Abstract
Wastewater is discharged in large amounts from different industries; thus, wastewater treatment is currently one of the main concerns, advanced oxidation is a promising technique for wastewater treatment. This research aims to synthesize magnetite nanoparticles and study their application in wastewater treatment via adsorption and advanced oxidation processes. Magnetite nanoparticles were synthesized via coprecipitation technique between ferric and ferrous sulfate at a molar ratio of 2:1. The prepared sample was characterized using FTIR, XRD, TEM, BET surface area, zeta potential, VSM, and UV‒visible spectroscopy. XRD confirmed the formation of a single face-centered cubic (FCC) spinel structure of Fe3O4. TEM revealed an average particle size of 29.2 nm and a BET surface area of 70.1 m2 g−1. UV‒visible spectroscopy revealed that the UV–visible peak of the sample was obtained at 410 nm. VSM confirmed the attraction of the sample to a magnet with a magnetization of 60 (emu/g). The removal efficiency of methylene blue was studied using adsorption and advanced oxidation methods. For adsorption, the studied parameters were dye concentration 2–10 ppm, 3–10 pH, and 50:300 mg Fe3O4/L. For advanced oxidation, peroxide was used with nanomagnetite as a catalyst, and the studied parameters were pH 2–11, magnetite dose 20–200 PPM, and peroxide dose 500–2000 PPM. The removal efficiency by adsorption reached 95.11% by adding 50 mg of Fe3O4/L and 10 ppm dye conc at 6.5 pH; on the other hand, in advanced oxidation, it reached 98.5% by adding 110 PPM magnetite and 2000 ppm H2O2 at pH 11. The magnetite nanoparticles were reused for ten cycles of advanced oxidation, for a 10% reduction in removal efficiency at the tenth cycle.
Similar content being viewed by others
Introduction
One of the main challenges facing most countries is the gap between water resources and water needs due to several factors, such as population growth and industrial activities. As a result of industrialization, a large amount of water is consumed, and a large amount of wastewater is produced, which poses a great threat to the environment1.
Environmental concerns related to the manufacture and use of dyes have greatly increased over the past years, and dyes are unquestionably one of the main factors influencing the textile dye industry today, which consumes huge amount of dye2.
The discharge of dyes resulting from textile manufacturing, paper making, and leather dyeing into water drains, canals, and rivers is considered the biggest problem facing the environment and threatening the safety of water and marine life.
Traditional treatment methods for effluent wastewater are physical, chemical, and biological methods. Adsorption transfers pollutants found in the liquid phase to the solid phase, after which further processing is needed to address the contaminated sludge. This is considered the main problem in all traditional methods3,4,5.
One of the most important techniques used in wastewater treatment is the advanced oxidation process (AOP). Heterogeneous photocatalysis is an AOP that has several advantages, including the ability to mineralize pollutants, low cost, waste-free nature, and eco-friendliness6,7,8. The mechanism of action of AOPs mainly depends on the generation of highly reactive free radicals such as hydroxyl radicals (·OH), sulfate radicals (SO4·−), and superoxide radicals (O2·−) by the activation of different oxidants (hydrogen peroxide, persulfate/peroxydisulfate, peroxymonosulfate and sodium percarbonate via oxidation and reduction reactions9,10,11,12. These released radicals cause the degradation of organic pollutants and subsequent mineralization to inorganic ions, water and carbon dioxide13,14.
Iron-based materials are recommended for use as catalysts for the activation of heterogeneous AOPs due to their environmental friendliness and cost effectiveness, which make them suitable for large-scale practical applications15,16.
Magnetite-based catalysts are effective Fenton catalysts for generating HO· for the removal of organic pollutants in wastewater17. The magnetite catalyst has a wider working pH range. In the Fenton system, a series of reactions that mainly occur on the surface of minerals are shown in Eqs. (1–4) 18.
