Abstract
This study aimed to investigate the relationship between hemodialysis duration (HDD) and retinal nerve fiber layer (RNFL) thickness. A total of 60 patients receiving maintenance hemodialysis and 67 healthy controls were analyzed. Spectral domain optical coherence tomography (SD-OCT) was employed to measure annular RNFL thicknesses. The hemodialysis group exhibited reduced temporal and inferior RNFL thicknesses relative to the control group. In hemodialysis patients, the inferior RNFL thickness was negatively correlated with HDD and positively correlated with intraocular pressure (IOP). Moreover, IOP was positively correlated with HDD. Mediation analysis showed that the negative correlation between HDD and inferior RNFL thickness was mediated by IOP. In conclusion, hemodialysis leads to temporal and inferior RNFL thinning, and the thickness reduction is proportional to hemodialysis duration. However, such changes are not induced by an increase in IOP.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) has become a significant global health concern worldwide. Over the past decades, the global prevalence of CKD has significantly increased, affecting approximately 10% of adults1. CKD is a continuous disease in which renal function declines for three months or more, including mild kidney injury and end-stage disease. Primary kidney disease or multiple system diseases involving the kidneys may lead to CKD. Regardless of the etiology, nervous system complications are common in CKD patients. Damage to various levels of nervous systems can occur due to several disorders, including central nervous system (CNS) disorders (e.g., uremic encephalopathy2, stroke3, and cognitive impairment4) and peripheral nervous system disorders (e.g., peripheral nerve and autonomic disorders5,6).
When CKD progresses to end-stage renal disease (ESRD), unless receiving a kidney transplant, patients need renal replacement therapy such as maintenance hemodialysis (MHD). Hemodialysis separates metabolic wastes, excess liquids, and electrolytes from the blood through a semipermeable membrane, which can maintain metabolic balance and improve the survival rate. However, the impact of hemodialysis on the nervous system is complex. Hemodialysis can clear toxins, alleviate fluid burden, and correct metabolic acidosis, greatly reducing the incidence of uremic encephalopathy. However, the hemodynamic instability caused by hemodialysis and the adverse effects of anti-coagulants may exacerbate the risk of stroke and cognitive impairment7,8. Therefore, it is critical to investigate whether MHD has a beneficial or detrimental effect on the nervous system.
Due to their homology during embryonic development, the retina and optic nerve are considered CNS components. Retinal ganglion cells collect neural activity signals of the retina and their axons aggregate to form the optic nerve, transmitting the signals to the lateral geniculate body and superior colliculus, ultimately reaching the visual processing center9. The close connection between the visual transmission pathway and the CNS in original and spatial structures suggests that any damage to the CNS may have an impact on the optic nerve and its related neurons. Therefore, changes in the optic nerve can partly reflect changes in the CNS, and eye imaging technology can provide valuable information about potential lesions10.
The transparency of ocular refractive media makes the retina the only optically accessible nervous tissue. Optical coherence tomography (OCT) is a non-invasive, non-contact, high-resolution optical scanning method that can quickly scan the microscopic imaging of the internal structure of the retina, completing visualization and accurate measurement of various layers of the retina11. The retinal nerve fiber layer (RNFL) is composed of unmyelinated axons of retinal ganglion cells and is an ideal structure for studying nerve injury and neurodegeneration. RNFL thinning has been reported in various CNS disorders12,13,14,15,16. Herein, we analyzed the changes in RNFL in patients receiving MHD, after excluding possible interfering factors, to elucidate the impact of long-term hemodialysis on the optic nerve and provide novel methods and basis for subsequent research on the impact of hemodialysis on the CNS.
Results
Clinical characteristics
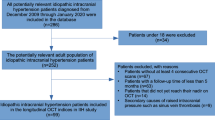
Sixty ESRD patients receiving MHD (119 eyes) (the hemodialysis group) and 67 healthy controls (120 eyes) (the control group) were enrolled. Some images of eyes were excluded due to poor image quality, low best corrected visual acuity (BCVA), and other factors. The demographic and clinical characteristics of the participants are shown in Table 1. No statistically significant difference in gender distribution, age, BCVA, and intraocular pressure (IOP) was found between the two groups, estimated glomerular filtration rate (eGFR), hemoglobin (Hb) and cardiovascular disease (CVD, including coronary heart disease, cerebrovascular disease, peripheral artery disease, and aortic atherosclerosis) showed statistically significant differences (p < 0.001). Meanwhile, hemodialysis duration (HDD) in the hemodialysis group did not conform to the normal distribution, which was expressed by the median (interquartile range).
