Abstract
Nonalcoholic fatty liver disease (NAFLD) is expanding as a global health problem with approximately 25% of the world's population affected by it. Dietary modification is one of the most important strategies for preventing NAFLD. The association between nutrient density and the Healthy Eating Index 2015 (HEI2015) with NAFLD demonstrates that nutrient density is an independent predictor of NAFLD in Iranian adults [fully adjusted model: OR (95% CI)tertile3vs.1: 0.68 (0.54–0.85), P for trend = 0.001]. However, a favorable association between NAFDL and diet quality (HEI 2015) is more pronounced in participants with abdominal obesity [fully adjusted model: OR (95% CI)tertile3vs.1: 0.63 (0.41–0.98), P for trend = 0.03]. Based on the gender-stratified path analysis, diet quality indirectly through Waist-to-Height Ratio (WHtR), C-reactive protein (CRP), and metabolic syndrome in women, and men through WHtR, hemoglobin A1c (HBA1c), CRP, and metabolic syndrome affects NAFLD. Nutrient density directly and indirectly in women through WHtR, CRP, and metabolic syndrome, and in men indirectly through WHtR, hemoglobin A1c, and metabolic syndrome negatively affect NAFLD. Hence, in these subjects; we can provide early dietary intervention and education to prevent progression to NAFLD.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is expanding as a global health problem in developed and developing countries1 with approximately 25% of the world's population affected by it. NAFLD and its progression can lead to liver cirrhosis or even liver cancer2. By 2025, it will be the major cause of liver transplants in America 3. Studies also provide evidence that NAFLD may operate as a stand-alone risk factor for cardiovascular disease mortality and morbidity4, therefore, it is important to understand the risk factors for NAFLD.
In the past, this disease was defined as the accumulation of fat in the liver, which was diagnosed by imaging methods or pathology, without excessive alcohol consumption or any other causes resulting in secondary hepatic fat infiltration5,6. But recently, a new definition has been considered, describing NAFLD as a metabolic-associated fatty liver disease (MAFLD) disease. In this new definition, besides the accumulation of fat in the liver, one of the following three factors is also necessary for diagnosing the disease: obesity or overweight, type 2 diabetes, or evidence of metabolic disorders7. This new definition shows a clearer picture of the relationship between this disease and many lifestyle-related factors.
Lifestyle modification is the first line of treatment for NAFLD, and adherence to a healthy diet is one of the main treatment pillars8. Studies have shown that improving diet quality plays an important role in the prevention and treatment of NAFLD. Diet can be effective through weight control, treatment of metabolic syndrome and cardiovascular risk factors, and improving the antioxidant capacity of the body9.
There are different indicators for examining diet quality10,11. Two of these indicators are Healthy Eating Index 2015 (HEI2015)12 and Nutrient-Rich Food 9.3. (NRF9.3) scores that can give us an accurate estimation of diet quality13.
HEI2015 index examines dietary adherence of people to the American Dietary Guideline (ADG)14, and previous studies indicate that the improvement of this index is associated with an improvement of NALD as well as the risk of cardiovascular diseases, cancer, type 2 diabetes, all-cause mortality15,16. NRF9.3 index, which is used to measure nutrient density in the diet, includes three nutrients to limit (saturated fat, added sugar, sodium) and nine nutrients to encourage (protein, fiber, vitamins A, C, D, calcium, magnesium, potassium, and iron). The results of the evidence show that affordable nutrient-rich dietary patterns are linked to preventing disease and/or positively influencing health.
NAFLD is a multifactorial disease. Besides diet, various risk factors, such as demographic, environmental, lifestyle, and clinical factors, contribute to its development and progression.17,18. NAFLD has garnered attention for its associations with metabolic abnormalities and cardiovascular risks. Specifically, C-reactive protein (CRP) and glycated hemoglobin (HbA1c) have been of interest as potential biomarkers for systemic inflammation and dysregulated glucose metabolism, respectively19,20,21. Extensive research has focused on the relationship between NAFLD and metabolic syndrome, a cluster of conditions like obesity, hypertension, dyslipidemia, and insulin resistance. Those with metabolic syndrome are more likely to develop NAFLD, emphasizing the complex interaction between liver fat buildup and metabolic irregularities22. Additionally, the impact of lifestyle choices, such as physical activity, on NAFLD risk has been a key area of investigation23. Studies suggest that regular exercise can mitigate hepatic fat accumulation and improve insulin sensitivity, potentially serving as a therapeutic strategy for managing NAFLD24.
Based on the literature review (Supplementary file 1), we proposed a schematic pathway involving interactions among HEI2015, NRF9.3, NAFLD, and possible mediating variables such as anthropometric parameters, and cardiometabolic risk factors (Fig. 1).
It is important to understand how these factors interact with each other to cause NAFLD to design the most effective interventions.
Path analysis appears well-suited to address the limitations of multiple regression models. This method places all effective, independent and dependent variables in a model, simultaneously examines how these variables relate to the outcome, detects missing paths, and computes indices for overall goodness of fit25. Path analysis has been used in a few studies on the association between diet quality and NAFLD26. Due to the diversity in dietary patterns of different countries, in this study, we intended to use the Path analysis method to evaluate the association between HEI2015, NRF9.3, NAFLD, and possible mediating factors in a community-based cross-sectional study, Amol, Iran (Fig. 2).
Results
Baseline characteristics of the study participants
Of 2956 (47.09 ± 14.46) recruited in the cohort, 1332 (45.1%) were women. The prevalence of NAFLD was 46% (n = 1360). Details of socio-demographic, anthropometric, biochemical parameters, physical activity and dietary assessment according to gender and disease status are presented in Tables 1 and 2. NAFLD participants had a higher BMI, WC, and WHtR compared to healthy adults. Compared to those without NAFLD, diabetes and metabolic syndrome comorbidities were much more highly prevalent in patients with NAFLD (all P < 0.001). In women with NAFLD, the medication history approached significance. This was significant for men who used glucose-lowering agents (p = 0.001). In both genders, there were high levels of TG, total cholesterol, and LDL in patients with NAFLD, whereas HDL levels were significantly lower in patients with NAFLD compared to patients without NAFLD. There was no difference between the groups in other variables.
The scores of nutrient adequacy (p < 0.001) and healthy eating index (p = 0.01) were significantly lower in women with NAFLD than in healthy women. Despite the low score of the healthy eating index and nutritional adequacy in men with NAFLD compared with healthy subjects, these differences were not significant (p > 0.05).
Additional baseline characteristics and dietary assessments across tertile categories of each dietary index (HEI2015 and NRF9.3) stratified based on gender and NAFLD status are included in Supplementary Tables 1 & 2. A comparison of anthropometric and biochemical parameters across categories of HEI2015 showed that in female participants, a higher HEI2015 score was associated with lower rates of abdominal obesity (p < 0.001), lower HBA1c (p = 0.04), and a lower intake of dietary energy density (DED) (p < 0.001).