The application of nanomagnetite as a catalyst in the AOP process is recommended because it has magnetic properties that result in high catalytic efficiency, and it can be separated easily from treated water19,20,21.
Response surface methodology (RSM) is a statistical software package that provides researchers with the ability to study the parameters affecting any process. On the other hand, process optimization can be performed using a predictive model based on a set of experiments that correlate the response to different variables22,23,24.
The response function (f) largely depends on the nature of the relationship between the response and the independent variables. The polynomial quadratic model is represented by Eq. (5):
where Y is the intercept or regression coefficient; βi, βii, and βij represent the linear quadratic and interaction coefficients, respectively; and Xi and Xj are the coded values of the process variables22.
In this research, the RSM Box–Behnkin method was used to model and optimize the use of synthetic magnetite nanoparticles for both adsorption and advanced oxidation. Depending on the magnetic properties of the synthesized nanomagnetite, it was separated by a magnet to study its reuse in water treatment for several cycles, after which the removal efficiency was calculated.
Experimental work
The raw material used, the method of preparation of nanomagnetite, the characterization methods, and the experimental work are explained.
Raw materials
Sodium hydroxide (NaOH) (96% purity), ferric sulfate pentahydrate (Fe2(SO4)3·5H2O) (98% purity), and ferrous sulfate heptahydrate (FeSO4·7H2O) (98% purity) were purchased from Alpha Chemical.
Preparation of magnetite nanoparticles
Magnetite nanoparticles (Fe3O4) were synthesized via the coprecipitation technique by mixing Fe2(SO4)3 and Fe(SO4) according to their stoichiometric ratios, as shown in Eq. (6), in distilled water. A solution of sodium hydroxide was added dropwise while stirring to reach a pH of 11. The mixture was continuously stirred and heated to 80 °C. Finally, the nanoparticles were separated, washed with distilled water to adjust the pH to 7.0, and then dried in an electrical dryer at 105 °C,25 as shown in Fig. 1.
Characterization of magnetite nanoparticles
Characterization techniques were used to verify the properties of the magnetite nanoparticles via X-ray diffraction (XRD) using a Bruker D8 Advance ECO diffractometer in reflection mode. Fourier transform infrared spectroscopy, which was performed on an ATR-IR instrument (400–4000 cm−1), was carried out using an ALPHA II BRUKER, USA. Transmission electron microscopy (TEM) was also performed on an FEI Talos F200S field-emission transmission electron microscope with an accelerating voltage of 200 kV. The Brunauer–Emmett–Teller (BET) surface area was determined from a nitrogen adsorption isotherm at 77 K using a Belsorp adsorption automatic specific surface area analyzer (Microtrac BEL, Japan), vibrating sample magnetometer analysis (VSM), chemical oxygen demand (COD), zeta potential, and ultraviolet spectroscopy (UV‒VIS spectrophotometer) were used, as discussed below.
Application of magnetite nanoparticles
The use of synthesized nanoparticles in adsorption and advanced oxidation was carried out. To study the adsorption of methylene blue using magnetite, three parameters were studied: dye concentration, 2:10 ppm; pH, 3:10 (effect of acidity and alkalinity of solution on removal efficiency); and magnetite dose in the range of 50:300 mg Fe3O4/L. RSM was used for the experiments, and both the adsorption isotherm and adsorption kinetics were studied. The concentrations of the different solutions were measured using a Spectro UV‒VIS Double-Model UVD-2950.
The oxidation process was performed using a sample of a synthetic solution with 100 ppm methylene blue dye (M. B.). The studied parameters were as follows: nanomagnetite dose, 20 to 200 ppm; peroxide dose, 500 to 2000 ppm; and pH 2:11 (from acidic to alkaline medium). The concentrations of the different solutions were measured using a Spectro UV‒VIS Double-Model UVD-2950. Table 4 shows the removal efficiency of dye from wastewater under different conditions. The removal efficiency was calculated as follows: (Ci − Cf) × 100/Ci, where Ci and Cf are the initial and final concentrations of dye, respectively. The highest removal efficiency (98.5%) was obtained by adding 110 mg of magnetite, 2000 ppm H2O2, and a pH of 11.
