Abstract
Vitamin D has shown antimicrobial effects. This study aimed to explore the antiviral effects of vitamin D3 on saliva samples collected from patients with coronavirus disease-19 (COVID-19) and compare saliva and swab results to aid in policy development. Saliva and swab samples were collected from adult patients with a positive test for COVID-19 at the King Faisal Specialist Hospital and Research Centre, Jeddah. Patients who were immunocompromised and pregnant and aged < 18 years were excluded. Vitamin D3 compound (100, 300, 800, and 1,200 IU) was added to the first saliva sample in the laboratory (n = 20); the rest of the swab specimens were compared with the saliva samples via real-time polymerase chain reaction. Of the 257 patients, 236 (94.8%) had positive saliva sample test results, 7 (2.8%) had errors, and 6 (2.4%) had negative results. Of the 236 positive tests, 235 (99.6%) had a cycle threshold (Ct) indicating strong positive reactions, and only one (Ct = 28.86) was weak. Among the 236 positive results, 235 (99.6%) exhibited robust positive reactions, indicating a substantial positive sample size. Thus, saliva might be a dependable alternative testing tool when obtaining swab samples from patients is inconvenient or challenging.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) pandemic poses an unparalleled challenge in medicine, fundamentally altering the course of history1. The novel severe acute respiratory syndrome 2 (SARS-CoV-2) was discovered in late 2019 and is part of an extensive family of coronaviruses responsible for various illnesses2. These illnesses span from an asymptomatic course to the common flu and can escalate to more severe conditions, including SARS and the potentially lethal Middle East respiratory syndrome. It affects mainly the respiratory system and is transmitted from animal to person and from person to another mainly through droplets, such as sneezing and coughing1.
Methods for viral detection include but are not limited to nasal or nasopharyngeal (NP) swabs3 and saliva4. Over the years, saliva has been shown to detect early stages of diseases, such as periodontal and autoimmune diseases, via its abundant biomarkers5,6,7, e.g., proteins and genetic components, hormones and infectious materials8 as it mirrors blood7,9. Extensive research show that COVID-19 is detected in saliva, thus shedding light on its invaluable uses10,11,12. Therefore, saliva is considered a diagnostic tool for periodontists and dental fields because of its noninvasiveness and greater acceptance of patients of all ages, particularly older adults, children, and patients with cancer than the gold standard (swabs)4. Oral presentations13 as discomfort, soreness, ulcerations, and dryness would reroute strategies toward innovative ways for increasing patient tolerance for diagnostic means. Moreover, the number of hospitalized patients during COVID-19 pandemic reached its peak in the Middle East. Thus the least invasive a sample obtaining is, the better outcome for patient acceptance and ease14
Studies have presented numerous advantages of vitamin D, including its antimicrobial properties, antiviral influences, and role in the immune system15. It regulates more than 200 genes that are involved in cell division, proliferation and apoptosis, and the synthesis of anti-microbial peptides (AMPs), cytokines, chemokines and interleukin responses that enhances innate immunity. It serves as the maestro for the respiratory process on a cellular level. This outlines the significance of vitamin D, being an antiviral agent16, a modulator of the innate17,18 and adaptive immune responses19. Many blood cells, e.g. macrophages and neutrophils may be recruited with the activation of viral neutralization by the active form of vitamin D (D3) and may prevent long-term innate immune response activation, through immunological tolerance induction. These signaling pathways have led to the hypothesis that adequate vitamin D levels20 may prevent a cytokine storm in COVID-19 patients. Accordingly, exploring the effect of vitamin D3 on saliva and swab samples in the laboratory would be novel.
Therefore, this study aimed to explore the antiviral effects of vitamin D3 on saliva samples10 collected from patients who tested positive to COVID-193, as a noninvasive screening tool. As the gold standard of practice is swab collection (NP), saliva and NP samples were compared to aid in making policy changes to better serve the patients.
Saliva collection is important in practice, particularly for dentists21, because most dental procedures require the use of rotary tools, which necessitate high-pressure water mist splatter. As a result, aerosols spread from the saliva to the environment10,22,23.
As COVID-19 has several manifestations, all clinics must practice utmost hygiene standards and ensure that all employees and patients are safe22,23.