RNFL thicknesses in hemodialysis and control groups
The measured RNFL thicknesses in the participants are displayed in Table 2. A statistically non-significant difference was found between the two groups for the average RNFL thicknesses. After subdividing the data into four quadrants, the temporal (p = 0.005) and inferior (p = 0.030) RNFL thickness was significantly thinner in the hemodialysis group than in the control group.
Correlation between clinical characteristics and RNFL thickness in hemodialysis patients
To determine factors influencing RNFL thinning in hemodialysis patients, the correlation between some clinical characteristics and measured RNFL thickness in the hemodialysis group was assessed with gender and age as control variables (Table 3). Results showed that the inferior RNFL thickness was significantly positively and negatively correlated with IOP (r = 0.210, p = 0.023) and HDD (r = − 0.198, p = 0.032), respectively. The IOP was significantly positively correlated with HDD (r = 0.232, p = 0.012). Additionally, the average RNFL thickness was significantly negatively correlated with HDD (r = − 0.203, p = 0.028), and temporal RNFL thickness was significantly positively correlated with BCVA (r = 0.248, p = 0.007).
Mediating effect of IOP in the correlation between HDD and inferior RNFL thickness
According to the above analysis, a pairwise correlation was found among IOP, HDD, and inferior RNFL thickness in MHD patients. It was hypothesized that HDD is associated with inferior RNFL thickness in MHD patients, with IOP as the mediating factor (Fig. 1).
Mediation analysis was performed using a multi-step regression analysis method17. First, the correlation between HDD and IOP was explored, and the indirect effect a was obtained. Then, the correlation between HDD and inferior RNFL thickness was evaluated to obtain the direct effect c'. Additionally, the correlation between IOP and inferior RNFL thickness was explored to obtain the indirect effect b. After validating the three steps, the correlations between HDD and IOP and inferior RNFL thickness were further tested, and the total effect c was obtained. The results are summarized in Table 4.
Model 1 showed that age had a significant positive impact on the IOP (β = − 0.189, p = 0.042). Model 2 revealed that HDD had a significant positive effect on the IOP (β = 0.232, p = 0.012), which was the indirect effect a. Model 3 demonstrated that gender (female) had a significant positive impact on the inferior RNFL thickness (β = 0.391, p < 0.001). Model 4 showed that HDD had a significant negative impact on the inferior RNFL thickness (β = − 0.186, p = 0.032), which was the total effect c; The direct effect c' is shown in Model 5 (β = − 0.245, p = 0.005). Model 5 revealed that IOP had a significant positive impact on the inferior RNFL thickness (β = 0.254, p = 0.004), which was the indirect effect b. To enhance the credibility of the test results, a mediation analysis was conducted on the same data through the PROCESS plugin in SPSS, which validated the above results. The simplified flowchart of the mediation analysis is shown in Fig. 2.
Due to the opposite sign of a * b and c', IOP played a suppression effect in the relationship between HDD and inferior RNFL thickness, with a suppression effect size of |ab/c'|= 24.05%.
Discussion
It has been established that age, male, diabetes, systemic hypertension, high IOP, and ametropia are the risk factors of RNFL thinning18,19. To avoid the influence of these factors, patients with diabetes and uncontrolled hypertension were excluded. Furthermore, gender and age were matched between the two groups and were considered as control variables in correlation analysis.
The present study demonstrated that the hemodialysis group exhibited reduced temporal and inferior RNFL thicknesses compared with the control group. Correlation analysis of RNFL thickness and clinical characteristics revealed that the IOP was proportional to HDD. Moreover, a reduction in RNFL thickness was proportional to HDD but this effect was not achieved by an increase in IOP.