Higher HEI2015 and NRF9.3 scores were associated with lower diastolic blood pressure among men compared to women (p = 0.02 and p = 0.01, respectively). A less energy-dense diet was also associated with higher HEI2015 and NRF9.3 scores (p < 0.001) in both genders.
Association among healthy eating index and nutrient density with NAFLD
The multiple-adjusted odds ratio (95% confidence interval) in Tables 3 and 4 demonstrated an inverse association of nutrient density with NAFLD for the highest (vs. lowest) tertile of NRF9.3 [0.68 (0.54–0.85), P for trend = 0.001]. When stratified by gender and abdominal obesity, greater nutrient adequacy was associated with a lower risk of NAFLD in participants with abdominal obesity [0.62 (0.40–0.95), P for trend = 0.03] compared to those without abdominal obesity [0.69 (0.52–0.90), P for trend = 0.007].
The results for nutrient quality and lower odds of NAFLD prevalence in both genders were also similar [in men: 0.68 (0.50–0.93), P for trend = 0.01; in women, 0.64 (0.46–0.89), P for trend = 0.01]. The findings of the full multiple-adjusted model of HEI2015, stratified by gender and abdominal obesity, showed that the favorable association was only presented on subjects with abdominal obesity [0.63, (0.41–0.98), P for trend = 0.03].
The link between Nutrient Density and HEI2015 with NAFLD and its determinants using path analysis
The best-fit/final mediation model of HEI2015 showed a good-fitting model: χ2/df = 4.08, P = 0.003, GFI = 0.999, AGFI = 0.975, CFI = 0.995, IFI = 0.995, SRMR = 0.015 and RMSEA = 0.032 (Fig. 3).
The final path analysis model for the relationship between HEI2015 and NAFLD. The numbers on the paths represent standardized regression coefficients (standardized effects). The bold-face coefficients represent values for women, and the coefficients below them represent values for men. The significance level of the comparison of each effect between men and women is depicted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Pink arrows refer to females whereas blue arrows refer to males. HEI Healthy Eating Index, HBA1c Hemoglobin A1c, MetS Metabolic syndrome, CRP c-reactive protein, NAFLD non-alcoholic fatty liver disease.
In both genders, WHtR was a risk factor for CRP (women: standardized β coefficient = 0.19, p < 0.001; men: standardized β coefficient = 0.11, p < 0.001), metabolic syndrome (women: standardized β coefficient = 0.34, p < 0.001; men: standardized β coefficient = 0.36, p < 0.001), and NAFLD (women: standardized β coefficient = 0.36, p < 0.001; men: standardized β coefficient = 0.46, p < 0.001), and was positively associated with HBA1c only in men (standardized β coefficient = 0.11, p < 0.001). Metabolic syndrome in both men and women was a risk factor for NAFLD (women: standardized β coefficient = 0.21, p < 0.001; men: standardized β coefficient = 0.15, p < 0.001). WHtR partially mediated the protective effects of HEI2015 on NAFLD (women: standardized β coefficient = –0.08, p < 0.001; men: standardized β coefficient = –0.05, p < 0.001).
The indirect relationship between HEI2015 and NAFLD was explained through WHtR, and metabolic syndrome, as well as WHtR, CRP, and metabolic syndrome in women. In men, these effects were partially mediated through WHtR, and metabolic syndrome, as well as WHtR, HBA1c, and metabolic syndrome.
In the final model of NRF9.3 in both genders, metabolic syndrome (women: β standardized coefficient = 0.21, p < 0.001; men: standardized β coefficient = 0.25, p < 0.001) was directly related to NAFLD.
WHtR was a risk factor for metabolic syndrome (women: standardized β coefficient = 0.34, p < 0.001; men: standardized β coefficient = 0.36, p < 0.001) and NAFLD (women: standardized β coefficient = 0.36, p < 0.001; men: standardized β coefficient = 0.47, p < 0.001), and there was a direct effect of NRF9.3 on WHtR in both genders.
In women, NRF9.3 directly (standardized β coefficient = –0.08, p < 0.001) and indirectly through mediating factors of HbA1c (standardized β coefficient = –0.06, p < 0.001), and metabolic syndrome (standardized β coefficient of HBA1c to metabolic syndrome = –0.13, p < 0.001) affected NAFLD. In contrast, in men, the protective effect of NRF9.3 on NAFLD only was exerted directly (standardized β coefficient = –0.09, p < 0.001).
The best-fit mediation model of NRF9.3 showed a well-fitting to the data: χ2/df = 3.97, P = 0.019, GFI = 0.999, AGFI = 0.976, CFI = 0.998, IFI = 0.998, SRMR = 0.012 and RMSEA = 0.032 (Fig. 4).
The final path analysis model for the relationship between NRF9.3 and NAFLD. The numbers on the paths represent standardized regression coefficients (standardized effects). The bold-face coefficients represent values for women, and the coefficients below them represent values for men. The significance level of the comparison of each effect between men and women is depicted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Pink arrows refer to females whereas blue arrows refer to males. NRF9.3, Nutrient-Rich Food Index 9.3, HBA1c Hemoglobin A1c, MetS Metabolic syndrome, CRP c-reactive protein, NAFLD non-alcoholic fatty liver disease.
The standardized path coefficients (β), standardized total effects, along with direct and indirect effects concerning HEI2015 and NRF9.3 among the participants of the study were documented in Supplementary Table 3.
Discussion
Association between nutrient density and diet quality with NAFLD
In the current cross-sectional study, nutrient density (high intake of vitamin A, vitamin C, calcium, potassium, and iron, and the low consumption of sodium and saturated fatty acid) was an independent predictor of reduced prevalence for NAFLD in adult Iranians. The association between diet quality and NAFDL was pronounced in participants with abdominal obesity, where better compliance with the healthy eating index was markedly linked to a lower risk of NAFLD.
Although previous studies have explored the role of individual nutrients in the development, progression, and treatment of NAFLD, nutrient pattern as a total has not been investigated27,28.
Total NRF scores (nutrient adequacy) significantly reduced the NAFLD risk by almost 50% in both genders, although there were differences for individual items between patients with NAFLD vs. those without NAFLD. In the current study, low overall intake of nutrients, including vitamin A, vitamin C, potassium, calcium, and iron, and the high consumption of sodium and saturated fatty acid in NAFLD participants, suggest lower the micronutrient density, particularly in women. This pattern was similar only to Iron and SFA among men (Supplementary Table 2).