Results and discussion
The characterization of the prepared nanomagnetite and its application in wastewater treatment are discussed in the following sections.
X-ray diffraction (XRD)
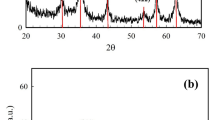
XRD was applied to determine the crystallinity of the tested sample. The peaks in the XRD pattern shown in Fig. 2 are between 30° and 62°, which correspond to standard magnetite26. These findings revealed that a single face-centered cubic (FCC) spinel structure of Fe3O4 was formed. (Device model SEIFERT XRD 3003 TT DIFRACTOR (GE, Germany) Equipped with a primary monochromator (CuK radiation, 2 ceta = 3 − 90°).
Transmission electron microscopy (TEM)
TEM is essential for material science and has many characteristics, such as particle size and morphology. As shown in Fig. 3, the particles are almost spherical in shape, and the average particle size is 29.2 nm, which is a reasonable size for obtaining a high surface area. (Device model: JEOL JEM-2100 (Origin. Japan). The BET surface area is 70.1 m2 g−1.
Zeta potential
The zeta potential is the charge that appears at the interface between a solid surface and the surrounding liquid medium. Figure 4 shows the results obtained from the examined sample, which indicate that the surface of magnetite has a negative charge when a particle is dispersed in a liquid, and the functional groups on its surface will react with the positively charged ions in the surrounding medium (device model: MALVERN ZETASIZER, USA).
Fourier transform infrared (FTIR) spectroscopy
The FTIR transmittance spectrum analysis is shown in Fig. 5. A peak is observed at 556 cm−1, which corresponds to the stretching vibration of the Fe–O bonds in the sublattice of Fe3O4. The peak at 2930 cm−1 is attributed to –CH2 and chemical group stretching vibrations. The values at 1629 cm−1 and 3389.64 cm−1 indicate the stretching of C–O, C=C, and OH–, respectively27. (Device model (FTIR) JASCO, FTIR-300 E.
UV–visible spectroscopy
The UV–visible spectra of the prepared magnetite nanoparticles are displayed in Fig. 6. The UV–visible peak of the sample was obtained at 410 nm. The reported UV–visible peak was found to be at 407 nm by Suresh Kumar et al.28. The peak in the near-IR region confirmed the presence of magnetite nanoparticles.
VSM of synthetic nanomagnetite
Vibrating sample magnetometer analysis (VSM) was performed using a Lake shore model 7410 instrument to analyze the synthesized nanomagnetite, as shown in Fig. 7. As shown in the figure, the magnetization of the sample revealed that it was completely attracted to the magnet and had a magnetization of 60 (emu/g)29.
Figure 8 shows the pattern taken by the nanoparticles when subjected to a magnetic field, which confirms its magnetic properties.
Adsorption of methylene blue dye using magnetite nanoparticles
The results obtained in different runs are tabulated in Table 1. The highest removal efficiency (95.11%) was obtained by adding 50 mg of Fe3O4/L, 10 ppm dye conc and 6.5 pH.
The reduced cubic model was the best model for representing the obtained data, with an R2 equal to 0.973 and an adjusted R2 of 0.94. Table 2 displays an ANOVA of the obtained model, which indicates that the model is significant with a p value of 0.0001. The suggested model that relates the studied parameters and removal efficiency according to the obtained results and statistical analysis was a reduced cubic model with R2 = 0.9697, adjusted R2 = 0.9395, and a p value lower than 0.0001, which confirmed that the model was significant. The ANOVA results of the models are displayed in Table 6. These results indicate that the model is significant and has a high confidence level, as the p value is lower than 0.05 and the F value is 32, which reveals the importance of the variance in each variable30.