Thinning of the RNFL of CKD patients receiving hemodialysis has been demonstrated. Wu et al. found that RNFL thinning was related to the severity of CKD, and patients with CKD experienced faster RNFL loss than healthy controls20,21. Chow et al. reported that patients with non-diabetic CKD had a thinned RNFL thickness in all other quadrants, except for the temporal quadrant, and such changes were associated with the severity of CKD22. Demir et al. claimed that the non-diabetic patients with conventional hemodialysis showed statistically significantly reduced average and all quadrant RNFL thicknesses23. Atilgan et al. demonstrated that inferior, temporal, and average RNFL were thinned in similar groups24. The current study found temporal and inferior RNFL thinning in hemodialysis patients; however, no significant difference was found in the average RNFL thickness, which might be related to differences in race, dialysis method, and inclusive criteria for subjects. Glaucoma studies consistently report thinning of the RNFL, and this vulnerability is particularly evident in the inferior and inferotemporal regions. Animal studies also further support these observations25,26,27,28. Among them, normal tension glaucoma (NTG) exhibits more localized inferior or inferotemporal RNFL defects compared to primary open angle glaucoma29,30. Although the reason underlying the increased vulnerability of the inferior RNFL is not known, some researchers propose a 'crowding hypothesis'. This hypothesis suggests that the inferior RNFL has a higher density of axons. While a thicker RNFL might be easier to detect changes in, this crowded organization of axons could also create a more fragile structural foundation, making them more susceptible to damage in glaucoma31. In addition to the influence of mechanical stress on the optic nerve, the pathogenesis of glaucoma (especially NTG) is also related to abnormal ocular hemodynamics32. This suggests that the mechanism driving the changes of RNFL thickness among hemodialysis patients may be similar to that of non-mechanical injury in glaucoma. Meanwhile, this finding suggests that ophthalmologists should be more cautious when screening or diagnosing glaucoma in dialysis patients to avoid underdiagnosis or misdiagnosis.
Anemia and cardiovascular disease (CVD) are common complications in CKD patients, especially in hemodialysis patients33,34. Studies have shown that patients with anemia exhibit a decrease in RNFL thickness. Furthermore, the data suggests a parallel trend, where the severity of anemia (as measured by Hb level) correlates with the degree of RNFL thinning35. Chen et al. demonstrated that RNFL thinning was independently associated with cardiovascular risk36. To exclude the effect of anemia and CVD, we measured the Hb concentration and the prevalence of CVD (including coronary heart disease, cerebrovascular disease, peripheral artery disease, and aortic atherosclerosis) in the patients. In addition, to investigate whether the adequacy of hemodialysis affected RNFL thickness, we collected Kt/V values of hemodialysis patients. The results of the correlation analysis showed that none of the above variables were correlated with RNFL thickness, and thus were not included in the subsequent mediation analysis.
According to the results of the mediation analysis, inferior RNFL thickness was significantly higher in females. Higher RNFL values in females have been reported in some population-based and clinical studies37,38,39,40. Dian Li et al. proposed that this may be attributed to the estrogen-mediated protective effect on the RNFL41. However, other studies showed no difference between the genders42,43. Further investigation is required to elucidate the relationship between gender and RNFL thickness. Nevertheless, it is imperative to consider gender as a variable in the measurement and analysis of RNFL thickness.
Correlation analysis of clinical characteristics and RNFL thickness revealed a significant pairwise correlation among HDD, IOP, and inferior RNFL thickness in MHD patients. Hemodialysis changes blood osmotic pressure and blood volume, which may alter the aqueous humor circulation. Although several studies have assessed the impact of hemodialysis on IOP, whether patients may experience an increase, decrease, or no change in IOP before and after a single hemodialysis remains controversial44,45,46,47. Besides, the above studies only focused on the changes in IOP before and after a single hemodialysis and did not investigate the long-term impact of hemodialysis on IOP. To the best of our knowledge, this is the first study to demonstrate that IOP increased with HDD, indicating that hemodialysis had a chronic effect on IOP. Further research is needed to elucidate whether an increase in IOP is associated with hemodynamic and osmotic pressure changes of long-term hemodialysis. Previous studies also demonstrated that RNFL thinning was proportional to the IOP48,49. Nevertheless, our data showed that IOP was positively correlated with inferior RNFL thickness in hemodialysis patients. Hence, elucidating the specific relationship among HDD, IOP, and inferior RNFL thickness is crucial to investigate whether RNFL thinning in hemodialysis patients is solely caused by optic nerve lesions in CKD or hemodialysis, or whether changes in IOP also have a partial impact.
