Abstract
The normal operation of the Three Gorges Reservoir, which involves periodic water storage and discharge, has led to strong disturbances in environmental conditions that alter soil microbial habitats in the riparian zones. Riparian zones are an important part of controlling pollution in the Three Gorges Reservoir area, since they act as a final ecological barrier that intercepts pollutants. Meanwhile, monitoring the health of microbial communities in the riparian zone is crucial for maintaining the ecological security of the reservoir area. We specifically investigate the Daning River, which are tributaries of the Three Gorges Reservoir and have typical riparian zones. Soil samples from these areas were subjected to high-throughput sequencing of 16S rRNA genes and 18S rRNA genes, in order to obtain the characteristics of the present microbial communities under strong disturbances in the riparian zones. We studied the characteristics and distribution patterns of microbial communities and their relationship with soil physicochemical properties. The study results indicate that microbial communities exhibit high diversity and evenness, and spatial heterogeneity is present. The ASV dataset contains many sequences not assigned to known genera, suggesting the presence of new fungal genera in the riparian zone. Redundancy analysis (RDA) revealed that pH and \({NH}_{4}^{+}\)-N were the primary environmental factors driving bacterial community variation in the riparian zone, while pH, total carbon (TC) content, and \({NO}_{3}^{-}\)-N were identified as the main drivers of soil archaeal community variation.
Similar content being viewed by others
Introduction
A riparian zone refers to land that is periodically exposed or submerged due to seasonal fluctuations of water levels in rivers, lakes, and reservoirs. These zones belong to the broad category of wetlands. Changing water levels in riparian zones may occur due to natural causes, such as seasonal water level fluctuations, or due to human activities, such as periodic water storage and release. As a transitional and connecting area between reservoirs and terrestrial ecosystems, the riparian zone acts as a vast ecological buffer zone around the reservoir, forming a unique aquatic-terrestrial wetland ecosystem1. The Three Gorges Reservoir adopts a regulation method of "winter storage and summer discharge." Starting from October each year, water is stored and gradually rises from 145 to 175 m. This rise generally takes about 60 days, during which the riparian zones and vegetation are gradually submerged under water. After the period of operation when the water level is 170–175 m (which lasts approximately 60 days) the water begins to recede until April of the following year, decreasing to 145 m. The recession process typically takes about 200 days, during which the riparian zones and vegetation gradually resurface. Subsequently, there is a period of operation at a lower water level of 145–150 m, lasting approximately 90 days2. Over 90% of the Daning Riparian Zone is covered by soil, with a soil layer thickness of about 1–5 m, and only a few areas have exposed rocks.The soil types are mainly yellow–brown soil and purple soil. Purple soil consists of weathered purple sandstone, siltstone, and mudstone, and was only subjected to weak chemical weathering. It has a high gravel content, weakly alkaline pH, abundant mineral elements (such as phosphorus and nitrogen), and strong microbial activity. Yellow–brown soil is formed from residual materials of weathered limestone, dolomite, sandstone, and shale. It has high moisture content, high organic matter content, and moderate levels of nitrogen and potassium.
The riparian zone, as a hybrid ecosystem, possesses characteristics of both terrestrial and aquatic habitats. Significant fluctuations in water levels, caused by periodic water storage and release, profoundly alter the microenvironment within the watershed. By regulating hydrological rhythms, these fluctuations significantly modify the physicochemical environment of the soil, thereby affecting vegetation biomass and diversity3, it also profoundly affects the structure of microbial communities. The alternation between wet and dry soil, as caused by hydrological fluctuations, can affect the composition and activity of soil microorganisms4. Research has shown that the process of soil drying and rewetting from hydrological fluctuations in riparian zones of the Three Gorges Reservoir may alter soil environmental factors, affecting the dynamics of bacterial communities5. Archaea are a diverse and abundant microbial group in soils, participating in various biogeochemical cycles6. For example, ammonia-oxidizing archaea drive the process of ammonia oxidation7, while methane-producing archaea drive methane production8. Archaeal communities in soil are influenced by various environmental factors, such as elevation, pH, salinity, and redox status9. Soil moisture also strongly affects soil archaeal communities10. Therefore, water level fluctuations may significantly affect the regulation of archaea communities in riparian zones and the associated nutrient cycles11.
Research in the riparian zone primarily focuses on soil physicochemical properties and plant community succession1. There is a lack of research on soil microbiology in the riparian zone, and even fewer studies on eukaryotic organisms in the riparian zone. Eukaryotic organisms typically have lower abundance compared to prokaryotes, leading to the common misconception that eukaryotes have no significant ecological role12. The response characteristics of soil microbial communities to highly disturbed environments and the long-term effects on the distribution and function of soil archaea communities in the riparian zone of the Three Gorges Reservoir have not been thoroughly investigated. Therefore, we focused on the most representative riparian zone in the Three Gorges Reservoir—the riparian zone of the Daning River, and used Illumina technology (NGS) to investigate the diversity of soil microbial communities, the characteristics of bacterial and eukaryotic microbial communities, and their spatiotemporal heterogeneity under highly disturbed environments, as well as their correlations with soil physicochemical properties, exploring the causes of the spatiotemporal heterogeneity of microbial community distribution and their potential ecological functions with the goal of providing technical references for formulating ecosystem protection policies in the Three Gorges Reservoir area.