These findings have been confirmed in other studies29. Panera et al. argued that the composition of the macro and micronutrients is more critical in the etiology and management of NAFLD than total calorie intake30. Imamura et al., in a systematic assessment of males and females in 187 countries, concluded that females had better dietary patterns than males31. However, there is a dearth of evidence indicating gender differences in the association between diet quality and adequacy and NAFLD risk, and the results remain controversial.
According to the HEI components, whole fruit intake was significantly lower in the women with NAFLD than the healthy women, which may suggest that fruit intake reduced NAFLD risk by functioning as a source of vitamin C, vitamin A, calcium, potassium, and dietary fiber32. NAFLD is associated with oxidative stress and low-grade inflammation33. Micronutrient adequacy protects hepatic cells from lipotoxicity-induced oxidative stress. This status can promote trigger inflammation known to contribute to metabolic dysfunction and disturbing vitamin E trafficking through the gut axis34. A higher intake of vitamin C, vitamin A, calcium, and potassium is a major preventive nutrient for metabolic syndrome and NAFLD35. Van Tien et al., in a Multi-institutional Collaborative Cohort of the Japanese population using 1588 subjects, proposed that a nutrient diet rich in vitamins, fiber, iron, and potassium was linked to a lower prevalence of NAFLD36. A cohort study assessing dietary intake in NAFLD patients found that recommended intakes of calcium, vitamin A, iron, vitamin B1, vitamin B2, zinc, and magnesium were not met37. Aktary et al.38, in a cross-sectional investigation of the dietary intake and health profile of a sample of Canadian adults with NAFLD (n = 42), demonstrated that NAFLD patients had poor micronutrients such as magnesium, calcium, vitamin D, and vitamin E. The increased consumption of saturated fatty acid has been shown to cause mitochondrial dysfunction, increased oxidative stress, and low-grade inflammation39. Moreover, a recent meta-analysis documented a positive association between high sodium consumption and a 60% greater risk of NAFLD40.
In both genders, NAFLD patients significantly had a lower intake of iron and higher consumption of saturated fatty acids than healthy participants did. The association between select micronutrients and NAFDL warrants further investigation.
The association between diet quality and NAFLD aligned with those in a US population-based study (n = 10,858, mean age = 42.9 years, 47.1% men)40. Women (healthy vs. NAFDL participants) had more consumption of seafood, plant proteins, and whole fruits, while healthy men consumed better fatty acid composition (greater ratio of polyunsaturated (PUFAs) and monounsaturated fatty acids (MUFAs) to SFA) relative to NAFLD participants.
The reason the relationship was significant only among those with abdominal obesity might be that these participants were more prone to have NAFLD41, and weight loss as primary therapy for most NAFLD patients has been documented42. Improving diet quality, for example, incorporating more vegetables, whole fruits, whole grains, and limiting the content of the SFA sources, may have important benefits in preventing weight gain or promoting weight loss in adults of both genders and even being predisposed to obesity43,44. Thus, implementing healthy dietary patterns in the clinical setting may be a viable alternative to encourage weight loss and reduce the risk of developing NAFLD; however, more research is required to identify short-term and long-term beneficial effects of these dietary interventions in the context of NAFLD.
Considering these findings, primary prevention of NAFLD by improving micronutrient adequacy may be a more effective and beneficial objective for the patient at risk of developing NAFLD.
Influencing factors of NAFLD
Based on the gender-stratified path analysis, in women, diet quality indirectly through WHtR, CRP, and metabolic syndrome, and in men through WHtR, Hemoglobin A1c, and metabolic syndrome affected NAFLD.
While nutrient density directly and indirectly (through HBA1c and metabolic syndrome) reduced NAFLD risk in women, it only had a direct protective effect on NAFLD in men.
The current findings apply to understanding how habitual diet shapes anthropometric indices, metabolic risk profiles, and health outcomes in the Iranian surveyed population.
The findings indicate age is a significant predictor of HEI, physical activity, cardiometabolic parameters (e.g., WHtR, HBA1c), and NAFLD risk. Previous studies revealed differences in lifestyle factors (e.g., eating behaviors and physical activity) based on age and gender45. Age directly influences diet quality, and is a significant predictor of physical activity46. Research findings affirmed that increased physical activity was correlated with better diet quality measured by HEI 201547,48.
NAFLD risk has been more prevalent in older people49. Asian studies also reported that under the age of 50 years, NAFLD was more prevalent in men, but in populations over 50 years, it was higher in women50,51. Processes associated with aging are considered possible contributing mechanisms in the pathogenesis of NAFLD and cardiometabolic disorders52.
In line with our results, growing evidence demonstrated obesity and metabolic syndrome were independently linked with NAFLD irrespective of other cardiometabolic risk factors53,54,55. The NAFLD's pathophysiology concerning obesity involves excess fat deposition in the liver and insulin resistance development, which are pivotal in the progression of NAFLD56. In a recent study, the correlation between cardiometabolic disorders and inflammation and the incidence of NAFLD was verified57.
Evaluating overall diet quality, rather than specific nutrients or food components, is more effective in identifying diet-disease associations5. HEI is an indicator of determining the nutritional balance and predicting health risks. However, there are discrepancies in the risk prediction of disease in diet quality because of unmeasured interactions with various effects of modifiers or mediators41. Furthermore, the relationship and interrelationship between diet quality and health risk cannot be precisely calculated using common statistical methods. Path analysis may help evaluate this connection within a conceptual framework by concurrently investigating all relevant regression pathways, including direct and indirect7. Applying Path analysis makes it easier to assess the mediating role of diet quality and adequacy, anthropometric and metabolic parameters, and NAFLD risk. Additionally, this method permits a thorough understanding of such a relationship and allows a more precise interpretation of results.
A higher HEI score signifies a more balanced nutritional intake, leading to improved insulin sensitivity, reduced inflammatory markers, and a decreased likelihood of metabolic syndrome58. The NRF index, unlike the HEI, evaluates individual foods and simultaneously provides a precise measure of overall diet nutrient density. Individuals can meet their nutrient requirements without excessive energy intake and gaining weight by choosing nutrient-dense foods59. Moreover, the NRF index prioritizes nutrients critical for preventing metabolic disorders and NAFLD60,61.
Although the rationale for WHtR, CRP, and HBA1c as strong predictors for NAFLD has been justified by several previous studies40, to our knowledge there is no research on metabolic dysfunction being a mediator of NAFLD as the present study is the only Path analysis modeling study in this regard. A meta-analysis reported the superiority of centralized obesity measures, particularly, WHtR, for NAFLD risk detection62. Evidence has shown that visceral adiposity is the main adipose depot responsible for fatty liver and is associated with it in a dose-dependent manner37. In the present study, high WHtR values were associated with CRP and HBA1c. Several epidemiologic studies have proved the causal link between obesity and increasing liver disease in individuals63. According to pathophysiology and clinical studies, the progression of NAFLD is caused by an imbalance between lipid intake and disposal, which leads to oxidative stress and hepatocyte injury63. This finding is important to broaden the discussion about the high level of early inflammatory markers in obese adults and clarify this relationship. CRP acts as a regulator of nitric oxide production in the endothelium and coordinates the production and secretion of various cytokines, increasing the pro-inflammatory activity of different adipokines. The measurement of CRP and HBA1c were independent predictors of metabolic syndrome in other cohort studies (OR 1.22, 95% CI; 1.12 to 1.35; OR 1.57, 95% CI; 1.35 to 1. 82, respectively)6,8. MetS was associated with oxidative stress and chronic low-grade inflammation63, and NAFLD is one criterion of MetS30.
