reduced peripheral MAIT cell abundance in SS: (1) recruitment of MAIT cells from the circulation into the inflamed tissue and (2) exhaustion-induced apoptosis of MAIT cells. To explore the possible contribution of MAIT cell migration into the inflamed tissue as a cause of peripheral MAIT deficiency, we analyzed MAIT cells in LSGB of SS patients. Notably, we observed the presence of MAIT cells in LSGB of SS patients, while they were indetectable in those of HC. This was confirmed using two different methods: immunofluorescence staining and flow cytometry analysis on LSGB mononuclear cells. These observations raises the hypothesis of MAIT cells migration towards target inflammatory tissues during SS as suggested by Wang and al. and others22. We did not observe any CD4+ MAIT cells within the SS LSGB, only CD8+ and double negative MAIT cells were identified. The significant increase in the CD4/CD8 ratio among MAIT cells observed in the blood of SS patients and the predominancy of CD8+ MAIT cells in tissue may be linked, suggesting a preferential migration of CD8+ MAIT cells. Further studies are needed to confirm such hypothesis. Besides, due to the small sample size of the current study, we were unable to establish a correlation between the level of circulating MAIT cells and the MAIT cell infiltration in the tissue. In sarcoidosis, MAIT cells are also enriched in inflamed tissues. Sarcoidosis patients with parenchymal infiltration in the lungs showed a significantly higher proportion and number of MAIT cells in BALF compared to patients without parenchymal infiltration26.
Studying the pathogenicity of MAIT cells in humans is complicated due to the functional plasticity of this cell subset, allowing them to obtain different effector functions depending on the local environment15. To explore microenvironment in inflamed tissue of SS, we performed immunohistochemistry on salivary glands. The immunohistochemistry analysis of the tissue cytokine environment showed an increased presence of IL-17 and IFNγ producing cells in the SS patients LSGB. The presence of these cytokines is consistent with the presence of activated MAIT cells. Unfortunately, because of the absence of co-staining of MAIT cell markers, we cannot draw conclusion regarding a direct link between the observed markers and MAIT secretion in the tissue. Moreover, both IL-17 and IFNγ play an important physiological role at mucosal barriers, and are involved in inflammatory responses to pathogens. IL-17 and IFNγ have been shown to promote chronic inflammation in several autoimmune diseases. We also observed the presence of IFNα secreting cells in the inflamed tissues and their absence in normal LSGB. IFNα belongs to the type 1 interferon family and has an activating role on MAIT cells33. In SS, there is a correlation between blood levels of IFNα and disease activity34. Further studies will be needed to establish a direct link between the interferon signature and the activation or migration of MAIT cells. Our results demonstrated that tissular MAIT cells were present in LSGB from SS patients with proinflammatory (IFNγ, IL17, IFNα) tissular cytokine environment, suggesting that MAIT cells might migrate to inflamed LSG and could thus further fuel the local inflammation that contribute to epithelial alternations observed during SS, thus explaining a part of the peripheral blood MAIT depletion. We postulate that inflammatory environment in LSGB of SS and functional plasticity towards MAIT cells producing proinflammatory cytokines and cytotoxic molecules, may enhance pathogenicity of MAIT cells at the main target sites of the disease, i.e. salivary and lacrimal glands15,18. Thus, altered MAIT cell functions and homeostasis might contribute to epithelial damage in SS, and their therapeutic manipulation to restore their normal phenotype and prevent deleterious mucosal inflammation during SS needs to be explored in future studies.
Altogether, our data suggest that changes in the abundance and phenotype of MAIT cells within the peripheral blood cell population, with significantly reduced MAIT levels but elevated activation, apoptosis, and expression of cytotoxic and pro-inflammatory molecules indicative of a dysfunctional and exhausted profile, characterize patients with Sjögren syndrome (Fig. 5). Significant decreases in circulating MAIT cells could suggest either an increased cell death and/or an enhanced MAIT cells migration into inflamed tissues.
The current pilot study conducted in patients with SS enrolled in real condition of treatment and follow-up, suggest for the first time that circulating MAIT cells with activated phenotype, proinflammatory and cytotoxic functions may migrate from the blood to the target mucosal tissues in SS patients. Their contribution to the epithelial damages observed during SS remains to be demonstrated. Whether such activated MAIT cells may be used as a biomarker of disease activity or progression in SS patients in clinical practice, to individualize treatment and follow-up is currently unknown but certainly deserves further studies that will be conducted in patients in the clinical setting.