Materials and methods
Study area and sampling
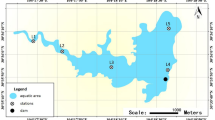
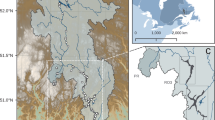
The present study was performed at the Daning River tributary of the Three Gorges Reservoir area in the municipality of Chongqing (Fig. 1). Six sampling points were selected, each with three elevations: 155 m, 165 m, and 175 m (Fig. 2). Three samples of soils were collected from each site at a depth of 0 ~ 20 cm on 8th and 9th July, 2023, and then subjected for high-throughput sequencing analysis. Larger root system and other debris were carefully removed from the samples.
A total of 54 soil samples were collected from the riparian zones of the Daning in the Three Gorges Reservoir area. Each sample had a mass of 20 g and was used for analysis of soil microbial community structure and physicochemical factors. The collected samples were stored at -80℃ conditions for 16S rRNA extraction. Microbial abundance, community composition, and diversity were investigated in response to different elevations and environmental factors.
Measurement of environmental factors
The retrieved soil samples were placed in a ventilated area for drying in natural air. After drying, the samples were ground and sieved through soil sieves of 2 mm, 1 mm, and 0.25 mm mesh sizes, for the determination of soil physicochemical properties. The pH was determined using the acidometer method with a soil-to-water ratio of 1:2.5 for water extraction. Organic matter (OM) content was determined using the potassium dichromate external heating method. Total nitrogen (TN) content was measured with an elemental analyzer (Elementar Vario EL, Germany). Total phosphorus (TP) and available phosphorus (AP) contents were determined utilizing the molybdenum antimony anti-colorimetric method (UV-2550, Shimadzu., CHN). Total potassium (TK) and available potassium (AK) contents in the soil were measured using inductively-coupled plasma optical emission spectrometry (ICP-OES, Thermo Fisher iCAP 6300, UK).
DNA extraction and high-throughput sequencing
DNA was extracted using a DNA extraction kit for each corresponding sample. The concentration and purity were measured using the NanoDrop One (Thermo Fisher Scientific, MA, USA). 16S rRNA/18SrRNA genes of distinct regions (e.g. Bac 16S: V3-V4/V4/V4-V5; Fug 18S: V4/V5) were amplified using specific primers (e.g. 16S: 338F and 806R/515F and 806R/515F and 907R; 18S: 528F and 706R/817F and 1196R with 12 bp barcode). These primers were synthesized by Invitrogen (Invitrogen, Carlsbad, CA, USA). PCR reactions, containing 25 μl 2 × Premix Taq (Takara Biotechnology, Dalian Co. Ltd., China), 1 μl for each primer (10 μM), and 3 μl DNA (20 ng/μl) template in a volume of 50 µl were amplified by thermocycling: 5 min at 94 °C for initialization; 30 cycles of 30 s denaturation at 94 °C, 30 s of annealing at 52 °C, and 30 s of extension at 72 °C; followed by 10 min of final elongation at 72 °C. The PCR instrument was a BioRad S1000 (Bio-Rad Laboratory, CA, USA). The PCR products were detected by electrophoresis using a 2% agarose gel, and purified by electrophoresis using a 1 × TAE agarose gel at a concentration of 2%, after thorough mixing using a E.Z.N.A. Gel Extraction Kit (Omega, USA). Sequencing libraries were generated using the NEBNext® Ultra™ II DNA Library Prep Kit for Illumina® (New England Biolabs, MA, USA) following the manufacturer's recommendations and adding index codes. The library quality was assessed using the Qubit@ 2.0 Fluorometer (Thermo Fisher Scientific, MA, USA). Lastly, the library was sequenced on an Illumina Nova6000 platform, and 250 bp paired-end reads were generated (Guangdong Magigene Biotechnology Co., Ltd., Guangzhou, China).
Results
Soil physicochemical properties at various elevations
Measurements were made of pH, organic matter (OM), total potassium (TK), total phosphorus (TP), total carbon (TC), total organic carbon (TOC), total nitrogen (TN), nitrate nitrogen (NO3--N), ammonium nitrogen (NH4+-N), and available phosphorus (AP) at three different elevations (Table. S1). The preliminary findings indicated no significant differences (P > 0.05) between soil samples from the Daning River tributary across the aforementioned indicators. However, when divided according to different elevations, some physicochemical indicators exhibited differences at altitudes of 155 m, 165 m, and 175 m, as indicated in Fig. 3. Soil pH ranged from 7.2 to 7.9, with an average of 7.58, showing weak alkalinity overall. Significantly higher pH levels were observed at 175 m, as compared to 155 m and 165 m (P < 0.05), with the pH peaking at 7.9 at 175 m. Conversely, TC showed significant differences depending on the elevation, as the total carbon content decreased with increasing altitude. The highest TC content was observed at 155 m, reaching 39.0 g/kg. Additionally, TK content at 175 m (24.49 g/kg) was significantly higher than at 165 m (18.68 g/kg).