Additionally, our results revealed a gender inequalities association of diet quality and adequacy with anthropometric and metabolic parameters and NAFLD. The beneficial effects of nutrient adequacy of NAFLD risk in women, directly and indirectly through changes in HBA1c and metabolic syndrome, and in men directly were potentially exerted.
These results reveal the importance of gender-specific interventions to control NAFLD and also the pivotal role of diet adequacy and adequacy in obesity and glycemic control and preventing low-grade systemic inflammation and metabolic syndrome among high-risk individuals.
Key strengths of the study include the relatively large community-based study sample recruited from rural and urban areas of Amol city that afforded us sufficient power to probe small effects, incorporating multiple potential anthropometric and biochemical mediators, and assessing their mediation role simultaneously in the relationship between diet quality and adequacy and the risk of NAFLD for the first time, using a reliable and validated semi-quantitative FFQ64 developed for the Iranian population, which results in a better representation of the participants' dietary habits. However, some potential limitations of this study need to be acknowledged. First, because of the cross-sectional design of the study, drawing any causal inference from the association would be incorrect. Second, although liver biopsy is a gold standard for diagnosing NAFLD, we used sonography for evaluating NAFLD due to the risks associated with liver biopsy and the impossibility of applying it in population-based studies. Furthermore, the sensitivity of the ultrasound for the detection of moderate to severe fatty liver is approximately 85%, which keeps it a preferred and practical modality for diagnosing NAFLD in epidemiological settings. Third, since dietary intake and other socio-demographic parameters in Amol may differ from those in other parts of the country, our results cannot be extended to all Iranians. Fourth, other effective factors, including meal and snack patterns and cooking methods, were not investigated in the current study, so the observed associations are not entirely explained. Finally, we could not completely rule out residual confounding due to unknown or unmeasured confounders in this study.
Conclusion
Nutrient density was an independent predictor of NAFLD prevalence in Iranian adults. The association between diet quality (assessed by the HEI2015) and NAFDL was more pronounced in participants with abdominal obesity. The beneficial indirect effects of diet quality and nutrient density on NAFLD prevention were mediated by changing WHtR, HBA1c, CRP, and metabolic syndrome. Therefore, for subjects with MetS, high WHtR, high HBA1c, and CRP, we can provide early dietary intervention and proper education to prevent progression to NAFLD. Future research assessing the longitudinal relationship using prospective study designs is needed to better understand these relationships and confirm the findings in the present study.
Methods
Study design, setting, and participants
This cross-sectional study was conducted within the framework of the Amol Cohort Study (AmolCS), a prospective study conducted on rural and urban residents of Amol City in the North of Iran, which evaluated obesity-related metabolic disorders and CVD. The AmolCS was set up in two phases. In the first phase started in 2009, 7104 participants aged 10–90 years through sixteen strata with ten-year intervals (10–19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80–89 years) were randomly selected across rural and urban health centers of Amol city. The second phase of the AmolCS, including 5147 adult participants ≥ 18 years of age, was launched in 2017, and the data from the second phase of the cohort study were used in the present analysis.
The exclusion criteria for the participants were pregnancy/lactation, following a specific dietary or physical activity regimen, history of disease including Wilson's disease, autoimmune liver disease, hemochromatosis, virus infection, alcoholic fatty liver, malignancy, thyroid disorder, and autoimmune diseases, as well as participants with significant alcohol consumption (> 30 g/d for men and > 20 g/d for women). Written informed consent was obtained from all participants before the study. Further details on the project are available in the previous studies65,66.
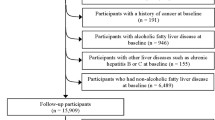
In total, 2956 subjects, including 1332 women (45.1%) and 1624 men (54.9%), were evaluated after excluding missing data for the abdominal ultrasonography (n = 166), covariates (n = 186), the food frequency questionnaire (n = 249), and misreported energy intake values (n = 492).
The study design and selection flowchart are outlined in Fig. 2. Approval for this study was received from the Iran University of Medical Sciences (IUMS) ethics committee (NO: IR.IUMS.REC.1399.1393).
Data collection
Written and verbal informed consent was obtained from participants. The documentation of participants' information in the second phase of the cohort study included demographic and lifestyle characteristics, clinical testing results, dietary assessment, and NAFLD diagnosis.
Dietary assessment
A validated semi-quantitative food-frequency questionnaire (FFQ) was used to evaluate the habitual intake of 168 food items64. For each food item on the list, participants were asked about the usual frequency of consumption in a commonly used unit or portion size (daily, weekly, and monthly) over the previous year. The consumption intake of each food item was calculated as grams/day by household measures67. Nutrient and energy intake was calculated using the food composition table (FCT) of the United States Department of Agriculture (USDA)68 and the Iranian FCT for traditional Iranian foodstuffs69.
Healthy eating index and nutrient density
In order to evaluate the quality of the diet, the Healthy Eating Index 2015 (HEI2015) was calculated using the method explained by the National Cancer Institute and the US Department of Agriculture (USDA) center70,71. In this index, nutritional intakes were compared with the US dietary guidelines. Scores could range between 0 and 100, with a higher score suggesting a healthier diet. The Nutrient Rich Food 9.3 score (NRF9.3) was calculated for the whole diet to measure nutrient density. Drewnowski et al.72 described the details of NRF9.3 calculation. In brief, the calculation of NRF9.3 is based on nine qualifying nutrients, including protein, fiber, vitamins A, C, and D, calcium, magnesium, potassium, and iron; and three disqualifying nutrients, including saturated fat, added sugar, and sodium. NRF9.3 was calculated as the sum of the percentage of the reference daily values (RDVs) for qualifying nutrients (NR9) minus the sum of the percentage of maximum recommended value (MRVs) for disqualifying nutrients (Lim3). All daily values calculated per 2000 kcal and the RDVs and MRVs suggested by Drewnowski et al. al. (based on several sources, i.e., WHO and FDA)72 were used in the present study.
Diagnosis and assessment of NAFLD
All the study subjects underwent ultrasonography of the abdomen to assess the hepatic parenchyma and biliary tree, performed by a single expert radiologist blinded to the clinical, and laboratory data of the participants using an ultrasound system (Esaote SpA, Genova, Italy) with transducer (frequency bandwidth 3–5 MHZ).