Dye removal equations by adsorption
The removal efficiency (Y) equation was obtained from the statistical analysis of the coded values
The removal efficiency (Y) equation was obtained from the statistical analysis of the actual values
Interaction between the studied parameters
A 2-D plot can be drawn for different variations in parameters, which exhibit a trend in which the response varies within the selected range of input parameters and the effect of each parameter over the other parameters. With the aid of statistical analysis, the interactions between the three studied parameters, namely, pH “A”, dye concentration “B” and dose “C”, can be studied using the obtained model graph contours.
-
a.
Effect of dye concentration and pH on dye removal
The effects of pH and concentration on removal efficiency are displayed in Fig. 9a–c. At a low magnetite dose, the removal efficiency increases as the pH and concentration increase simultaneously. At an average dose of magnetite, increasing the concentration of dye increases the removal efficiency even at a low pH. At a high dose of magnetite, the removal efficiency increases with increasing pH and concentration simultaneously, which confirms the interaction between the studied parameters.
-
b.
Effect of the magnetite dose and pH on dye removal
The effect of magnetite dose and pH on dye removal efficiency is illustrated in Fig. 10a–c. As the concentration of dye increases from the minimum to the maximum value, the removal efficiency increases even at low pH and magnetite doses.
Adsorption isotherm
Synthetic wastewater solutions with concentrations of 10, 20, 40, 60, 80, and 100 ppm methylene blue dye were prepared. The dose of magnetite was 0.05 g/L for all the samples. All the samples were agitated on a mechanical shaker at a speed equal to 100 rpm. The concentrations of all the samples were measured over time until they reached equilibrium. The results are listed in Table 3.
The adsorption capacity increased with increasing concentration up to 80 ppm and then decreased at 100 ppm, possibly due to the saturation of magnetite.
Three models were applied: the Langmuir isotherm model, Freundlich isotherm model, and Dubinin–Radushkevich isotherm model.
As shown in Figs. 11 and 12, the Langmuir isotherm was fitted with the experimental data, as it had an R2 of 0.9295; in contrast, the Freundlich isotherm had an R2 of 0.6787.
The maximum adsorption capacity was calculated from the line slope, which was qmax = 1/slope = 50 mg/g, and the Langmuir constant KL = 2 L/mg.
The Langmuir model indicates that a monolayer is formed, the heat of adsorption Q is constant and independent of coverage, each adsorbate molecule occupies only one site, and the adsorption is localized (molecules remain at the site of adsorption until desorption).
The Dubinin–Radushkevich isotherm model (D–R) can be applied to determine whether the adsorption process is physical or chemical and is expressed as31
where KD−R (mg g−1) is the Dubinin–Radushkevich constant and the Polanyi potential is ε (mol2 J−2), which is equal to
where T is the absolute temperature (K) and R is the universal gas constant (8.314 J K−1 mol−1). The constant β is related to E (kJ mol−1). The energy E is defined as the free energy change required to transfer 1 mol of ions from the solution to the solid:
The linear relation of (ln qe) against ε2 is carried out as shown in Fig. 13, and the values β and KD−R are obtained from the slope and intercept of the line. The value of E represents the information adsorption mechanism; an E value less than 8 kJ mol−1 represents the physisorption process, and an E value within the range of 8–16 kJ mol−1 is assigned to the chemisorption process31. The calculated value of E is 2.23 kJ/mol, which indicates that the adsorption of M.B by the nanomagnetite physisorption process.
Adsorption kinetics
Adsorption kinetics were studied for a sample with an 80 ppm concentration, as it maintained its maximum adsorption capacity. Two kinetic models were applied: a pseudo-first-order model and a pseudo-second-order model. Figures 14 and 15 show that the pseudo first-order model fit the experimental data, as it had an R2 of 0.9269 versus an R2 of 0.0198 for the pseudo second-order model.