Regarding physicochemical properties and flooding duration, it can be found that with increasing altitude, flooding time, water content, and clay content decrease, while conductivity increases with altitude (Table S2).Throughout the year, the flooding duration at 155 m is 249 days, while at 175 m it is only 1 day.The clay content at 155 m is 36.5% higher than at 175 m. The conductivity at 175 m is twice that at 155 m.
Microbial community diversity and phylotypes
After quality filtering, deletion of chimeras, and sequence assembling, a total of 3,174,738 high quality sequences (n = 36), with an average number of 88,187 per sample (max = 92,346, min = 84,231, SD = 2,448), were retrieved by Illumina MiSeq sequencing of the bacterial 16S rRNA genes. The overall community Chao index was 2465.09 ± 303.18 (Fig. 4A), the Simpson index was 0.010 ± 0.013 (Fig. 4B), the Shannon index was 9.754 ± 0.546 (Fig. 4C), and the Pielou index was 0.886 ± 0.039 (Fig. 4D), with a significant variation between 155 m and the 165 m, 175 m sites. Although slight deviations were observed in the 165 m and 175 m sites, there were no significant differences in their parameters.
Characteristics of bacteria communities
A total of 53 microbial phyla, 120 classes, 251 orders, 276 families, and 1602 genera were detected in the ASVs dataset. Bacteria were much more abundant than archaea in the kingdom classification, as the relative abundance of bacteria was 90.39% to 86.20%, while for archaea it was 9.61% to 13.80%. Furthermore, the community structure was similar at different sites with no significant difference (P > 0.05) (Fig. 5A). The community included five dominant phyla (relative abundance > 5%) of Proteobacteria (20.15% ~ 31.89%), Acidobacteriota (16.50% ~ 24.08%), Chloroflexi (6.90% ~ 12.44%), Thaumarchaeota (9.55% ~ 13.58%), and Planctomycetes (6.00% ~ 8.34%).
With an increase in altitude, the relative abundance of Proteobacteria and Chloroflexi gradually decreases, whereas the relative abundance of Acidobacteria, Thaumarchaeota, and Planctomycetes gradually increases (Fig. 5B).
However with increasing altitude, there is also a general upward trend in the proportion of archaea (Fig. 5A). The classes that dominate within the archaeal community are Nitrososphaeria, Methanomicrobia, and Bathyarchaeia. Moreover, as altitude increases, the relative abundance of Nitrososphaeria becomes higher, accounting for 69.78%, 85.74%, and 97.62% at the three sampling points, respectively. Meanwhile, it is also clear that the relative abundance of Methanomicrobia and Bathyarchaeia decreases significantly with increasing altitude (Fig. 5C).
Characteristics of eukaryotic microbial communities
A total of 46 microbial phyla, 62 classes, 251 orders, 57 families, and 202 genera were detected in the ASV dataset. It can be observed that the dominant populations at the phylum level are Phragmoplastophyta (25.53% to 46.02%), Ascomycota (20.58% ~ 43.44%), and Cercozoa (6.60% ~ 11.29%). The relative abundance in each sample was greater than 5%. We found that the proportion of Phragmoplastophyta in the community decreases with increasing altitude, whereas the proportion of Ascomycota increases with increasing altitude.
Furthermore, significant differences were found in the relative abundance of fungal communities between the altitude of 175 m and 155 m or 165 m (0.01 < P < 0.05), and the proportion of fungi in the environment gradually increased with altitude. The predominant fungal genus in the samples was Chaetomium, accounting for 20.29% to 49.60% of the total, with its abundance initially decreasing and then increasing with elevation (Fig. 6A). At the genus level, a considerable proportion of sequences remained unassigned to known genera (44.34% at 155 m, 72.72% at 165 m, and 73.60% at 175 m), potentially indicating the presence new fungal genera at the sampling sites (Fig. 6B).
Relationships between bacteria communities and environmental factors
A redundancy analysis (RDA) ordination plot (Fig. 7) was made to discern possible linkages between the environmental factors (pH, TC, TK, TOC, OM, AP, TP, TN, NO3--N, NH4+-N) and bacterial (Fig. 7A) and archaeal (Fig. 7B) community structures. This was done after detrended correspondence analysis with axis lengths of 0.80, 0.72, 0.96, and 0.50 for bacterial community structure and 1.12, 0.39, 0.55, and 0.56 for archaeal community structure. Subsequently, the influence of riparian biochemical characteristics on microbial communities was evaluated through RDA (Fig. 7). We found that different physicochemical parameters were correlated with microbial community structure. Soil pH and NH4+-N were significantly correlated with bacterial community structure (P < 0.05), with the latter being highly significant (P = 0.001). As shown in Fig. 7, the first and second principal axes explained 30.8% and 49.1% of the total variation in bacterial communities, respectively, indicating that the variation in bacterial communities in the Three Gorges Reservoir riparian zone is mainly caused by changes in soil salinity and ammonium nitrogen content. Further analysis revealed a positive correlation between pH and bacterial communities at an elevation of 175 m (r2 = 0.038, P = 0.03), while a negative correlation was observed with bacterial communities at 155 m. Conversely, bacterial communities at 155 m showed a significant positive correlation with NH4+-N (r2 = 0.070, P = 0.001), although there were unclear correlations between this environmental factor and bacterial communities at 165 m and 175 m.