The mediation analysis was used to analyze the relationship among HDD, IOP, and inferior RNFL thickness. The results demonstrated that the negative impact of the HDD on inferior RNFL thickness was independent, suggesting that RNFL thinning in hemodialysis patients was induced by neurologic disorders instead of intraocular factors. While the IOP was proportional to the HDD, an increase in IOP did not exacerbate inferior RNFL thinning.
Occurrence of retinal nerve damage in patients with CKD may be driven by several mechanisms. CKD patients often exhibit systemic diseases such as hypertension and diabetes, which are themselves associated with neurodegeneration50,51,52. Our analysis excluded patients with diabetes and uncontrolled hypertension, minimizing the potential influence of these systemic diseases on the results. However, CKD patients are often accompanied by chronic inflammation, oxidative stress, elevated uremic toxins, and disruptions in the Renin-Angiotensin system. These factors may directly or indirectly contribute to nerve damage, including potential effects on the RNFL thickness we investigated. Therefore, the impact of CKD on our findings cannot be entirely ruled out and warrants further consideration in future studies53,54. In addition, hemodialysis patients experience blood pressure variability both during and between hemodialysis treatments, which could result in peripheral dysfunction and arterial stiffness, leading to ocular microcirculatory disruption and further exacerbating retinal neurodegeneration55,56.
Although the emergence and development of hemodialysis has greatly prolonged the survival of ESRD patients, the nervous system impairment caused by uremia remains a serious concern57. Hemodialysis has changed the neurological disease spectrum of ESRD. Some patients still develop chronic uremic neuropathy, with elusive root causes, which may be related to a series of middle molecule toxins that cannot be cleared by standard dialysis58. Our results demonstrated that the RNFL thickness was thinner in hemodialysis patients than in normal individuals, and a reduction in RNFL thickness was proportional to HDD. RNFL is a combination of unmyelinated axons of retinal ganglion cells, serving as an extension of the optic nerve and partly reflecting changes in the CNS. Chow et al. reported that RNFL thinning was related to the severity of CKD (i.e., the stage of CKD) instead of the duration of CKD22. Therefore, our study suggested the following two possibilities: (1) hemodialysis was both beneficial and disadvantageous to the nervous system of ESRD patients but the demerits outweighed the benefits. The involvement of the nervous system gradually intensified with an increase in the HDD. (2) Although hemodialysis may partially replace renal function, neurologic disorders in ESRD still developed and hemodialysis could not prevent or alleviate neurologic disorders after CKD progressed to the ESRD stage.
This study presents two inspiring findings. (1) The impact of hemodialysis on the nervous system is complex, and neuropathy caused by hemodialysis deserves further attention, which may be monitored through RNFL thickness, providing a non-invasive and convenient monitoring method for neuropathic changes in hemodialysis patients. (2) The RNFL thickness is thinner in hemodialysis patients than in normal individuals, and the relationship between RNFL thickness and IOP is different from that of patients with simple glaucoma. Moreover, considerable attention should be paid to analyzing the reasons for RNFL thinning in diagnosis and follow-up of hemodialysis patients undergoing glaucoma evaluation to avoid misdiagnosis.
Nevertheless, this study has several limitations. Firstly, hemodialysis patients are generally complicated with other systemic diseases. We excluded diabetes and uncontrolled hypertension, which are two factors that undoubtedly affect the experimental results. Besides, although both age and gender were controlled during data analysis, it is barely possible to control all potential factors affecting RNFL. Our study involved hemodialysis patients with varying HDD. Ideally, residual kidney function should be assessed using 24-h urine collection in such patients. However, this method was not feasible in the present study due to the recruitment setting (outpatient clinics) and potential limitations with patient compliance and urine collection standardization. Consequently, residual kidney function remains a potential confounding factor that we were unable to exclude. In future studies, we will place a stronger emphasis on evaluating residual kidney function to minimize the influence of confounding variables and strengthen the validity of our results. Secondly, this is a cross-sectional study, and it can barely clarify the causal relationship between hemodialysis and RNFL thickness. Thus, further large-scale longitudinal studies are still warranted.
In summary, temporal and inferior RNFL thicknesses were lower in hemodialysis patients than in the control group, and a reduction in inferior RNFL thickness was proportional to the HDD. However, such changes were not induced by an increase in IOP.