Thermodynamic study
A thermodynamic study was carried out by changing the temperature (20, 30, 40 °C) during the adsorption of methylene blue dye with nanomagnetite. Determination of the equilibrium concentration of different samples and thermodynamic parameters such as the free energy change (ΔG0), enthalpy change (ΔH0) and entropy change (ΔS0) were carried out using the following equations32:
where T is the temperature in K, R is the universal gas constant (R = 8.314 J mol–1 K–1), and Kc is the equilibrium constant (kc = qe/Ce). A linear plot between ln (Kc) and 1/T is displayed in Fig. 16. The values of ΔS0 and ΔH0 were determined from the intercept and slope, respectively, as presented in Table 4. The value of ΔH0 is positive, which points to an endothermic reaction. The value of ΔS0 is positive, which indicates that the degrees of freedom increased at the solid–liquid interface during dye adsorption.
Application of advanced oxidation in removing methylene blue dye using nanomagnetite as a catalyst
The parameters and removal efficiency are tabulated in Table 5. The suggested model that relates the studied parameters and removal efficiency according to the obtained results and statistical analysis was a reduced cubic model with R2 = 0.9697, adjusted R2 = 0.9395, and a p value lower than 0.0001, which confirmed that the model was significant. The ANOVA results of the models are displayed in Table 6. These results indicate that the model is significant and has a high confidence level, as the p value is lower than 0.05 and the F value is 32, which reveals the importance of the variance in each variable30. The maximum removal percentage reached 98.5% according to the tabulated values; this value is greater than that of most of the methods found in the literature33, and not only does this removal occur because the AOP does not produce any sludge.
Dye removal efficiency equations using the advanced oxidation approach
The removal efficiency (Y) equation obtained from the statistical analysis of the coded values is shown below:
The actual removal efficiency is as follows:
Effect of the catalyst dose and hydrogen peroxide on dye removal (interaction between study parameters)
The catalyst dose “A” on the X-axis and hydrogen peroxide “B” on the Y-axis were studied while varying the pH.C. to its minimum, average, and maximum values to study its effect on dye removal. Figure 17a–c indicates that at a minimum pH, the removal efficiency increases as the peroxide dose increases; at the maximum pH, increasing the catalyst dose or peroxide dose increases the removal efficiency of the dye. Figure 18 shows photos of several treated samples after different runs.
Figure 19a–c shows the separation of magnetite nanoparticles from treated water using a magnet. The solution was very clear after approximately 5 min, as shown in Fig. 20c. All the magnetite nanoparticles were attracted to the magnet below the beaker, which confirmed the magnetic characteristics of the produced nanomagnetite and the ease of separation from treated water, which distinguishes it as a catalyst for advanced oxidation in wastewater treatment.
Effect of UV radiation on the removal efficiency of methylene blue solution using advanced oxidation
Two samples of methylene blue dye (100 ppm) were prepared, and the same conditions were applied for treatment via advanced oxidation under the optimum conditions, as determined from the abovementioned study. One of the samples was subjected to UV radiation at different time intervals—10, 20, 30, 40, 50, 60, and 70 min—and the other sample was not. The concentration was measured for both samples at different times, and the removal efficiency was calculated as listed in Table 7. The removal efficiency was improved by using UV radiation and reached a maximum value of approximately 99% after 50 min.
Kinetics of the photocatalytic oxidation of M.B dye using nanomagnetite
To investigate the photocatalytic degradation abilities of nanomagnetite, a quasifirst-order kinetic model was applied to analyze the kinetics of dye degradation, and the correlation equation is expressed as follows34,35:
Co is the initial concentration of dye, C is the concentration of dye at any given time, and k is the rate constant. The linear relation between ln(C/Co) is represented by Fig. 20, and the rate constant k = 0.0316 min−1 is calculated from the slope of the line. The kinetic model is highly fitted to quasifirst-order kinetics, as R2 = 0.904.