Methods
Patients and healthy control subjects
Peripheral blood mononuclear cells (PBMCs) were collected from 23 SS patients diagnosed according to the 2016 ACR-EULAR criteria1 and 23 age- and gender-matched HC. Healthy controls were excluded if they had any autoimmune disease, chronic infection, or had been infected with SARS-CoV-2 over the past 3 months. All participants were recruited from the Internal Medicine Department at Lariboisière University Hospital and provided written inform consent. Blood samples were collected in lithium-heparin tubes and PBMC were isolated using density gradient centrifugation with Pancoll human separating Solution (PAN Biotech®). The study was approved by the institutional ethics committee of Pitié-Salpêtrière Hospital and was performed in accordance with the Declaration of Helsinki and good clinical practices. All participants provided informed consents.
Two labial salivary gland biopsies (LSGB) were obtained from SS patients with grade 4 sialadenitis according to the Chisholm and Mason classification and two from HC for tissue immunofluorescence staining. A HC who underwent a salivary glands biopsy, presented clinical manifestations such as sicca syndrome and/or arthralgia, suggesting Sjögren syndrome. However, specific autoimmune antibodies were negative and, finally, histological analysis of salivary glands biopsy did not show inflammatory infiltrates. Flow cytometry analysis was further performed in four SS patients with grade 3 or 4 sialadenitis, eight HC with normal LSGB. Tissue samples were collected in culture media containing RPMI 1640 middle supplemented with GlutaMAX (Gibco®), 100 U/ml penicillin, 100 mg/ml streptomycin and 5% bovine serum albumin. For immunohistochemistry, paraffin-embedded LSG tissue was obtained from five SS patients with grade 4 sialadenitis as well as from five HC.
Flow cytometry analysis of MAIT cell phenotype and function
PBMC were washed with 1 mL buffer containing 2% fetal calf serum Gibco® and 98% DPBS Gibco®. Subsequently, they were incubated with a mix of antibodies for 30 min at 4 °C. The following monoclonal antibodies (mAbs) and reagents were used: AF700-conjugated anti-CD3 (1:20), APC-conjugated anti-CD161 (1:20), BV605-conjugated anti-TCRVα7.2 (1:20), FITC-conjugated anti-CD4 (1:20), BV510-conjugated anti-CD8 (1:20), PECY7-conjugated anti-CD69 (1:20), 7-AAD (1:20) (Biolegend®). MAIT cells were identified based on their expression of surface markers CD3+ , TCRVα 7.2+ and CD161high. CD4 and CD8 phenotypes, activation marker (CD69) expression, and viability (7-AAD) were analyzed. For functional analysis, after isolation, two million PBMCs were incubated in 1 mL complete media, consisting of RPMI 1640 medium supplemented with GlutaMAX Gibco®, 100 U/ml penicillin, 100 mg/ml streptomycin and 5% bovine serum albumin. They were then stimulated with 2 µl/ml of cell stimulation cocktail containing PMA and ionomycin with Golgi Inhibitor (eBioscience®) for 5 h. Following stimulation, the cells were stained with AF700-conjugated anti-CD3 (1:20), APC-conjugated anti-CD161 (1:20), BV605-conjugated anti-TCRVα7.2 (1:20), 7-AAD (1:20) (Biolegend®). Cells were then permeabilized using the fixation/permeabilization Kit BDCytofix/Cytoperm®. Subsequently, the cells were stained with PB-conjugated anti-GzA (1:100), BV510-conjugated anti-TNFα (1:20), PECY7-conjugated anti-IFNy (1:20), APCFire750-conjugated anti-GzB (1:20) and analyzed by flow cytometry. The cell staining was performed with appropriate mAbs dilution for 30 min at 4 °C. The stained cells were analyzed using a BD FACSCelesta flow cytometer (BD Bioscience®), and the data were analyzed using FlowJo® software.
Labial salivary gland cells isolation and analysis with flow cytometry
LSGB were obtained from four patients with sialadenitis along with eight normal LSGB. The biopsies were washed with RPMI 1640 medium supplemented with GlutaMAX Gibco®, 100 U/ml penicillin, 100 mg/ml streptomycin and 5% bovine serum albumin. Subsequently, the biopsies were ground against a Falcon® nylon filter with a diameter of 70 μm and the filter was washed five times. The resulting cell pellets were stained with the following mAbs and reagents, using the same dilution and protocol as explained above for PBMC: AF700-conjugated anti-CD3, APC- conjugated anti-CD161, BV605-conjugated anti-TCRVα7.2, FITC-conjugated anti-CD4, BV510-conjugated anti-CD8, PECY7-conjugated anti-CD69, 7-AAD (Biolegend®). The stained cells were analyzed using a BD Celesta® flow cytometer, and the data were further analyzed with FlowJo software®.