Methods
This observational cross-sectional study was conducted in the Ophthalmology and Nephrology Department of the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China. The study was conducted according to the tenets of the Declaration of Helsinki and the principles of good clinical practice guidelines. All participants signed informed consent before participating in this study. This study was approved by the Ethics Committee of the People’s Hospital of Guangxi Zhuang Autonomous Region (KY-IIT-2023–31) and registered in the Chinese Clinical Trial Registry (ChiCTR2300075469).
The subjects included ESRD patients receiving MHD and healthy controls. The exclusive criteria included (1) eye diseases that affected the examination results, such as diabetes, uncontrolled hypertension (Blood pressure ≥ 140/90 mmHg regardless of medication use or treatment), glaucoma, macular degeneration, optic disc edema, or atrophy; (2) a history of intraocular surgery; best corrected visual acuity (BCVA) below 20/80; (3) a high refractive error (± 5.0 diopters spherical or ± 2 diopters cylinder); (4) unable to cooperate in ophthalmic examinations; (5) under 18 years old. The demographic data were collected from all subjects, and the latest (no more than one month) serum creatinine results were selected to obtain the Hb and eGFR (based on the Cockcroft-Gault formula59). To investigate the prevalence of CVD, we collected subjects' medical histories, specifically inquiring about coronary heart disease, cerebrovascular disease, peripheral artery disease, and aortic atherosclerosis. Patients in the hemodialysis group received the MHD treatment thrice a week, for 3–4 h each time, and the last Kt/V values were collected. Kt/V was calculated by the second generation Daugirdas formula60: Kt/V = − ln(R − 0.008 × t) + (4 − 3.5 × R) × UFV/W [ln, natural logarithm; R, post-dialysis/pre-dialysis blood urea nitrogen; t, effective dialysis time in hours; UFV, ultrafiltration volume in liters; W, post-dialysis body weight in kg]. The ophthalmic examinations were conducted on a non-hemodialysis day to avoid any parameter changes owing to hemodialysis effects. Ophthalmic examinations were conducted by an experienced inspector in a dark room, without mydriasis, including BCVA, intraocular pressure (IOP), slit lamp examination, fundus examination, and OCT. BCVA was measured using Snellen’s test chart and converted into the logarithm of the minimum angle of resolution (LogMAR) data format. IOP was measured three times for each eye using the slit-lamp mounted Goldmann applanation tonometer and the average value was recorded. Annular RNFL thicknesses (with the optic disc as the core, diameter = 3.45 mm) were measured using the optic nerve head scanning of spectral domain optical coherence tomography (SD-OCT, RTVue-XR2 Avanti, Optovue Inc. Fremont, CA, USA) and the average RNFL thickness and mean RNFL thickness in each quadrant were recorded. The four quadrants were defined as temporal (316°–45°), superior (46°–135°), nasal (136°–225°), and inferior (226°–315°) (Fig. 3). Eyes with an average signal strength index (SSI) of < 48 were excluded.
Optical coherence tomography images of RNFL thickness measurement. (a) RNFL thickness was measured on a circle (black circle) with a diameter of 3.45 mm centered on the optic disc. (b) RNFL thickness was measured on the b-scan image in the black circle in (a) and compared with the standard database. The ranges of normal, borderline, and outside normal values are represented in green, yellow, and red colors, respectively. (c) Quadrant division and corresponding RNFL thickness (the average RNFL thickness was 102 μm). T temporal, S superior, N nasal, I inferior, RNFL retinal nerve fiber layer.
Descriptive statistics, correlation analysis, and regression analysis were conducted using SPSS 23.0. Perform normality test using Shapiro Wilk test (S-W test). For measurement data that conforms to a normal distribution and has homogeneity of variance, independent sample t-test is chosen for comparison between the two groups. For measurement data with non-normal distribution or uneven variance, Mann Whitney U test is chosen for comparison between the two groups. Choose chi square test for comparison between categorical data, such as comparing gender distribution. Partial correlation analysis (control variables: gender and age) was selected for the correlation analysis between variables. Normal distribution measurement data is represented by mean ± standard deviation, while non-normal distribution measurement data is represented by median (interquartile range). A multi-step regression analysis method17 was performed for the mediation analysis, and the results were verified using the resampling-based bootstrapping approach via the PROCESS plugin in SPSS. p < 0.05 was considered statistically significant.
Ethics approval
This study was approved by the Ethics Committee of the People’s Hospital of Guangxi Zhuang Autonomous Region (KY-IIT-2023-31) and registered in the Chinese Clinical Trial Registry (ChiCTR2300075469).