Assessment of the reuse of synthesized magnetite nanoparticles
A stock of 20 ppm methylene blue solution was prepared, and advanced oxidation was applied for the treatment of synthetic wastewater. Ten samples were prepared at the same concentration. The optimum conditions were applied after 24 h. The solution was withdrawn, and fresh synthetic wastewater was added to the same dose of magnetite. This process was repeated ten times to measure the efficiency of using reused magnetite as a catalyst. As shown in Table 8, the removal efficiency decreased from 100 to 90% after using magnetite ten times, which supports that the treatment process is economical, as shown in Fig. 21.
Industrial samples
Industrial waste samples were taken from the industrial zone at the tenth Ramadan textile factory, and several experiments were conducted. The initial and final COD concentrations were 1500 PPM and 195 PPM, respectively, which corresponds to 87.5% removal efficiency at this pH 2, 1250 peroxide dose and 200 ppm magnetite.
Conclusion
Magnetite nanoparticles were prepared via the coprecipitation of ferrous and ferric oxides. The prepared nanomagnetite powder was characterized using XRD, which confirmed the formation of single-phase magnetite, and the zeta potential data indicated that the particles had a negative charge when dispersed in solution. TEM confirmed the formation of spherical nanoscale magnetite particles with an average size of 29.2 nm. The magnetic properties of the prepared sample were tested via VSM, which confirmed the attraction of the sample to a magnet with a magnetization of 60 (emu/g).
The prepared nanomagnetite powder was applied in wastewater contaminated with methylene blue dye using both adsorption and advanced oxidation techniques.
During the treatment of wastewater via adsorption, the highest removal efficiency (95.11%) was obtained by adding 50 mg of Fe3O4/L, 10 ppm dye conc, and a pH of 6.5. The adsorption results were fitted to the Langmuir model, which indicated that a monolayer was formed, and the Dubinin–Radushkevich isotherm had an activation energy of 2.23 kJ/mol, which indicated that the adsorption of M.B by nanomagnetite was a physisorption process. The adsorption kinetics and thermodynamics were studied, and the results showed that the pseudo first-order model was fitted with the experimental data, and the value of ΔH0 was positive, which indicated an endothermic reaction. The value of ΔS0 was positive, which indicated that the degrees of freedom increased at the solid–liquid interface during dye adsorption.
An advanced oxidation process using peroxide and magnetite nanoparticles was applied for the treatment of methylene blue dye solution (100 ppm). RSM was applied using different parameters: pH 2:11, 20:200 mg Fe3O4/L and 500:2000 ppm of peroxide. The highest removal efficiency (98.5%) was obtained by adding 110 PPM magnetite to 2000 ppm H2O2 at a pH = 11. The kinetic model was studied and found to be highly fit to quasifirst-order kinetics, with R2 = 0.904.
COD was measured for various samples, and the removal efficiency of the contaminated organic matter was 92.2% for the measured optimum sample. The magnetite was reused for ten cycles under optimum conditions, and the removal efficiency was reduced by 10%.
Data availability
Data is provided within the main manuscript file.
References
Pratap, B. et al. Wastewater generation and treatment by various eco-friendly technologies: Possible health hazards and further reuse for environmental safety. Chemosphere 313, 137547 (2023).
Herbst, W., Hunger, K., Wilker, G., Ohleier, H. & Winter, R. Industrial Organic Pigments (Wiley, 2004). https://doi.org/10.1002/3527602429.
Yerramilli, A., Chary, N. & Dasary, S. Decolorization of industrial effluents–available methods and emerging technologies–a review. Rev. Environ. Sci. Biotechnol. 4, 245–273 (2005).
Barakat, M. A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 4, 361–377 (2011).
Gosavi, V. & Sharma, S. A general review on various treatment methods for textile wastewater. J. Environ. Sci. Comput. Sci. Eng. Technol. 3, 29–39 (2013).