Laboratory testing
After an overnight fast of at least 12 h, intravenous blood samples of each participant were collected, with one tube for Ethylene Diamine Tetraacetic Acid (EDTA) anticoagulation and one tube for separation gel coagulation, and then centrifuged at 3000 rpm for 10 min at 4 °C; the aliquots were stored at -80 °C until use. Fasting blood sugar (FBS) was measured by the hexokinase method, and lipid profile containing total cholesterol (TC), high-density lipoprotein cholesterol (HDLc), and low-density lipoprotein cholesterol (LDLc) were measured by the enzymatic method using an Auto-analyzer BS200 (Mindray, Shenzhen, China) and diagnostic kits (Pars Azmoon Co., Tehran, Iran). Alanine transaminase (ALT), aspartate transaminase (AST), γ-glutamyl transaminase (GGT), and CRP were measured with a rating method.
Hepatitis B surface antibody, hepatitis B surface antigen, hepatitis C virus antibody, and hepatitis B core antibody were assessed by Enzyme-linked immunosorbent assay (ELIZA) kits (Pishtaz Teb Co., Tehran, Iran). Ten percent of the blood samples were re-evaluated by the Iranian National Reference Laboratory. The coefficients of variations ranged from 1.7% to 3.8% for all laboratory values.
Anthropometric variables and covariates
For each participant, covariates of demographic and lifestyle characteristics, smoking and alcohol drinking status, and physical activity were collected with the questionnaire-based interview. Data on physical activity was completed using the validated international physical activity questionnaire (IPAQ), as metabolic equivalent minutes per minute per week (MET-min/week)73.
Trained assistants employed medical equipment to measure height (cm), weight (kg), waist circumference (WC, cm), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Height and weight were measured with the subjects wearing light clothing and no shoes. Height was recorded at the nearest 0.1 cm and weight was to the nearest 0.1 kg. Body mass index (BMI) was computed as weight (kg) divided by height squared (m2). The girth of the midpoint between the lowest point of the rib and the upper edge of the iliac crest was calculated as waist circumference (WC). The WC measurement was taken to the nearest 0.1 cm. WHtR was respectively calculated as WC divided by height74.
Blood pressure was measured 2 times after at least 5 min of rest using the standardized desktop sphygmomanometer. The average blood pressure derived from two measurement readings was used75. All variables were collected according to standard interview guidelines and standard protocols75,76.
Data analysis
Descriptive analysis
Descriptive statistics included the frequency count (percent) for categorical variables and mean and standard deviation (SD) for continuous variables. The normality of continuous variables was evaluated by the Shapiro–Wilk statistical test. Baseline characteristics and dietary intake of the participants across tertile categories of each dietary index (HEI2015 and NRF9.3) were compared by conducting a one-way analysis of variance (ANOVA) with a Bonferroni post-hoc analysis to make multiple comparisons for continuous variables and chi-square test for categorical variables. The associations were adjusted for energy intake.
Logistic regression
To examine the association between nutrient density and healthy eating indices with NAFLD, multiple logistic regression was used in several models for all participants. The obtained findings were adjusted for confounding factors, including age, sex, WC, BMI, energy intake, physical activity, and smoking. Further adjustment for lowering serum lipid drugs, lowering hypertension (HPTN) drugs, lowering serum glucose drugs, residual areas, the presence of heart disease, and diabetes was applied in the last model. Stratified analyses by gender, as well as waist circumference status, were also conducted. We used tertile categories as an ordinal variable to assess the trend of odds ratios across increasing tertiles of dietary indxes scores. For potential confounding factors, a univariate analysis was applied, and those with a P-value for entry (Pe) lower than 0.20 were included in the final multiple models.
Path analysis model
A path analysis model was utilized to assess the hypothesized model. Path analysis is based on the maximum-likelihood estimation of the entire system of the hypothesized model and assesses the degree to which the data fits the specified model77. In the present analysis, we performed a two-step strategy outlined by Anderson and Gerbing78. The initial hypothesized model was evaluated by Path analysis to measure the fit and path coefficients. We computed the standardized regression weights, standardized total effects, as well as direct and indirect effects.
The goodness-of-fit indices of models and their corresponding suggested thresholds were: Goodness-of-fit Index (GFI) > 0.90, adjusted goodness-of-fit index (AGFI) > 0.90, comparative fit index (CFI) > 0.90, incremental fit index (IFI) ≥ 0.9, root-mean-square error of approximation (RMSEA) ≤ 0.08, and standardized root mean square residual (SRMR) < 0.0879,80.
In this study, the significance level was set at 5%, and all reported P-values are based on two-sided tests and the corresponding 95% confidence interval (CI). All the statistical analyses were done via SPSS version 24 (Statistical Package for Social Science, SPSS Inc, Chicago, IL, USA) software. A path analysis model was employed using AMOS 23.0 to build a measurement model and verify the structural relationship between nutrient density and healthy eating index with NAFLD.
Ethics statement
The current study was conducted according to the guidelines in the Declaration of Helsinki, and procedures involving human subjects/patients were approved by the Iran University of Medical Sciences (IUMS) ethical committee (No.IR.IUMS.REC.1399.1393). Written informed consent was obtained from all participants before the study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. Interested researchers may contact the corresponding author, Prof. Farhad Zamani, email address: zamani.farhad@gmail.com.
Abbreviations
- ADG:
-
American Dietary Guideline
- AGFI:
-
Adjusted goodness-of-fit index
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- BMI:
-
Body mass index
- CFI:
-
Comparative fit index
- CRP:
-
C-reactive protein
- DBP:
-
Diastolic blood pressure
- FBS:
-
Fasting blood sugar
- FCT:
-
Food composition table
- FFQ:
-
Food-frequency questionnaire
- GFI:
-
Goodness-of-fit Index
- GGT:
-
γ-Glutamyl transaminase
- HBA1c:
-
Hemoglobin A1c
- HDLc:
-
High-density lipoprotein cholesterol
- HEI2015 :
-
Healthy Eating Index 2015
- IFI:
-
Incremental fit index
- IPAQ:
-
International physical activity questionnaire
- LDLc:
-
Low-density lipoprotein cholesterol
- MetS:
-
Metabolic syndrome
- MRVs:
-
Maximum recommended value
- NRF9.3 :
-
NuFtrient-Rich Food 9.3
- MAFLD:
-
Metabolic-associated fatty liver disease
- NAFLD:
-
Nonalcoholic Fatty liver disease
- RDVs:
-
Reference daily values
- RMSEA:
-
Root-mean-square error of approximation
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- SRMR:
-
Standardized root mean square residual
- TC:
-
Total cholesterol
- USDA:
-
US Department of Agriculture
- WC:
-
Waist circumference
- WHtR:
-
Waist-to-height ratio
References
Wong, V.W.-S., Ekstedt, M., Wong, G.L.-H. & Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. https://doi.org/10.1016/j.jhep.2023.04.036 (2023).