Redundancy analysis (RDA) between microbial biomass and soil properties in soil. (A) Bacteria (B) Archaea. (Arrows indicate soil physicochemical properties and different coloured spheres indicate microbial communities at different elevations. ("***" and "**" indicate significant differences at the level of P < 0.001 and P < 0.01, respectively) .
Observing the archaea separately within the community reveals certain ways in which they differ from the bacterial community as a whole. As depicted in the graph, the first and second principal axes respectively account for 96.2% and 3.5% of the total variation in the bacterial community. Among them, soil pH, total carbon (TC), and nitrate nitrogen (NO3--N) are all significantly correlated with the archaeal community structure, indicating that the variation in these communities is mainly caused by changes in soil salinity, carbon content, and nitrate nitrogen content. The bacterial community at an elevation of 175 m shows a positive correlation with pH (r2 = 0.046, P = 0.008) and a negative correlation with NO3- (r2 = 0.054, P = 0.004), with overlapping communities at 165 m and 175 m. Notably, total carbon (TC) has a highly significant impact on the archaeal communities (r2 = 0.103, P = 0.002). Additionally, the distribution of soil salinity and alkalinity falls within the first quadrant, while the nitrate nitrogen distribution is in the second quadrant, indicating that a synergistic effect of reducing soil salinity and nitrate nitrogen levels drives soil archaeal community succession.
To observe further connections between archaea and the environment, we determine the correlations between environmental factors and various orders of archaea, as shown in Fig. 8. Among them, the order Bathyarchaeia is significantly positively correlated with AP, OM, TN, and TOC (p < 0.01). This order possesses diverse metabolic pathways: it is capable of degrading recalcitrant macromolecules in the environment such as aromatic compounds, chitin, cellulose, and proteins; it can ferment to produce small molecules like acetic acid; and it can utilize CO2 and H2 for autotrophic production of acetic acid as an energy source. The order Nitrososphaeria exhibits similar properties, being strongly positively correlated with AP, OM, TN, and TOC (p < 0.01), and significantly positively correlated with TP and K2O (p < 0.05). It is notable that the pH shows a significant negative correlation with Thermoprotei, indicating sensitivity of this population to soil pH. NH4+-N, AP, OM, and TOC also exhibit significant negative correlations with the Thermoprotei, unlike most other factors.
To further elucidate the response of different soil bacterial groups to lithology-related factors in various soils, this study selected four environmental factors: MC (Moist content), DF (Duration of flooding), CC (Clay content), and Co (Conductivity) as explanatory variables, with soil bacterial community groups as response variables for redundancy analysis (RDA).
As illustrated in Figure 9, the first and second principal axes accounted for 67.3% and 22.7% of the total variation in the bacterial community, respectively, indicating that the variation in the bacterial community in the riparian zone of the Three Gorges Reservoir area is primarily driven by changes in moisture content. Further analysis indicated that the moisture content at 155 meters altitude was positively correlated with the bacterial community (r2=0.160, P=0.016), with no significant differences in other indices.
Discussion
Soil physicochemical properties
Different degrees of flooding can affect the soil contents of C, N, P, and K in the Riparian Zone, as well as their ecological stoichiometric characteristics. Changes in water levels result in varying degrees of vegetation cover and soil erosion at different elevations, thereby causing alterations in soil nutrient content.
The degree of flooding alters the soil pH. Our findings indicate that after flooding, the pH of acidic soil increases while that of alkaline soil decreases, ultimately trending towards neutrality. The rise in pH in acidic soil is attributed to proton consumption, whereas the pH decrease in alkaline soil is due to the accumulation of carbon dioxide post-flooding, neutralizing the alkalinity13,14. Soils subjected to longer periods of flooding exhibit significantly higher total carbon (TC) content compared to regions with shorter flooding durations. In the riparian zone, abundant growth of annual or perennial herbaceous plants occurs, and the process of seasonal water storage leads to the decomposition of flood-intolerant plants from previous years, which releases both organic matter and nutrients into the water15. Generally, the carbon-to-nitrogen (C:N) ratio of soil organic matter is inversely related to its decomposition rate; thus, a lower C:N ratio indicates a faster mineralization rate, resulting in a higher fraction of available nitrogen in soil organic matter. The findings of this study indicate that with increasing elevation in the riparian zone, the C:N ratio gradually decreases. This is consistent with the results of regarding the gradual increase in C: N during ecological succession16. We also found that nitrate nitrogen content was slightly higher at lower elevations compared to higher elevations, possibly due to the recent exposure of the sampling time17. During the initial period of water level decline, leaching processes in the soil may lead to downward nitrogen migration, potentially accumulating in soils at the 155 m elevation. Additionally, compared to other natural ecotones, the reservoir’s riparian zone is mainly distinguished by artificial water level regulation, which may affect the timing of certain nitrogen transformation processes. Organic matter exhibits significant potassium retention capability, and thus there were no significant differences in total phosphorus and total potassium content at different elevations before and after the impact of water level fluctuations. Our preliminary observations suggest an increase in potassium and phosphorous content with elevation. This could be related to the promotion of weathering of potassium- or phosphorus-containing minerals, and the release of potassium and phosphorus elements as facilitated by water immersion18. A warm and humid climate aids in the weathering of minerals and the formation of clay particles, whereas a dry and cold climate hinders clay formation. This might explain the high clay content at lower altitudes19. The dissolved salts in the soil (including sodium salts, potassium salts, calcium salts, and magnesium salts) are the primary determinants of conductivity. The higher the concentration of dissolved salts, the greater the conductivity of the soil. Conductivity can be used to monitor the pollution levels in soil and groundwater. The soil at 175 m altitude might have a higher risk of pollution compared to lower altitudes20.