Data availability
The data that support the findings of this study are not openly available due to reasons of study participant privacy and are available from the corresponding author upon reasonable request.
Abbreviations
- BCVA:
-
Best corrected visual acuity
- CKD:
-
Chronic kidney disease
- CNS:
-
Central nervous system
- CVD:
-
Cardiovascular disease
- eGFR:
-
Estimated glomerular filtration rate
- ESRD:
-
End-stage renal disease
- Hb:
-
Hemoglobin
- HDD:
-
Hemodialysis duration
- IOP:
-
Intraocular pressure
- Kt/V:
-
A product of dialyzer urea clearance (K) and treatment time (t) divided by urea distribution volume (V)
- LogMAR:
-
Logarithm of the minimum angle of resolution
- MHD:
-
Maintenance hemodialysis
- NTG:
-
Normal tension glaucoma
- OCT:
-
Optical coherence tomography
- RNFL:
-
Retinal nerve fiber layer
- SD-OCT:
-
Spectral domain optical coherence tomography
- SSI:
-
Signal strength index
References
Bikbov, B. et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet 395, 709–733 (2020).
Rosner, M. H., Husain-Syed, F., Reis, T., Ronco, C. & Vanholder, R. Uremic encephalopathy. Kidney Int. 101, 227–241 (2022).
Masson, P. et al. Chronic kidney disease and the risk of stroke: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 30, 1162–1169 (2015).
Xie, Z., Tong, S., Chu, X., Feng, T. & Geng, M. Chronic kidney disease and cognitive impairment: The kidney-brain axis. Kidney Dis. (Basel) 8, 275–285 (2022).
Hamed, S. A. Neurologic conditions and disorders of uremic syndrome of chronic kidney disease: Presentations, causes, and treatment strategies. Expert Rev. Clin. Pharmacol. 12, 61–90 (2019).
Salman, I. M. Cardiovascular autonomic dysfunction in chronic kidney disease: A comprehensive review. Curr. Hypertens. Rep. 17, 59 (2015).
Karunaratne, K., Taube, D., Khalil, N., Perry, R. & Malhotra, P. A. Neurological complications of renal dialysis and transplantation. Pract. Neurol. 18, 115–125 (2018).
Bansal, V. K. & Bansal, S. Nervous system disorders in dialysis patients. Handb. Clin. Neurol. 119, 395–404 (2014).
Prasad, S. & Galetta, S. L. Anatomy and physiology of the afferent visual system. In Handbook of Clinical Neurology vol. 102 3–19 (Elsevier, 2011).
London, A., Benhar, I. & Schwartz, M. The retina as a window to the brain—from eye research to CNS disorders. Nat. Rev. Neurol. 9, 44–53 (2013).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
Xie, J. S., Donaldson, L. & Margolin, E. The use of optical coherence tomography in neurology: A review. Brain 145, 4160–4177 (2022).
Rohani, M., Meysamie, A., Zamani, B., Sowlat, M. M. & Akhoundi, F. H. Reduced retinal nerve fiber layer (RNFL) thickness in ALS patients: A window to disease progression. J. Neurol. 265, 1557–1562 (2018).
Petzold, A. et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 16, 797–812 (2017).
Chrysou, A., Jansonius, N. M. & Van Laar, T. Retinal layers in Parkinson’s disease: A meta-analysis of spectral-domain optical coherence tomography studies. Parkinson. Relat. Disord. 64, 40–49 (2019).
Wang, M., Zhu, Y., Shi, Z., Li, C. & Shen, Y. Meta-analysis of the relationship of peripheral retinal nerve fiber layer thickness to Alzheimer’s disease and mild cognitive impairment. Shanghai Arch. Psychiatry 27, 263–279 (2015).
Baron, R. M. & Kenny, D. A. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182 (1986).
Ho, H. et al. Retinal nerve fiber layer thickness in a multiethnic normal asian population. Ophthalmology 126, 702–711 (2019).
Majithia, S. et al. Retinal nerve fiber layer thickness and rim area profiles in Asians. Ophthalmology 129, 552–561 (2022).
Wu, I. et al. Retinal neurovascular changes in chronic kidney disease. Acta Ophthalmol. 98, (2020).