Among the few studies on saliva24,25,26 one was published in June 202024. The study reported the effect of iodine (Povidone) as a mouthwash on saliva samples in patients with COVID-19. The study compared saliva samples before and after the use of povidone in the clinic, and the results showed positive outcomes. Thus, the povidone rinse was used before any procedure to minimize aerosol contamination in the dental clinic24. Others examined the effect of different mouthwashes on covid-19 samples either in vivo27 or in vitro, to assess its antiviral properties. Chlorhexidine also showed 99.9% in viral load reduction in covid-19 patients, when used in different mouthwash concentrations (0.2%, 0.12% and 0.1%) for 30 s28. Cetylpyridinium (CPC) or iodine‐povidone (PVP‐I) ‐based mouthwashes are also mentioned to lower SARS‐CoV‐2 viral load in droplets and aerosols produced during dental procedures29,30.
In conclusion, the use of different mouthwashes showed a decrease of SARS‐CoV‐2 infectivity and its variants with the mouthwashes tested27. In view of such reviews, chlorohexidine or iodine-based mouth washes are the commonly used in dental clinics or hospitals.
This study aimed to explore the effect of vitamin D3 (as a compound biomaterial) on COVID-19 samples, if any, and should there be positive veridical effects observed and evaluate the results of both nasal swab and saliva samples from patients with COVID-19 and compare similar outcomes. That is, if the results are equal or not, the proposed approach could aid in deciding whether saliva should be used solely as a noninvasive diagnostic tool.
Although numerous studies have investigated the implications of vitamin D3, research examining its direct effect on saliva and nasal swabs is limited. Thus, investigations to ascertain the effects of vitamin D3 on such samples in the context of COVID-19 are needed. Should vitamin D3 have any influence on saliva and/or nasal swab samples, it could introduce novel applications beyond existing formulations24,31. These may include utilization in the oral cavity as a mouth rinse and/or in the nasal cavity as a nasal spray. Consequently, laboratory studies should be conducted to experimentally assess the effect of vitamin D3 on COVID-19 samples in the aforementioned context.
Methods
Patients who tested positive for COVID-19 through nasal swab collection as the standard of practice at their respective departments (emergency room ER, family medicine FM, and inpatient) in King Faisal Specialist Hospital and Research Center (KFSH&RC), Jeddah were included in the study. In this study, nurses would communicate positive testing to the principal investigator from July 2021 until October 2023. Before sample collection, each patient was asked if they agreed to participate in the study after receiving a thorough explanation. Upon agreement, signed informed consent was obtained once at the collection time for both the nasal swab and whole-passive saliva, ensuring that sputum or phlegm were not collected.
Adult patients with a positive test for COVID-19 were included. Conversely, patients who had an immunocompromised stage, pregnant women, and patients aged < 18 years were excluded.
Samples were collected by patients with nursing assistance from each designated department, as follows:
Nasal swab sample collection
The protocol32 was established according to the Centers for Disease Control. The swab was placed after collection in a viral transport media (VTM) tube, sealed immediately after collection, and kept on the bench side for ≤ 1 day until it was used for laboratory transfer for RNA extraction and rapid antigen/antibody and/or reverse-transcription polymerase chain reaction (RT-PCR, Abbott Laboratories, IL, USA).
Saliva sample collection
Following standard precautionary hygiene measures, the patient was given a 10-mL collecting tube (Falcon). After informed consent obtained, the patient was asked to pool saliva in his/her mouth without swallowing the saliva. Once the mouth was full, the patient was instructed to lean his/her head forward to allow for the saliva to flow into33 (from the tip of the lip: a seal was created with the tube) the tube that contained 1–2 mL of VTM for sample stability. The tube was sealed, placed at the bench side for ≤ 1 day, and frozen at − 80 °C until RNA extraction. After which the RNA, was amplified via Abbott RT-PCR at the molecular laboratory of KFSHRC.
Vitamin D3 preparation
The biomaterials intended for analysis were selected from the pharmacy and prepared at the KFSHRC Research Center. Specific elements of the compounds were previously approved from the Formulary List at KFSHRC-J. Vitamin D3 was generated at different concentrations (100, 300, 800, and 1,200 IU), which were subsequently added to the samples in the laboratory.