Paik, J. M. et al. The burden of nonalcoholic fatty liver disease (NAFLD) is rapidly growing in every region of the world from 1990 to 2019. Hepatol. Commun. https://doi.org/10.1097/HC9.0000000000000251 (2023).
Feng, S., Roll, G. R., Rouhani, F. J. & Fueyo, A. S. The future of Liver Transplantation. Hepatology, 10.1097. https://doi.org/10.1097/HEP.0000000000000873 (2024).
Mantovani, A. et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 6, 903–913. https://doi.org/10.1016/S2468-1253(21)00308-3 (2021).
Nasreddine, L. et al. Dietary, lifestyle and socio-economic correlates of overweight, obesity and central adiposity in Lebanese children and adolescents. Nutrients 6, 1038–1062. https://doi.org/10.3390/nu6031038 (2014).
Lee, O., Lee, D.-C., Lee, S. & Kim, Y. S. Associations between physical activity and obesity defined by waist-to-height ratio and body mass index in the Korean population. PloS one https://doi.org/10.1371/journal.pone.0158245 (2016).
Hovestadt, I. et al. HbA1c percentiles and the association between BMI, age, gender, puberty, and HbA1c levels in healthy German children and adolescents. Pediatric Diabetes 23, 194–202. https://doi.org/10.1111/pedi.13297 (2022).
Mellergård, E., Johnsson, P. & Eek, F. Sociodemographic factors associated with HbA1c variability in type 2 diabetes: A prospective exploratory cohort study. BMC Endocr. Disord. 20, 1–8. https://doi.org/10.1186/s12902-020-00585-6 (2020).
Kim, J. H., Kim, H. L., Battushig, B. & Yoo, J. Y. Relationship between socio-demographics, body composition, emotional state, and social support on metabolic syndrome risk among adults in rural Mongolia. PloS one https://doi.org/10.1371/journal.pone.0254141 (2021).
Cai, J. et al. Waist-to-height ratio, an optimal anthropometric indicator for metabolic dysfunction associated fatty liver disease in the Western Chinese male population. Lipids Health Disease 20, 1–12. https://doi.org/10.1186/s12944-021-01568-9 (2021).
Summart, U. et al. Gender differences in the prevalence of nonalcoholic fatty liver disease in the Northeast of Thailand: a population-based cross-sectional study. F1000Research https://doi.org/10.12688/f1000research.12417.1 (2017).
Zhou, Y.-J. et al. Prevalence of fatty liver disease and its risk factors in the population of South China. World J. Gastroenterol. WJG 13, 6419. https://doi.org/10.3748/wjg.v13.i47.6419 (2007).
Helvacı, G., Kartal, F. T. & Ayhan, N. Y. Healthy Eating Index (HEI-2015) of Female College Students According to Obesity and Exercise Participation. J. Obesity Metabolic Syndrome 30, 296. https://doi.org/10.7570/jomes21018 (2021).
Garcia-Hermoso, A., Sanchez-Lopez, M., Escalante, Y., Saavedra, J. M. & Martinez-Vizcaino, V. Exercise-based interventions and C-reactive protein in overweight and obese youths: a meta-analysis of randomized controlled trials. Pediatric Res. 79, 522–527. https://doi.org/10.1038/pr.2015.274 (2016).
Cavero-Redondo, I. et al. The effect of physical activity interventions on glycosylated haemoglobin (HbA1c) in non-diabetic populations: a systematic review and meta-analysis. Sports Med. 48, 1151–1164. https://doi.org/10.1007/s40279-018-0861-0 (2018).
Yamaoka, K. & Tango, T. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med. 10, 1–10. https://doi.org/10.1186/1741-7015-10-138 (2012).
Lee, Y., Kang, D. & Lee, S.-A. Effect of dietary patterns on serum C-reactive protein level. Nutrition Metabol. Cardiovasc. Diseases 24, 1004–1011. https://doi.org/10.1016/j.numecd.2014.05.001 (2014).
Denova-Gutierrez, E., Tucker, K. L., Flores, M., Barquera, S. & Salmeron, J. Dietary patterns are associated with predicted cardiovascular disease risk in an urban Mexican adult population. J. Nutrition 146, 90–97. https://doi.org/10.3945/jn.115.217539 (2016).
Doustmohammadian, A. et al. The association between dietary inflammatory index (DII) scores and c-reactive protein (CRP) and nonalcoholic fatty liver disease (NAFLD) in a general population cohort. Clin. Nutrition ESPEN 60, 156–164. https://doi.org/10.1016/j.clnesp.2024.01.017 (2024).
Doustmohammadian, A. et al. Dietary Acid Load (DAL), Glycated Hemoglobin A1c (HbA1c), and Metabolic Syndrome (MeS) Mediate the Association of the Adherence to the Dietary Approaches to Stopping Hypertension (DASH) and Mediterranean Diet (MeD) With Nonalcoholic Fatty Liver Disease. Front. Nutrition https://doi.org/10.3389/fnut.2022.921415 (2022).
Alasadi, A., Humaish, H. H. & Al-hraishawi, H. Evaluation the predictors of non-alcoholic fatty liver disease (NAFLD) in type 2 diabetes mellitus (T2DM) patients. Syst. Rev. Pharm 11, 421–430. https://doi.org/10.31838/srp.2020.5.58 (2020).
Radu, F. et al. The link between NAFLD and metabolic syndrome. Diagnostics 13, 614. https://doi.org/10.3390/diagnostics13040614 (2023).
Chun, H. S. et al. Association of physical activity with risk of liver fibrosis, sarcopenia, and cardiovascular disease in nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2021.12.043 (2023).
Younossi, Z. M., Zelber-Sagi, S., Henry, L. & Gerber, L. H. Lifestyle interventions in nonalcoholic fatty liver disease. Nat. Rev.Gastroenterol. Hepatol. 20, 708–722. https://doi.org/10.1038/s41575-023-00800-4 (2023).
Streiner, D. L. Finding our way: an introduction to path analysis. Can. J. Psychiatry 50, 115–122. https://doi.org/10.1177/070674370505000207 (2005).
George, E. S. et al. Exploring the Path of Mediterranean Diet, Non-Alcoholic Fatty Liver Disease (NAFLD) and Inflammation towards 10-Year Cardiovascular Disease (CVD) Risk: The ATTICA Study 10-Year Follow-Up (2002–2012). Nutrients 14, 2367. https://doi.org/10.3390/nu14122367 (2022).