Tissular immunofluorescence staining
Frozen LSGB sections from two patients with sialadenitis grade 4 sialadenitis and two HC were used. Cryostat sections with a thickness of 5 μm were fixed in 4% paraformaldehyde (PFA), moisturized with 1× phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100 (Fisher Scientific®), and blocked in a solution containing 5% donkey serum (UDS 20%). The samples were then incubated with primary antibodies: anti-CD3 (1:400, A0452, Agilent Technology), anti-TCRVα7.2 (1:100, 3C10, BioLegend®) and anti-IL-18Rα (1:100, AF840, R&D Systems®) during 12 h at 4 °C. After washing, slides were incubated in the dark for 2 h with the following secondary antibodies: anti-rabbit Alexa 647 (dark red), anti-mouse Cy3 (red), and anti-goat Alexa (green) at dilution of 1:400. Nuclei were stained with DAPI at a dilution of 1:1000. The slides were mounted with Immuno-Mount®. Image acquisition was performed using a Leica TCS SP8 fluorescence microscope (Leica Microsystems®), and the data were analyzed with the Leica Application Suite X (Leica Microsystems®).
Tissular Immunohistochemistry staining
The tissue cytokine environment was characterized in LSGB using the immunoperoxidase method. Serial sections of paraffin-embedded tissues from five SS patients and five HC were stained using the Dako Omnis robot (Dako®). The sections were deparaffinized with clearify clearing agent, washed, and then underwent antigen retrieval using water bath (30 min for anti-IFNy and anti-IFNα, 20 min for anti-IL17 at 97 °C) and EnVision FLEX TRS solution (low pH for anti-IFNy and anti-IL17 and high pH for anti-IFNα). Endogenous peroxidase activity was blocked by EnV FLEX peroxydase-blocking reagent. Primary antibodies: mouse anti-human IL-17 (1:500; Abcam®), mouse anti-human IFNy (1:200; Abcam®), mouse anti-human IFNα F-7 (1:100; Santa Cruz Biotechnology®) were incubated for 30 min. EnvFlex + Rabbit LINKER, stained polymer EnV FLEX/HRP and EnV FLEX substrate working solution were used to visualize the reaction (30 min each). Slides were counterstained with hematoxylin and mounted with tissue-Tek® robot after immersion in ethanol (100%) and xylene bath. Each histochemical labeling run included positive controls (human inflammatory tonsils) and negative controls (omission of primary antibody). The sections were viewed using the Eclipse Ci-S Nikon® microscope and images were acquired with the digital slight 1000 Nikon® camera.
Statistical analysis
Data are expressed as medians [interquartile range] for continuous variables and number (%) for categorical variables. Differences between groups were analyzed using the non-parametric Mann–Whitney test performed in GraphPad Prism (GraphPad, San Diego, CA, USA). Statistical significance was defined as p < 0.05 (two-tailed).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Shiboski, C. H. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 76(1), 9–16 (2017).
Daniels, T. E. et al. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren’s syndrome among 1726 registry participants. Arthritis Rheum. 63(7), 2021–2030 (2011).
Flament, H. et al. Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat. Immunol. 5, 1 (2021).
du Halgouet, A. et al. Role of MR1-driven signals and amphiregulin on the recruitment and repair function of MAIT cells during skin wound healing. Immunity 56(1), 78-92.e6 (2023).
Gnirck, A. C. et al. Mucosal-associated invariant T cells contribute to suppression of inflammatory myeloid cells in immune-mediated kidney disease. Nat. Commun. 14(1), 7372 (2023).
Bugaut, H. et al. A conserved transcriptional program for MAIT cells across mammalian evolution. J. Exp. Med. 221(2), e20231487 (2024).
Hinks, T. S. C. et al. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J. Allergy Clin. Immunol. 136(2), 323–333 (2015).
Toubal, A., Nel, I., Lotersztajn, S. & Lehuen, A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 19(10), 643–657 (2019).
Le Bourhis, L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11(8), 701–708 (2010).
Salou, M. et al. Neuropathologic, phenotypic and functional analyses of mucosal associated invariant T cells in multiple sclerosis. Clin. Immunol. 166–167, 1–11 (2016).
Cho, Y. N. et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J. Immunol. 193(8), 3891–3901 (2014).
Koppejan, H. et al. Altered composition and phenotype of mucosal-associated invariant T cells in early untreated rheumatoid arthritis. Arthritis Res. Ther. 21(1), 3 (2019).
Ghesquière, T. et al. Mucosal-associated invariant T cells in giant cell arteritis. J. Autoimmun. 121, 102652 (2021).
Li, J. et al. The frequency of mucosal-associated invariant T cells is selectively increased in dermatitis herpetiformis. Australas. J. Dermatol. 58(3), 200–204 (2017).