Spatiotemporal dynamics of prokaryotic microbial communities
Bacteria are the most abundant and diverse group among soil microorganisms. They play a crucial role in riparian zone ecosystems through processes such as organic matter mineralization, humus compound formation, and the cycling of elements like carbon and nitrogen. Therefore, understanding the diversity and community composition of soil bacteria is of the utmost importance.
In this study, we found that the α diversity of eukaryotic microbial communities in the Three Gorges Reservoir first increased with elevation and then decreased. Some studies have found that microbial diversity at high elevations shows a positive correlation with elevation19,20, which differs from the results in this study. The soil at 155 m elevation had been fully exposed for approximately one month at the time of sampling, and it was close to the water source. Therefore, the recovery of this community was effective, showcasing the highest diversity and evenness.
In our investigated bacterial communities, Proteobacteria, Acidobacteria, and Chloroflexi are the dominant populations. Similarly, both Qin21 and Wang22 found Proteobacteria, Chloroflexi, and Acidobacteria to be predominant in the Three Gorges Reservoir area. Among these, the relative abundance of Proteobacteria and Chloroflexi gradually decreases with increasing elevation, whereas the relative abundance of Acidobacteria gradually increases. The high prevalence of Proteobacteria in the reservoir area and other rivers might be due to its high absorption capacity and growth rate, as most Proteobacteria play important roles in organic matter decomposition and cycling. Recent studies have also found that nitrogen addition can increase the relative abundance of Proteobacteria23. At the genus level, the most dominant groups are RB41 (1.37%-3.83%) and Candidatus_Methanoperedens (0.58%-1.72%). The former plays an important role in carbon cycling in soil and material metabolism in nutrient-poor soil24,25, while the latter can convert NO3--N to NO2--N and oxidize CH4 under anaerobic conditions, thus accelerating the reduction of NO3--N in flooded soil26,27,28.
The composition and functionality of archaeal communities in the riparian zone exhibit distinct spatial distribution trends, which are associated with altitude. Prolonged flooding in low-altitude areas creates a cold anaerobic environment that inhibits the growth and reproduction of certain microorganisms, thereby limiting the decomposition of soil carbon29. Accumulated soil nutrients and organic matter promote the growth and diversity of microbial communities. In contrast, soils at high altitudes that experience shorter flooding durations may favor the growth and reproduction of archaea30 High-altitude areas are primarily inhabited by the aerobic and nitrogen-transforming Nitrososphaeria, which play a crucial role in soil and water bodies. They participate in nitrogen transformation and cycling, thereby influencing the nitrogen balance of ecosystems and aiding plant growth31.
In low-altitude areas, there exist archaea that are capable of survival and carbon transformation under anaerobic conditions. This includes Methanomicrobia, which produce methane under anaerobic conditions. Flooding results in organic matter accumulation in low-altitude soils, providing abundant substrates for some methane-producing archaea. To a certain extent, prolonged flooding promotes methane generation in the riparian zones of the Three Gorges Reservoi32. Bathyarchaeia, which thrive in either anaerobic or microaerobic conditions, also constitute a significant proportion. They possess the potential for anaerobic lignin degradation33 and are believed to play important roles in carbon cycling and methane metabolism. Furthermore, research indicates that abundant Bathyarchaeia in low-altitude soils can produce methane by reducing methyl compounds with H234. These methane-producing archaea significantly enhance the methane production capacity of soils in the fluctuating water level zones of reservoirs35.
With decreasing altitude, the soil gradually trends towards pH neutrality. Soil acidification is the main driver behind the regulation of nitrification by ammonia-oxidizing microorganisms36. Weakly acidic soils enhance nitrification as mediated by ammonia-oxidizing archaea. Ammonia-oxidizing archaea play a crucial role in nitrification, as they influence the accumulation of nitrate in soil. Prolonged cycles of wetness and dryness weaken soil nitrification processes within fluctuating water level zones, leading to lower nitrate nitrogen levels in low-altitude areas.