Xie, F., Zhou, H. & Wang, Y. Atherogenic index of plasma is a novel and strong predictor associated with fatty liver: A cross-sectional study in the Chinese Han population. Lipids Health Disease 18, 1–6. https://doi.org/10.1186/s12944-019-1112-6 (2019).
Alavian, S. M., Esmaillzadeh, A., Adibi, P. & Azadbakht, L. Dietary quality indices and biochemical parameters among patients with non alcoholic fatty liver disease (NAFLD). Hepatitis Monthly https://doi.org/10.5812/hepatmon.10943 (2013).
Byrne, C. D. & Targher, G. NAFLD: a multisystem disease. J. Hepatol. 62, S47–S64. https://doi.org/10.1016/j.jhep.2014.12.012 (2015).
Sy, R. G. et al. Socio-demographic factors and the prevalence of metabolic syndrome among Filipinos from the LIFECARE cohort. J. Atherosc. Thromb. 21, S9–S17. https://doi.org/10.5551/jat.21_Sup.1-S9 (2014).
Vahid, F., Rahmani, D. & Hekmatdoost, A. The association between dietary antioxidant index (DAI) and nonalcoholic fatty liver disease (NAFLD) onset; new findings from an incident case-control study. Clin. Nutrition ESPEN 41, 360–364. https://doi.org/10.1016/j.clnesp.2020.10.020 (2021).
Muthiah, M. D., Cheng Han, N. & Sanyal, A. J. A clinical overview of non-alcoholic fatty liver disease: a guide to diagnosis, the clinical features, and complications—what the non-specialist needs to know. Diabetes Obes. Metabolism 24, 3–14. https://doi.org/10.1111/dom.14521 (2022).
Wong, R. J. et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148, 547–555. https://doi.org/10.1053/j.gastro.2014.11.039 (2015).
Krebs-Smith, S. M. et al. Update of the healthy eating index: HEI-2015. J. Acad. Nutrition Dietetics 118, 1591–1602. https://doi.org/10.1016/j.jand.2018.05.021 (2018).
Chaturvedi, S. et al. Association of nutrient intake with non-alcoholic fatty liver disease and liver steatosis in adult Indian population–A case control study. Hum. Nutr. Metab. https://doi.org/10.1016/j.hnm.2023.200188 (2023).
Van Tien, N., Arisawa, K., Uemura, H. & Imaeda, N. Association Between Nutrient Patterns and Fatty Liver Index: Baseline Survey of the Japan Multi-Institutional Collaborative Cohort Study in Tokushima. Japan 32, 376–383. https://doi.org/10.2188/jea.JE20200447 (2022).
Vranešić Bender, D. et al. Nutritional status and nutrition quality in patients with non-alcoholic fatty liver disease. Acta Clin. Croatica 56, 625–634. https://doi.org/10.20471/acc.2017.56.04.07 (2017).
Franco, I. et al. Physical Activity and Low Glycemic Index Mediterranean Diet: Main and Modification Effects on NAFLD Score. Results from a Randomized Clinical Trial. Nutrients https://doi.org/10.3390/nu13010066 (2020).
Ge, X., Zheng, L., Wang, M., Du, Y. & Jiang, J. Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990–2017: A population-based observational study. BMJ open https://doi.org/10.1136/bmjopen-2019-036663 (2020).
Moraeus, L., Lindroos, A. K., Lemming, E. W. & Mattisson, I. Diet diversity score and healthy eating index in relation to diet quality and socio-demographic factors: results from a cross-sectional national dietary survey of Swedish adolescents. Public Health Nutrition 23, 1754–1765. https://doi.org/10.1017/S1368980019004671 (2020).
Man, S. et al. Association between metabolically healthy obesity and non-alcoholic fatty liver disease. Hepatol. Int. 16, 1412–1423. https://doi.org/10.1007/s12072-022-10395-8.10.1007/s12072-022-10395-8 (2022).
Younossi, Z. et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 69, 2672–2682. https://doi.org/10.1002/hep.30251 (2019).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209. https://doi.org/10.1016/j.jhep.2020.07.045 (2020).
Chalasani, N. et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67, 328–357. https://doi.org/10.1002/hep.29367 (2018).
Wrottesley, S. V. et al. Age and gender influence healthy eating and physical activity behaviours in South African adolescents and their caregivers: Transforming Adolescent Lives through Nutrition Initiative (TALENT). Public Health Nutrition 24, 5187–5206. https://doi.org/10.1017/S1368980019002829 (2021).
Rhodes, R. E. & Quinlan, A. Predictors of physical activity change among adults using observational designs. Sports Med. 45, 423–441. https://doi.org/10.1007/s40279-014-0275-6 (2015).
Xu, F. et al. Relationship between Diet Quality, Physical Activity and Health-Related Quality of Life in Older Adults: Findings from 2007–2014 National Health and Nutrition Examination Survey. J. Nutr. Health Aging 22, 1072–1079. https://doi.org/10.1007/s12603-018-1050-4 (2018).
Liang, J. et al. Association between joint physical activity and dietary quality and lower risk of depression symptoms in US adults: cross-sectional NHANES study. JMIR Public Health Surveillance https://doi.org/10.2196/45776 (2023).
Li, Y., Adeniji, N. T., Fan, W., Kunimoto, K. & Török, N. J. Non-alcoholic fatty liver disease and liver fibrosis during aging. Aging Disease 13, 1239. https://doi.org/10.14336/AD.2022.0318 (2022).
Wang, Z., Xu, M., Hu, Z., Hultström, M. & Lai, E. Sex-specific prevalence of fatty liver disease and associated metabolic factors in Wuhan, south central China. Eur. J gastroenterol. Hepatol. 26, 1015–1021. https://doi.org/10.1097/MEG.0000000000000151 (2014).
Yuan, L., Kardashian, A. & Sarkar, M. NAFLD in women: Unique pathways, biomarkers, and therapeutic opportunities. Curr. hepatol. Rep. 18, 425–432. https://doi.org/10.1007/s11901-019-00495-9 (2019).
Muzurović, E. et al. Nonalcoholic fatty liver disease and cardiovascular disease: A review of shared cardiometabolic risk factors. Hypertension 79, 1319–1326. https://doi.org/10.1161/HYPERTENSIONAHA.122.17982 (2022).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 92, 82–97. https://doi.org/10.1016/j.metabol.2018.11.014 (2019).
Kouvari, M. et al. Skeletal muscle mass and abdominal obesity are independent predictors of hepatic steatosis and interact to predict ten-year cardiovascular disease incidence: data from the ATTICA cohort study. Clinical Nutrition 41, 1281–1289. https://doi.org/10.1016/j.clnu.2022.03.022 (2022).