Nasseh, N., Khosravi, R., Mazari Moghaddam, N. S. & Rezania, S. Effect of UVC and UVA photocatalytic processes on tetracycline removal using CuS-coated magnetic activated carbon nanocomposite: A comparative study. Int. J. Environ. Res. Public Health 18, 11163 (2021).
Ubhi, M. K. et al. Insight into photocatalytic behavior of magnesium ferrite-bentonite nanocomposite for the degradation of organic contaminants. J. Mater. Res. 38, 990–1006 (2023).
Grewal, J. et al. Structural, magnetic, and photocatalytic properties of core–shell reversal nanocomposites of titanium-doped strontium ferrite and silica. J. Mater. Res. https://doi.org/10.1557/s43578-022-00855-0 (2023).
Brillas, E., Sirés, I. & Oturan, M. A. Electro-fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 109, 6570–6631 (2009).
Arellano, M., Sanromán, M. & Pazos, M. Electroassisted activation of peroxymonosulfate by iron-based minerals for the degradation of 1-butyl-1-methylpyrrolidinium chloride. Sep. Purif. Technol. 208, 34–41 (2018).
Sablas, M. M. et al. Percarbonate mediated advanced oxidation completely degrades recalcitrant pesticide imidacloprid: Role of reactive oxygen species and transformation products. Sep Purif. Technol. 250, 117269 (2020).
Al-Musawi, T. J., Sadat Mazari Moghaddam, N., Masoomeh Rahimi, S., Hajjizadeh, M. & Nasseh, N. Hexadecyltrimethylammonium-activated and zinc oxide-coated nanobentonite: A promising photocatalyst for tetracycline degradation. Sustain. Energy Technol. Assess. 53, 102451 (2022).
Ribeiro, A. R., Nunes, O. C., Pereira, M. F. R. & Silva, A. M. T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 75, 33–51 (2015).
Wang, J. & Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 334, 1502–1517 (2018).
Luo, H., Zeng, Y., He, D. & Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 407, 127191 (2021).
Khan, S., Sayed, M., Sohail, M., Shah, L. A. & Raja, M. A. Chapter 6-advanced oxidation and reduction processes. In Advances in Water Purification Techniques (ed. Ahuja, S.) 135–164 (Elsevier, 2019). https://doi.org/10.1016/B978-0-12-814790-0.00006-5.
Gao, L. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. https://doi.org/10.1038/nnano.2007.260 (2007).
Xue, X., Hanna, K., Abdelmoula, M. & Deng, N. Adsorption and oxidation of PCP on the surface of magnetite: Kinetic experiments and spectroscopic investigations. Appl. Catal. B 89, 432–440 (2009).
Azqandi, M., Shahryari, T., Fanaei, F. & Nasseh, N. Green construction of magnetic MnFe2O4/ZIF-8 nanocomposite utilizing extract of Melissa officinalis plant for the photodegradation of tetracycline under UV illumination. Catal. Commun. 185, 106798 (2023).
Vieira, Y. et al. New insights into the mechanism of heterogeneous activation of nano–magnetite by microwave irradiation for use as Fenton catalyst. J. Environ. Chem. Eng. 8, 103787 (2020).
Pérez-Poyatos, L., Morales-Torres, S., Maldonado-Hódar, F. J. & Pastrana Martinez, L. Magnetite nanoparticles as solar photo-fenton catalysts for the degradation of the 5-fluorouracil cytostatic drug. Nanomaterials 12, 4438 (2022).
Karmoker, J. R., Hasan, I., Ahmed, N., Saifuddin, M. & Reza, M. S. Development and optimization of acyclovir loaded mucoadhesive microspheres by box–behnken design. Dhaka Univ. J. Pharm. Sci. 18, 1–12 (2019).
Sarabia, L. A. & Ortiz, M. C. 1.12-response surface methodology. In Comprehensive Chemometrics (eds Brown, S. D. et al.) 345–390 (Elsevier, 2009). https://doi.org/10.1016/B978-044452701-1.00083-1.