Yeung, L. et al. Accelerated peripapillary retinal nerve fiber layer degeneration in patients with chronic kidney disease: A 2-year longitudinal study. Trans. Vis. Sci. Tech. 11, 10 (2022).
Chow, J. Y., She, P. F., Pee, X. K., Wan Muda, W. N. & Catherine Bastion, M.-L. Comparison of peripapillary retinal nerve fiber layer and macular thickness in non-diabetic chronic kidney disease and controls. PLoS ONE 17, e0266607 (2022).
Demir, M. N. et al. Retinal nerve fiber layer thickness in chronic renal failure without diabetes mellitus. Eur. J. Ophthalmol. 19, 1034–1038 (2009).
Atilgan, C. U. et al. Effects of hemodialysis on macular and retinal nerve fiber layer thicknesses in non-diabetic patients with end stage renal failure. SMJ 37, 641–647 (2016).
Hood, D. C. et al. Early glaucoma involves both deep local, and shallow widespread, retinal nerve fiber damage of the macular region. Invest. Ophthalmol. Vis. Sci. 55, 632 (2014).
Hood, D. C., Raza, A. S., De Moraes, C. G. V., Liebmann, J. M. & Ritch, R. Glaucomatous damage of the macula. Prog. Retinal Eye Res. 32, 1–21 (2013).
Leung, C.K.-S. et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography. Ophthalmology 119, 1858–1866 (2012).
Tu, S. et al. Relationship between intraocular pressure and retinal nerve fibre thickness loss in a monkey model of chronic ocular hypertension. Eye 33, 1833–1841 (2019).
Yamazaki, Y., Koide, C., Miyazawa, T., Kuwagaki, N. & Yamada, H. Comparison of retinal nerve-fiber layer in high- and normal-tension glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 229, 517–520 (1991).
Kubota, T. et al. Comparative study of retinal nerve fiber layer damage in Japanese patients with normal- and high-tension glaucoma. J. Glaucoma 8, 363–366 (1999).
Hood, D. C. et al. The nature of macular damage in glaucoma as revealed by averaging optical coherence tomography data. Trans. Vis. Sci. Tech. 1, 3 (2012).
Wu, X. et al. Role of ocular blood flow in normal tension glaucoma. Adv. Ophthalmol. Pract. Res. 2, 100036 (2022).
Mikhail, A. et al. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 18, 345 (2017).
Ahmadmehrabi, S. & Tang, W. H. W. Hemodialysis-induced cardiovascular disease. Semin. Dial. 31, 258–267 (2018).
Datta, S., Baidya, K., Banerjee, M., Mahapatra, S. & Mukherjee, S. Retinal nerve fibre layer thinning in patients with thalassaemia, iron deficiency anaemia, and anaemia of chronic diseases. J. Ophthalmol. 2020, 1–7 (2020).
Chen, Y. et al. Retinal nerve fiber layer thinning as a novel fingerprint for cardiovascular events: Results from the prospective cohorts in UK and China. BMC Med. 21, 24 (2023).
Wang, Y. X. et al. Retinal nerve fiber layer thickness. The Beijing Eye Study 2011. PLoS One 8, e66763 (2013).
Zhao, L., Wang, Y., Chen, C. X., Xu, L. & Jonas, J. B. Retinal nerve fibre layer thickness measured by Spectralis spectral-domain optical coherence tomography: The Beijing Eye Study. Acta Ophthalmol. 92, 1 (2014).
Wu, J. et al. Retinal nerve fibre layer thickness measured with SD-OCT in a population-based study: The Handan Eye Study. Br. J. Ophthalmol. 107, 1156–1164 (2023).
Li, C. et al. Variation in retinal nerve fiber layer and ganglion cell complex associated with optic nerve head size in healthy eyes. Trans. Vis. Sci. Tech. 12, 26 (2023).
Li, D. et al. Sex-specific differences in circumpapillary retinal nerve fiber layer thickness. Ophthalmology 127, 357–368 (2020).
Alasil, T. et al. Analysis of normal retinal nerve fiber layer thickness by age sex, and race using spectral domain optical coherence tomography. J. Glaucoma 22, 532–541 (2013).
Zha, Y. et al. Evaluation of myopia on retinal nerve fiber layer thickness measured by Spectralis optical coherence tomography. Exp. Ther. Med. 14, 2716–2720 (2017).