Yari, Z., Fotros, D. & Hekmatdoost, A. Comparison of cardiometabolic risk factors between obese and non-obese patients with nonalcoholic fatty liver disease. Sci. Rep. 13, 14531. https://doi.org/10.1038/s41598-023-41893-w (2023).
Fujii, H., Kawada, N. & Nafld, J. S. G. O. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 21, 3863. https://doi.org/10.3390/ijms21113863 (2020).
Aneni, E. C. et al. Cardiometabolic disorders, inflammation and the incidence of non-alcoholic fatty liver disease: A longitudinal study comparing lean and non-lean individuals. PloS one 17, e0266505. https://doi.org/10.1371/journal.pone.0266505 (2022).
Yuguang, L. et al. Inflammation mediates the relationship between diet quality assessed by healthy eating index-2015 and metabolic syndrome. Front. Endocrinol. 15, 1293850. https://doi.org/10.3389/fendo.2024.1293850 (2024).
Murakami, K., Livingstone, M. B. E., Fujiwara, A. & Sasaki, S. Application of the Healthy Eating Index-2015 and the Nutrient-Rich Food Index 9.3 for assessing overall diet quality in the Japanese context: different nutritional concerns from the US. PLoS One https://doi.org/10.1371/journal.pone.0228318 (2020).
Kramer, C. S. et al. The elderly-nutrient rich food score is associated with biochemical markers of nutritional status in European older adults. Front. Nutrition 6, 150. https://doi.org/10.3389/fnut.2019.00150 (2019).
Streppel, M. et al. Nutrient-rich foods, cardiovascular diseases and all-cause mortality: the Rotterdam study. Eur. J. Clin. Nutrition 68, 741–747. https://doi.org/10.1038/ejcn.2014.35 (2014).
Sluik, D., Streppel, M. T., van Lee, L., Geelen, A. & Feskens, E. J. Evaluation of a nutrient-rich food index score in the Netherlands. J. Nutr. Sci. https://doi.org/10.1017/jns.2015.4 (2015).
O’Donoghue, G. et al. Socio-economic determinants of physical activity across the life course: A" DEterminants of DIet and Physical ACtivity"(DEDIPAC) umbrella literature review. PloS one 13, e0190737. https://doi.org/10.1371/journal.pone.0190737 (2018).
Fung, T. T. et al. Long-Term Change in Diet Quality Is Associated with Body Weight Change in Men and Women. J. Nutr. 145, 1850–1856. https://doi.org/10.3945/jn.114.208785.10.3945/jn.114.208785 (2015).
Doustmohammadian, A. et al. Favorable association between Mediterranean diet (MeD) and DASH with NAFLD among Iranian adults of the Amol Cohort Study (AmolCS). Sci. ntific Rep. 12, 1–9. https://doi.org/10.1038/s41598-022-06035-8 (2022).
Motamed, N. et al. A population-based prospective study on obesity-related non-communicable diseases in northern Iran: rationale, study design, and baseline analysis. Front. Endocrinol. 15, 1329380. https://doi.org/10.21203/rs.3.rs-1266308/v1 (2023).
Ghaffarpour, M., Houshiar-Rad, A. & Kianfar, H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran Nashre Olume Keshavarzy 7, 42–58 (1999).
US Department of Agriculture Agricultural Research Service, Nutrient Data.http://www.ars.usda.gov/main/site_main.htm?modecode=12-35-45-00. (2011);
Azar, M. & Sarkisian, E. Food composition table of Iran (National Nutrition and Food Research Institute, Shaheed Beheshti University, 1980).
Reedy, J. et al. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutrition Dietetics 118, 1622–1633. https://doi.org/10.1016/j.jand.2018.05.019 (2018).
Ashoori, M. et al. Food and nutrition literacy: A predictor for diet quality and nutrient density among late adolescents. Turkish J. Pediatr. 65, 290–300. https://doi.org/10.24953/turkjped.2022.607 (2023).
Drewnowski, A. & Fulgoni, V. L. 3rd. New Nutrient Rich Food Nutrient Density Models That Include Nutrients and MyPlate Food Groups. Front. Nutrition 7, 107. https://doi.org/10.3389/fnut.2020.00107 (2020).
Hagströmer, M., Oja, P. & Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutrition 9, 755–762. https://doi.org/10.1079/PHN2005898 (2006).
Browning, L. M., Hsieh, S. D. & Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0· 5 could be a suitable global boundary value. Nutrition Res. Rev. 23, 247–269. https://doi.org/10.1017/S0954422410000144 (2010).
Schoettker, P. et al. Blood pressure measurements with the OptiBP smartphone app validated against reference auscultatory measurements. Sci. Rep. 10, 17827. https://doi.org/10.1038/s41598-020-74955-4 (2020).
Group, W. M. G. R. S. & de Onis, M. Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatrica 95, 38–46, https://doi.org/10.1111/j.1651-2227.2006.tb02374.x, (2006).
McCoach, D. B. SEM Isn’t just the Schoolwide Enrichment Model anymore: Structural Equation Modeling (SEM) in gifted education. J. Educ. Gifted 27, 36–61. https://doi.org/10.1177/016235320302700104 (2003).
Anderson, J. C. & Gerbing, D. W. Structural equation modeling in practice: A review and recommended two-step approach. Psychol. Psychol. Bull. 103, 411–423 (1988).
Bollen, K. A. A new incremental fit index for general structural equation models. Sociol. Methods. Res. 17, 303–316. https://doi.org/10.1177/0049124189017003004 (1989).
Ryu, E. Model fit evaluation in multilevel structural equation models. Front. Psychol. 5, 81. https://doi.org/10.3389/fpsyg.2014.00081 (2014).
Acknowledgements
We greatly appreciate the participants, healthcare executives in public health centers in Amol, and, the GILDRC staff (www.gildrc.ac.ir), without whom the study would not have been possible.
Funding
This research was conducted with a grant from the Gastrointestinal and Liver Diseases Research Center (GILDRC), Iran University of Medical Sciences (IUMS) (grant NO: 99–2-30–19054). The funder had no role in the design of the study, data collection, analysis, interpretation of data, and the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
FZ, NM, and AD were responsible for the study concept and design. AD, BA, and NM had full access to all data and took responsibility for the integrity of the data and the accuracy of the data analysis. MM and EG were involved in data collection. AD, NM, and SE analyzed and interpreted the data, and SC took responsibility for the accuracy of the SEM analysis. AD and BA wrote the initial draft of the manuscript. All authors revised the manuscript critically for important intellectual content and approved the final manuscript. FZ is the guarantor and takes responsibility for the paper as a whole.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doustmohammadian, A., Amirkalali, B., de Courten, B. et al. Path analysis model to identify the effect of poor diet quality on NAFLD among Iranian adults from Amol Cohort Study. Sci Rep 14, 19935 (2024). https://doi.org/10.1038/s41598-024-70181-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70181-4