Research has shown that long-term alternation between wet and dry conditions reduces the abundance of archaea with aerobic ammonia oxidation capabilities36. In soils at low altitudes, the abundance of archaea with aerobic ammonia oxidation functionality is lower than that at high altitudes. This suggests that prolonged flooding weakens the nitrification capacity of the soil, thus reducing the production of NO3-. The sampling location in this study was exposed for one month at the 155 m elevation, which may also contribute to the slightly higher NO3- levels at that point.
In the RDA analysis, we discovered a strong correlation between the bacterial community and pH, NH4-N, and moisture content. Soil moisture content not only directly impacts the physicochemical properties and nutrient content of the soil but also may affect the diversity of microbial communities by changing the habitat of soil microorganisms. A study conducted in the upper reaches of the Yellow River revealed that land use type and distance from the river significantly influenced the diversity of soil microbial communities. Regions with higher humidity exhibited greater microbial diversity, whereas regions with lower humidity showed reduced diversity37 consistent with the findings of this study. Moreover, microbial activity reached its maximum at 80% water-filled pore space (WFPS), suggesting that humidity markedly influences the gas and liquid diffusion rates in microorganisms, as well as the availability of oxygen and substrates38.
Spatiotemporal dynamics of eukaryotic microbial communities
In the riparian zone soils, we found significant differences in community composition depending on the flooding duration. This applied not only to bacterial communities, but also to eukaryotic communities and their associated compositions39.
The study of eukaryotic microbial communities in water bodies is an important facet of water ecology research40. Freshwater fungi are ubiquitous and diverse, occupying an important position as saprotrophs or parasites in freshwater ecosystems41 In this study, eight fungus classes were observed in the eukaryotic community: Ascomycota, Basidiomycota, Cryptomycota, Chytridiomycota, LKM15, Zoopagomycota, Blastocladiomycota and Mucoromycota. Specifically, Ascomycota was the most dominant in the well-lit aerobic surface layer, whereas Phragmoplastophyta was more abundant in the microoxidative lower layer.
It was found that Ascomycota and Basidiomycota exhibited high proportions in all samples (Fig. 6A). The fungal phyla Ascomycota and Basidiomycota have attracted widespread attention due to their biomineralization capabilities, and their ability to accumulate toxic heavy metals within their cells. Previous studies have indicated a higher prevalence of Ascomycota in aquatic environments, while our results showed a greater prevalence of Ascomycota as elevation increased. Differences in community composition may be attributed to primer amplification biases or geographical variations in the populations. As the most abundant phylum in soil fungal communities, Ascomycota are susceptible to the degree of nutrient availability in the environment. They play a crucial role in decomposing lignocellulosic materials, and serve as primary decomposers of organic matter in soil, thus accelerating nutrient cycling and promoting the growth and reproduction of soil microorganisms. Additionally, as elevation increases, the higher oxygen content becomes more conducive to the growth of eukaryotic organisms42.
Chaetomium sp. occupies a dominant position in the fungal community, being in the highest proportions in soils at low altitudes43. Research indicates that Chaetomium sp. can produce large amounts of cellulases, which play a role in the degradation of recalcitrant organic compounds such as cellulose and lignin. This may be one of the reasons why the total carbon content was the highest at low altitudes. However, Chaetomium sp. can also experience antagonistic effects. For instance, soil at the 165 m elevation likely contains microorganisms that are antagonistic to Chaetomium sp., which are unfavorable for its growth and reproduction, resulting in a significant decrease in its abundance.
Conclusion
By investigating soils from the riparian zone of the Three Gorges Reservoir area, we revealed spatiotemporal variations of microbial communities, and elucidated environmental factors affecting prokaryotic and eukaryotic microbial communities in these soils.
Specifically, we describe the structure and diversity of bacterial and eukaryotic communities in the riparian zones of the Daning River tributary. An important finding is that microbial communities vary depending on the elevation, as they are influenced by elevation-dependent factors such as water content, oxygen content, and other physicochemical parameters. Redundancy analysis (RDA) suggests that pH, \({NH}_{4}^{+}\)-N and moist content are the main environmental factors driving variation in soil bacterial communities, while different flooding times,pH, total carbon (TC), and \({NO}_{3}^{-}\)-N are the main environmental factors driving variation in soil archaeal communities. There are also significant differences in the community structures of eukaryotic organisms in soils at varying altitudes. These findings emphasize the connection between environmental variables and community assembly processes, and may contribute to our understanding of carbon and nitrogen cycling processes in the riparian zones of the Three Gorges Reservoir, thus providing insights for reservoir operation and riparian zone management.
However, our current research lacks an in-depth investigation about the impact of human activities such as agriculture on the diversity, function and ecological balance of microbial communities in the riparian zone. It is hoped that this aspect can be enriched in the future.
Data availability
Data is provided within supplementary information files.
References
Vidon, P. G., Welsh, M. K. & Hassanzadeh, Y. T. Twenty years of riparian zone research (1997–2017): Where to next?. J. Environ. Qual. 48(2), 248–260. https://doi.org/10.2134/jeq2018.01.0009 (2019).