Caglayan, M. et al. Effects of hemodialysis on corneal and anterior chamber morphometry and intraocular pressure in patients with end-stage renal disease. Arquivos Brasileiros de Oftalmologia 80, (2017).
Bahadir Kilavuzoglu, A. E. et al. The effect of hemodialysis on intraocular pressure. Adv. Clin. Exp. Med. 27, 105–110 (2018).
Yang, S. J., Han, Y. H., Song, G. I., Lee, C. H. & Sohn, S. W. Changes of choroidal thickness, intraocular pressure and other optical coherence tomographic parameters after haemodialysis. Clin. Exp. Optometry 96, 494–499 (2013).
Chen, S.-H. et al. Changes in intraocular pressure during hemodialysis: A meta-analysis. J. Glaucoma 30, 866–873 (2021).
Jammal, A. A. et al. Impact of intraocular pressure control on rates of retinal nerve fiber layer loss in a large clinical population. Ophthalmology 128, 48–57 (2021).
Diniz-Filho, A. et al. Association between intraocular pressure and rates of retinal nerve fiber layer loss measured by optical coherence tomography. Ophthalmology 123, 2058–2065 (2016).
Sahin, O. Z. et al. The impact of hypertension on retinal nerve fiber layer thickness and its association with carotid intima media thickness. Blood Pressure 24, 178–184 (2015).
Lee, M.-W. et al. Effect of systemic hypertension on peripapillary RNFL thickness in patients with diabetes without diabetic retinopathy. Diabetes 70, 2663–2667 (2021).
Lee, M.-W., Lee, J.-W., Lee, K.-H., Lee, Y.-H. & Kim, J.-Y. Peripapillary RNFL/vessel density ratio in patients with type2 diabetes without clinical diabetic retinopathy. Sci. Rep. 12, 9463 (2022).
Wong, C. W., Wong, T. Y., Cheng, C.-Y. & Sabanayagam, C. Kidney and eye diseases: Common risk factors, etiological mechanisms, and pathways. Kidney Int. 85, 1290–1302 (2014).
Arnold, R., Issar, T., Krishnan, A. V. & Pussell, B. A. Neurological complications in chronic kidney disease. JRSM Cardiovasc. Dis. 5, 2048004016677687 (2016).
Dubin, R., Owens, C., Gasper, W., Ganz, P. & Johansen, K. Associations of endothelial dysfunction and arterial stiffness with intradialytic hypotension and hypertension: Vascular dysfunction and hemodynamic instability. Hemodial. Int. 15, 350–358 (2011).
Roskal-Wałek, J. et al. The haemodialysis session effect on the choroidal thickness and retinal and choroidal microcirculation—a literature review. JCM 12, 7729 (2023).
Rizzo, M. A. et al. Neurological complications of hemodialysis: state of the art. JN 25, 170–182 (2012).
Babb, A. L., Ahmad, S., Bergström, J. & Scribner, B. H. The middle molecule hypothesis in perspective. Am. J. Kidney Dis. 1, 46–50 (1981).
Cockcroft, D. W. & Gault, H. Prediction of creatinine clearance from serum creatinine. Nephron 16, 31–41 (1976).
Daugirdas, J. T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 4, 1205–1213 (1993).
Acknowledgements
The authors would thank all the reviewers and editors who participated in the review, and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript. The author “Yiyi Hong” was jointly trained by the Postdoctoral Programme of Wuhan University and the Postdoctoral Programme of The People’s Hospital of Guangxi Zhuang Autonomous Region.
Funding
This work was supported by the Guangxi clinical ophthalmic research center under Grant [No. Guike AD19245193].
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.H. and F.X.; data curation, Y.H. and L.L.; formal analysis, Y.H.; funding acquisition, M.L.; investigation, L.L., X.H. and Y.Z.; methodology, K.Y., C.C. and M.L.; Project administration, K.Y. and F.X.; resources, M.D. and K.Y.; supervision, C.C. and M.L.; validation, Y.H. and M.D.; visualization, Y.H. and L.L.; writing—original draft, Y.H.; writing—review and editing, F.X. and C.C. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hong, Y., Lan, L., Hu, X. et al. A cross-sectional study on the impact of hemodialysis duration on retinal nerve fiber layer thinning in hemodialysis patients. Sci Rep 14, 17824 (2024). https://doi.org/10.1038/s41598-024-68589-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-68589-z