Laboratory testing
The samples were collected and stored at − 80 °C for analysis to determine the effect of the D3 compound on the samples. Freezing for up to 90 days was the maximum time needed for sample stability. All samples were labeled, with a code that was used to identify each patient, and electronic documents were used to access the results.
The samples were tested for the presence of COVID-19 (Ct \(\le \) 27) by RT-qPCR using Abbott RTM2000, and positive samples were further tested. Moreover, the positive samples were subjected to different doses of vitamin D3 in the test group. In the control group, normal saline was added to the samples to ensure that the effect of vitamin D3 was the causal factor. Indeed, if a true veridical effect on the COVID-19-positive samples was found, no effect on the pilot (n = 20) samples was noted. Subsequently, the swab and saliva results were compared.
In the laboratory, viral RNA extraction was safely performed. Thereafter, D3 was added to the samples. The laboratory team was blinded to the grouping of the samples.
Because this study used one-time sample collection, no follow-up was needed. If home isolation was insufficient, patients with COVID-19 were managed by their respective doctors within their departments according to their needs.
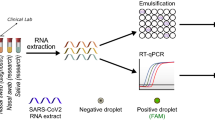
Workflow of RT-PCR-based diagnostic methods
After NP or oropharyngeal swab and saliva collection, the samples were transported to the molecular laboratory for storage and handling to preserve the viral RNA integrity. SARS-CoV-2 heat inactivation and RNA extraction were performed through custom or commercial protocols and viral RNA retro-transcription into double-stranded cDNA. Then, RT-PCR amplification and RT-fluorescent signal detection was performed, amplification signals were interpreted, and the positivity threshold was set.
Materials and samples
In this study, saliva was used for the detection of SARS-CoV-2 by RT-PCR. All saliva specimens were collected from patients with known positive results via PCR or antigen tests.
Saliva specimens were collected from suspected patients and subsequently placed in a sterile container that contained a universal virus transport medium to preserve the specimen. Initially, approximately 20 saliva specimens were tested for the presence of vitamin D3 compounds, and their corresponding NP swabs were positive. The remaining saliva specimens had no additional compounds.
Nucleic acid extraction
RNA was extracted using an ExiPrep™96 Lite device from BIONEER. First, a mixture of 2000 µL of protein kinase with 1,000 µL of the SARS-CoV-2 internal control was prepared, and this mixture was added to buffer cartridge 1 provided by the company. Then, to evaluate the validity of the assay, 200 µL of a negative or positive control solution (Abbott Laboratories) was dispensed, after which the same volume of the sample was loaded as a control. This step was performed in a negative pressure room inside a Biosafety Cabinet Level-2 type A2 while wearing PPE, including gloves, gowns, and N95 masks, because these samples were considered infectious. Then, accessories and cartridges were inserted into the machine. After the device was initialized and allowed to self-test, the samples were ready to run. Buffer cartridge 1 undergoes lysis, which breaks down the cell wall, allowing the release of viral RNA. Buffer cartridge 2 had silica-coated magnetic beads that bind to the viral RNA. Then, to remove any proteins or remaining particles, the bound RNA was washed several times using buffer cartridges from number 3 to number 6. Finally, the viral RNA was eluted using an elution buffer in cartridge 7. The extraction was performed in approximately 45 min.
Product amplification
After RNA extraction, cartridge buffer number 7, which contained the final product, was added to Abbott m2000sp for master mix addition. A master mix (Abbott Laboratories), which included SARS-CoV-2 oligonucleotide reagent, thermostable rTth polymerase enzyme, and activation reagent, was used. Using Abbott m2000sp, the master mix and aliquots of the nucleic acid samples were transferred to the Abbott 96-well optical reaction plate, which was subsequently placed in Abbott m2000rt to start amplification.