Li, X., Li, S., Xie, Y., Wei, Z. & Li, Z. What Drives the Morphological Traits of Stress-Tolerant Plant Cynodon dactylon in a Riparian Zone of the Three Gorges Reservoir China. Water 15(18), 3183. https://doi.org/10.3390/w15183183 (2023).
Zhang, M., O’Connor, P. J., Zhang, J. & Ye, X. Linking soil nutrient cycling and microbial community with vegetation cover in riparian zone. Geoderma https://doi.org/10.1016/j.geoderma.2020.114801 (2021).
Eggleston, E. M. et al. Key respiratory genes elucidate bacterial community respiration in a seasonally anoxic estuary. Environ. Microbiol. 17(7), 2306–2318. https://doi.org/10.1111/1462-2920.12690 (2015).
Chen, Z. et al. Effects of wet and dry seasons on the aquatic bacterial community structure of the Three Gorges Reservoir. World J. Microbiol. Biotechnol. 29(1), 841–853 (2013).
Bates, S. T. et al. Examining the global distribution of dominant archaeal populations in soil. ISME J. 5, 908–917. https://doi.org/10.1038/ismej.2010.171 (2011).
Wang, J. et al. Large Chinese land carbon sink estimated from atmospheric carbon dioxide data. Nature 586(7829), 720–723. https://doi.org/10.1038/s41586-020-2849-9 (2020).
Singh, A., Vishwakarma, P., Adhya, T. K., Inubushi, K. & Dubey, S. K. Molecular ecological perspective of methanogenic archaeal community in rice agroecosystem. Sci. Total Environ. 596–597, 136–146. https://doi.org/10.1016/j.scitotenv.2017.04.011 (2017).
DeAngelis, K. M., Silver, W. L., Thompson, A. W. & Firestone, M. K. Microbial communities acclimate to recurring changes in soil redox potential status. Environ. Microbiol. 12(12), 3137–3149. https://doi.org/10.1111/j.1462-2920.2010.02286.x (2010).
Høj, L., Olsen, R. A. & Torsvik, V. L. Effects of temperature on the diversity and community structure of known methanogenic groups and other archaea in high Arctic peat. ISME J. 2, 37–48. https://doi.org/10.1038/ismej.2007.84 (2008).
Ye, F. et al. Shifts of archaeal community structure in soil along an elevation gradient in a reservoir water level fluctuation zone. J. Soils Sediments 16, 2728–2739 (2016).
Wu, S., Zhao, W., Liu, M., Gao, F. & Chen, H. Prokaryotic and Eukaryotic Communities Characteristic in the Water Column and Sediment along the Xiangjiang River. China. Water. 15(12), 2189. https://doi.org/10.3390/w15122189 (2023).
Ding, C. F. et al. Changes in the pH of paddy soils after flooding and drainage: Modeling and validation. Geoderma 337, 511–513. https://doi.org/10.1016/j.geoderma.2018.10.012 (2019).
Ren, C. J. et al. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau China. Geoderma 282, 103–111. https://doi.org/10.1016/j.geoderma.2016.07.018 (2016).
Ostojić, A., Rosado, J., Miliša, M., Morais, M. & Tockner, K. Release of Nutrients and Organic Matter from River Floodplain Habitats: Simulating seasonal inundation dynamics. Wetlands 33, 847–859 (2013).
Hume, A., Chen, H. Y. H., Taylor, A. R., Kayahara, G. J. & Man, R. Z. Soil C:N: P dynamics during secondary succession following fire in the boreal forest of central Canada. Forest Ecol. Manag. 369, 1–9. https://doi.org/10.1016/j.foreco.2016.03.033 (2016).
Ren, C. J. et al. Linkages of C:N: P stoichiometry and bacterial community in soil following afforestation of former farmland. Forest Ecol. Manag. 376, 59–66. https://doi.org/10.1016/j.foreco.2016.06.004 (2016).
Chang, C., Xie, Z.-Q., Xiong, G.-M. & Chu, L.-M. The Effect of Flooding on Soil Physical and Chemical Properties of Riparian Zone in the Three Gorges Reservoir. J. Nat. Resourc. 26(7), 1236–1244. https://doi.org/10.1184/zrzyxb.2011.07.016 (2011).
Shigyo, N., Umeki, K. & Hirao, T. Seasonal dynamics of soil fungal and bacterial communities in cool-temperate montane forests. Front. Microbiol. https://doi.org/10.3389/fmicb.2019.01944 (2019).
Xing, R., Gao, Q.-B., Zhang, F.-Q., Wang, J.-L. & Chen, S.-L. Environmental filtering affects fungal communities more than dispersal limitation in a high-elevation hyperarid basin on Qinghai-Tibet Plateau. FEMS Microbiol. Lett. https://doi.org/10.1093/femsle/fnab033 (2021).
Qin, Y. et al. Changes in planktonic and sediment bacterial communities under the highly regulated dam in the mid-part of the Three Gorges Reservoir. Appl. Microbiol. Biotech. 105(2), 839–852. https://doi.org/10.1007/s00253-020-11047-3 (2021).
Wang, S. et al. Microbial diversity accumulates in a downstream direction in the Three Gorges Reservoir. J. Environ. Sci. 101, 156–167. https://doi.org/10.1016/j.jes.2020.08.006 (2021).