Waterhölter, A., Wunderlich, M. & Turner, J. E. MAIT cells in immune-mediated tissue injury and repair. Eur. J. Immunol. 53(12), e2350483 (2023).
Godfrey, D. I., Koay, H. F., McCluskey, J. & Gherardin, N. A. The biology and functional importance of MAIT cells. Nat. Immunol. 20(9), 1110–1128 (2019).
Talvard-Balland, N. et al. Human MAIT cells inhibit alloreactive T-cell responses and protect from acute graft-versus-host disease. JCI Insight 2024, e166310 (2024).
Li, Y., Du, J. & Wei, W. Emerging roles of mucosal-associated invariant T cells in rheumatology. Front. Immunol. 13, 819992 (2022).
Guggino, G. et al. IL-17 polarization of MAIT cells is derived from the activation of two different pathways. Eur. J. Immunol. 47(11), 2002–2003 (2017).
Hinrichs, A. C., Kruize, A. A., Leavis, H. L. & van Roon, J. A. G. In patients with primary Sjögren’s syndrome innate-like MAIT cells display upregulated IL-7R, IFN-γ, and IL-21 expression and have increased proportions of CCR9 and CXCR5-expressing cells. Front. Immunol. 13, 1017157 (2022).
Wang, J. J., Macardle, C., Weedon, H., Beroukas, D. & Banovic, T. Mucosal-associated invariant T cells are reduced and functionally immature in the peripheral blood of primary Sjögren’s syndrome patients. Eur. J. Immunol. 46(10), 2444–2453 (2016).
Haga, K. et al. MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J. Gastroenterol. Hepatol. 31(5), 965–972 (2016).
Yasutomi, Y. et al. Activated mucosal-associated invariant T cells have a pathogenic role in a murine model of inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 13(1), 81–93 (2022).
Ye, L. et al. Mucosal-associated invariant T cells restrict allergic airway inflammation. J. Allergy Clin. Immunol. 145(5), 1469-1473.e4 (2020).
Matsuyama, H. et al. Activation of mucosal-associated invariant T cells in the lungs of sarcoidosis patients. Sci. Rep. 9(1), 13181 (2019).
Ebert, L. M. & McColl, S. R. Up-regulation of CCR5 and CCR6 on distinct subpopulations of antigen-activated CD4+ T lymphocytes. J. Immunol. 168(1), 65–72 (2002).
Legoux, F., Salou, M. & Lantz, O. MAIT cell development and functions: the microbial connection. Immunity 53(4), 710–723 (2020).
Chiba, A. et al. Activation status of mucosal-associated invariant T cells reflects disease activity and pathology of systemic lupus erythematosus. Arthritis Res. Ther. 19(1), 58 (2017).
Ioannidis, M., Cerundolo, V. & Salio, M. The immune modulating properties of mucosal-associated invariant T Cells. Front. Immunol. 11, 1556 (2020).
Pavlovic, M., Gross, C., Chili, C., Secher, T. & Treiner, E. MAIT cells display a specific response to type 1 IFN underlying the adjuvant effect of TLR7/8 ligands. Front. Immunol. 11, 2097 (2020).
Trutschel, D. et al. Variability of primary Sjögren’s syndrome is driven by interferon-α and interferon-α blood levels are associated with the class II HLA-DQ locus. Arthritis Rheumatol. 74(12), 1991–2002 (2022).
Single-cell diversity and functional plasticity of human MAIT cells. Nat Immunol. 24(9), 1409–1410. https://doi.org/10.1038/s41590-023-01600-3 (2023).
Acknowledgements
Jeanne Chauffier was supported by grant from Société Nationale Française de Médecine Interne and grant from Groupe Pasteur Mutualité (Villa M). Aïcha Kante was supported by grant from Société Nationale Française de Médecine Interne.
Author information
Authors and Affiliations
Contributions
J.C., D.S. and C.C. developed the research concept; J.C and H.B. performed the experiments; MF.C., R.K., H.A., D.S. and C.C. validated results; MF.C., M.L., H.A., R.K., A.S., M.C., A.C., S.C. provided reagents and technical support; J.C. and C.C. performed statistical analyses; A.K., X.C., E.A., R.B., K.C., B.A., W.B., S.M., D.S. and C.C. recruited patients; J.C., H.B., A.S. and A.C. performed sample processing; J.C., D.S. and C.C. wrote the main manuscript; J.C., MF.C., S.M., D.S. and C.C. reviewed and edited the main manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chauffier, J., Berger de Gallardo, H., Chevalier, M.F. et al. Role of mucosal-associated invariant T cells dynamics in pathogenesis of Sjögren syndrome. Sci Rep 14, 17256 (2024). https://doi.org/10.1038/s41598-024-67901-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-67901-1