In Abbott m2000rt, SARS-CoV-2 and internal control reverse primers were hybridized to their targets and subsequently amplified in the presence of a Thermostable rTth polymerase Enzyme, which has reverse-transcriptase action to convert the target RNA into cDNA. The PCR steps used for denaturation, annealing, and extension were performed using the thermal cycler system m2000rt. In the denaturation step, the reaction temperature was raised above the melting point of the double-stranded cDNA product, after which the temperature was decreased to allow for the annealing of the second primer to cDNA. Afterward, the temperature was slightly increased to start the extension step using the rTth enzyme, which has DNA polymerase activity that provides double-stranded DNA. This cycle is repeated 35 times. Viral quantitative detection was possible using SARS-CoV-2 and internal control fluorescent probes, which had a fluorescent signal at the 5′ end and a quencher at the 3′ end and could hybridize to their targets. When the target RdRP was present, the fluorophore and quencher were separated, allowing for fluorescent detection.
Ethics and contributions
This study was approved by the Human Subjects Ethics Board of King Faisal Specialist Hospital and Research Centre, Jeddah (IRB approval no. 2020-25) and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Results
In total, 271 samples were collected, 256 were positive, 8 were negative, and 7 specimens yielded errors.
The results of the pilot study (n = 20) were negative to D3 additions on both NP and saliva samples (Table 1). In the additional 249 participants, 236 (94.8%) were positive, 7 (2.8%) resulted as errors, and 6 (2.4%) were negative.
Discussion
This study using a large sample provides a strong foundation for considering saliva as a reliable diagnostic tool. A total of 99.6% strong positive reactions indicate consistent and accurate detection of the target analyte in the saliva samples. This high success rate is particularly noteworthy, especially that this study was the first to initiate and establish saliva testing at KFSH&RC, J with the available resources and it indicates that saliva can be a dependable alternative when obtaining other sample types, such as blood or urine, becomes inconvenient or challenging, particularly in situations where patient cooperation is limited.
These encouraging results show that people may rely solely on saliva. However, to introduce this immature diagnostic tool, saliva testing may require further scrutiny and validation through comprehensive laboratory testing to establish the robustness of saliva-based testing protocols and facilitate a gradual shift in policy.
In addition to technical considerations, the success of saliva-based diagnostics pivots on patient acceptance and experience. Despite the appealing noninvasive nature of saliva collection, efforts should be directed toward educating both healthcare professionals and the general public about the efficacy and reliability of saliva-based tests. Enhancing patient confidence in this diagnostic approach is crucial for widespread adoption.
Another key consideration is the need for standardization and quality control measures in saliva collection and processing procedures. Standardized protocols can ensure consistency across different testing environments, mitigating potential variations that may arise because of differences in collection methods or storage conditions. Moreover, further research should focus on understanding the dynamics of analyte stability in saliva over time, addressing any potential degradation that may affect the accuracy of test results.
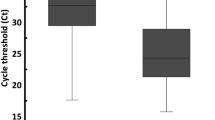
In this study, testing for the effect of D3 on COVID-19 in the first 20 samples (Fig. 1), enrolled between July 2021 and March 2022, where there was no effect, shows that the dynamics of all saliva proteins34 and mucosal cells may play a role if D3 was used as a mouthwash versus using the compound bench side. To further evaluate the effectiveness of saliva as a diagnostic test for COVID-19, 237 adults aged ≥ 18 years were recruited (Fig. 2) and enrolled from November 2022 until October 2023, and they provided saliva samples in addition to nasal swabs positive for COVID-19. This value was based on an estimate from previous studies showing that 81% of patients had a positive saliva sample 1–5 days after COVID-19 diagnosis3. All 237 patients had a positive nasal swab for COVID-19 and a saliva sample, including a cycle threshold (Ct) value for positive results. Therefore, the rest of the 249 samples were only used to compare NP swabs to saliva (Fig. 2) in such patients in whom D3 was not introduced to the samples.
The results of the (n = 20) show that vitamin D3 treatment did not affect NP and saliva samples were inconclusive (Table 1) and error results may be related to the high viscosity of the saliva specimens. With regard to the Ct values of the saliva tests, of the 236 positive tests, 235 (99.6%) showed strong positive reactions, and one was much weaker.