Nie, Y. X. et al. Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci. Total Environ. 624, 407–415. https://doi.org/10.1016/j.scitotenv.2017.12.142 (2018).
He, J. Z., Hu, H. W. & Zhang, L. M. Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biol. Biochem. 55, 146–154. https://doi.org/10.1016/j.soilbio.2012.06.006 (2012).
Stone, B. W. et al. Nutrients cause consolidation of soil carbon flux to small proportion of bacterial community. Nat. Commun. https://doi.org/10.1038/s41467-021-23676-x (2021).
Chen, X. L. et al. Metagenomic analysis reveals the response of microbial community in river sediment to accidental antimony contamination. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2021.152484 (2022).
Haroon, M. F. et al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500(7464), 567. https://doi.org/10.1038/nature12375 (2013).
Shen, L. D. et al. Different responses of nitrite- and nitrate-dependent anaerobic methanotrophs to increasing nitrogen loading in a freshwater reservoir. J. Environ. Pollut. https://doi.org/10.1016/j.envpol.2020.114623 (2020).
Zhang, D. D. et al. Linkages between soil organic carbon fractions and carbon-hydrolyzing enzyme activities across riparian zones in the Three Gorges of China. Sci. Rep. https://doi.org/10.1038/s41598-020-65200-z (2020).
Hu, H. et al. Significant association between soil dissolved organic matter and soil microbial communities following vegetation restoration in the Loess Plateau. Ecol. Eng. https://doi.org/10.1016/j.ecoleng.2021.106305 (2021).
Luo, Z.-H. et al. Temperature, pH, and oxygen availability contributed to the functional differentiation of ancient Nitrososphaeria. ISME J. https://doi.org/10.1093/ismejo/wrad031 (2024).
Shi, W. J., Du, M., Ye, C. & Zhang, Q. F. Divergent effects of hydrological alteration and nutrient addition on greenhouse gas emissions in the water level fluctuation zone of the Three Gorges Reservoir China. Water Res. https://doi.org/10.1016/j.watres.2021.117308 (2021).
Knief, C. Diversity of methane-cycling microorganisms in soils and their relation to oxygen. Curr. Issues Mol. Biol. https://doi.org/10.21775/cimb.033.023 (2019).
Evans, P. N. et al. An evolving view of methane metabolism in the Archaea. Nat. Rev. Microbiol. 17(4), 219–232. https://doi.org/10.1038/s41579-018-0136-7 (2019).
Evans, P. N. et al. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 350(6259), 434–438. https://doi.org/10.1126/science.aac7745 (2015).
Jung, M. Y. et al. Indications for enzymatic denitrification to N2O at low pH in an ammonia-oxidizing archaeon. ISME J. 13(10), 2633–2638. https://doi.org/10.1038/s41396-019-0460-6 (2019).
Liu, S. L. et al. Composition and diversity of soil microbial community associated with land use types in the agro-pastoral area in the Upper Yellow River Basin. Front. Plant Sci. https://doi.org/10.3389/fpls.2022.819661 (2022).
Fan, S., Qin, J., Sun, H., Jia, Z. & Chen, Y. Alpine soil microbial community structure and diversity are largely influenced by moisture content in the Zoige wetland. Int. J. Environ. Sci. Technol. 19(5), 4369–4378. https://doi.org/10.1007/s13762-021-03287-1 (2022).
Weigel, B. et al. Local eukaryotic and bacterial stream community assembly is shaped by regional land use effects. ISME Commun. https://doi.org/10.1038/s43705-023-00272-2 (2023).
Sprong, P. A. A. et al. Spatial dynamics of eukaryotic microbial communities in the German Bight. J. Sea Res. https://doi.org/10.1016/j.seares.2020.10194 (2020).
Sun, J.-Y. et al. Fungal community dynamics during a marine dinoflagellate (Noctiluca scintillans) bloom. Marine Environ. Res. 131, 183–194. https://doi.org/10.1016/j.marenvres.2017.10.002 (2017).
Souza, R. C. et al. Shifts in taxonomic and functional microbial diversity with agriculture: How fragile is the Brazilian Cerrado?. BMC Microbiol. 16(1), 42. https://doi.org/10.1186/s12866-016-0657-z (2016).
Yue, H.-M., Wang, M., Gong, W.-F. & Zhang, L.-Q. The screening and identification of the biological control fungi Chaetomium spp. against wheat common root rot. FEMS Microbiol. Lett. https://doi.org/10.1093/femsle/fny242 (2018).
Author information
Authors and Affiliations
Contributions
XL wrote the main manuscript text. QH prepared all the illustrations. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, X., Gong, Q. & Li, Z. Response characteristics of soil microorganisms under strong disturbance conditions in the riparian zone of the three Gorges reservoir Area. Sci Rep 14, 18394 (2024). https://doi.org/10.1038/s41598-024-69533-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-69533-x
Keywords
This article is cited by
-
Activities of soil carbon and nitrogen enzymes during charcoal production in derived savanna of Nigeria
Discover Applied Sciences (2025)