Moslehi, M. H. et al. Statistical computational optimization approach for photocatalytic-ozonation decontamination of metronidazole in aqueous media using CuFe2O4/SiO2/ZnO nanocomposite. Environ. Res. 242, 117747 (2024).
Thabet, R. H., Fouad, M. K., Ali, I. A., El Sherbiney, S. A. & Tony, M. A. Magnetite-based nanoparticles as an efficient hybrid heterogeneous adsorption/oxidation process for reactive textile dye removal from wastewater matrix. Int. J. Environ. Anal. Chem. 103, 2636–2658 (2023).
Fadli, A. et al. Synthesis of magnetite nanoparticles via coprecipitation method. IOP Conf. Ser. Mater. Sci. Eng. 622, 012013 (2019).
Morsy, R. H. T. Sustainable Wastewater Treatment Based on the Principle of Advanced Oxidation by Rahman Hussein Thabet Morsy Sustainable Wastewater Treatment Based on the Principle of Advanced Oxidation (Cairo University, 2022).
Sureshkumar, V., Kiruba Daniel, S. C. G., Ruckmani, K. & Sivakumar, M. Fabrication of chitosan–magnetite nanocomposite strip for chromium removal. Appl. Nanosci. 6, 277–285 (2016).
Abdullah, N. H., Shameli, K., Etesami, M., Chan Abdullah, E. & Abdullah, L. C. Facile and green preparation of magnetite/zeolite nanocomposites for energy application in a single-step procedure. J. Alloys Compd. 719, 218–226 (2017).
Amarzadeh, M. et al. Statistical modeling optimization for antibiotics decomposition by ultrasound/electro-Fenton integrated process: Noncarcinogenic risk assessment of drinking water. J. Environ. Manage. 324, 116333 (2022).
Zaki, E. R., Ahmed, S. M., Ali, O. I. & Abdalla, M. S. Adsorption properties of magnetite nanoparticles for the removal of heavy metals from aqueous solution. Egypt. J. Appl. Sci. 37, 11–29 (2022).
Abate, G. Y., Alene, A. N., Habte, A. T. & Addis, Y. A. Adsorptive removal of basic green dye from aqueous solution using humic acid modified magnetite nanoparticles: Kinetics, equilibrium and thermodynamic studies. J. Polym. Environ. 29, 967–984 (2021).
Rahimian, R. & Zarinabadi, S. A review of studies on the removal of methylene blue dye from industrial wastewater using activated carbon adsorbents made from almond bark. Progress Chem. Biochem. Res. 3, 251–268 (2020).
Liu, Y., Liu, X., Zhao, Y. & Dionysiou, D. D. Aligned α-FeOOH nanorods anchored on a graphene oxide-carbon nanotubes aerogel can serve as an effective Fenton-like oxidation catalyst. Appl. Catal. B 213, 74–86 (2017).
Wang, C. et al. Synthesis and structure of semirigid tetracarboxylate copper(II) porous coordination polymers and their versatile high-efficiency catalytic dye degradation in neutral aqueous solution. Cryst. Growth Des. 16, 2277–2288 (2016).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.S. and A.M. prepared the nano material, S.S. and S.Y. have conducted experiments on wastewater, M.B. worked on RSM, S.T. worked on adsorption isotherm and kinetics in addition to writing this manuscript with K.H. the manuscript was revised by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aly, S.T., Saed, A., Mahmoud, A. et al. Preparation of magnetite nanoparticles and their application in the removal of methylene blue dye from wastewater. Sci Rep 14, 20100 (2024). https://doi.org/10.1038/s41598-024-69790-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-69790-w
Keywords
This article is cited by
-
Exploring chemically processed Symplocos racemosa sustainable material feasibility for sorptive amputation of methylene blue dye from waste water by green technology
Biomass Conversion and Biorefinery (2025)