Reflecting on rt-PCR results between the samples that received D3 or did not, the following measures (Rdrp & N gene, E gene-19, and N gene-21 and its CT values), differed from NP swabs when compared to saliva. In most samples, saliva had higher values but differed between samples. Other samples were the opposite, and a few showed similar values in both samples. Perhaps such is dependable on the amount or consistency and nature of saliva samples received (1–2 ml) versus the NP swab samples, and the level of symptoms the patients expressed before sample collection, i.e. during or after the incubation period.
In a recent study, a high single dose of vitamin D3 was used on hospitalized covid-19 patients, as an intervention to observe any improvement in the length of stay in moderate to severe cases of such patients20,35. While in this study, four single doses were used, on each of the samples bench-side, where also there was no effect of vitamin D3 on covid-19 patients or samples.
Limitations of this study include the high viscosity of saliva in a few samples, thus may have given the results in “errors”, as well as the high demands on hospital staff during the pandemic with longer time to finish the study for patient recruitment. Despite such challenges, the sample size was sufficient to suggest saliva as a convenient alternative for such positive outcomes, when comparing between NP and saliva samples.
Conclusion
This study provides compelling evidence supporting the reliability of saliva as a diagnostic tool, particularly given the overwhelmingly high number of positive reactions observed. Therefore, saliva may be utilized as an alternative testing tool when obtaining relevant information from patients is inconvenient or challenging. Accordingly, whether we solely rely on saliva as a diagnostic tool is unclear. Thus, further laboratory testing is needed to establish such information for a gradual change in policy with greater patient acceptance and experience.
In summary, vitamin D3 had no effect on COVID-19 samples, therefore a future study to explore its effect as antiviral properties as a mouthwash in covid-19 patients would be an area for research.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Wu, F. et al. A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020).
Almaghrabi, R. S. et al. Outcome of SARS-CoV-2 variant breakthrough infection in fully immunized solid organ transplant recipients. J. Infect. Public Health 15(1), 51–55 (2022).
Wyllie, A. L. et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N. Engl. J. Med. 383, 1283–1286 (2020).
Tsujimoto, Y. et al. Diagnostic accuracy of nasopharyngeal swab, nasal swab and saliva swab samples for the detection of SARS-CoV-2 using RT-PCR. Infect. Dis. Lond. 53(8), 581–589 (2021).
Javaid, M. A., Ahmed, A. S., Durand, R. & Tran, S. D. Saliva as a diagnostic tool for oral and systemic diseases. J. Oral Biol. Craniofac. Res. 6(1), 66–75 (2016).
Lee, Y. H. & Wong, D. T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 22(4), 241–248 (2009).
Ghosh, S. et al. Role of saliva as a non-invasive diagnostic method for detection of COVID-19. Cureus 14(7), e27471 (2022).
Bibi, T. et al. Gingival crevicular fluid (GCF): A diagnostic tool for the detection of periodontal health and diseases. Molecules 26(5), 1208 (2021).
Han, P. & Ivanovski, S. Saliva-friend and foe in the COVID-19 outbreak. Diagnostics (Basel) 10, 290 (2020).
Czumbel, L. M. et al. Saliva as a candidate for COVID-19 diagnostic testing: A meta-analysis. Front. Med. 7, 465 (2020).
Yang, Q. et al. Saliva TwoStep for rapid detection of asymptomatic SARS-CoV-2 carriers. eLife 10, e65113 (2021).
Butler-Laporte, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2. JAMA Intern. Med. 181, 353 (2021).
Chen, X. et al. Oral health in adult patients receiving palliative care: A mixed method study. Am. J. Hosp. Palliat. Care 38(12), 1516–1525 (2021).
Al-Rajhi, A. et al. Data-driven prediction for COVID-19 severity in hospitalized patients. Int. J. Environ. Res. Public Health 19(5), 2958 (2022).
Beard, J. A., Bearden, B. & Striker, R. Vitamin D and the antiviral state. J. Clin. Virol. 50(3), 194–200 (2011).
Ghosh, J. Potential role of vitamin D as an antiviral agent. Med. J. Dr. D. Y. Patil Vidyapeeth 14(1), 3–12 (2021).
Liu, P. T. et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311(5768), 1770–1773 (2006).
Aglipay, M. et al. Effect of high-dose vs standard-dose wintertime vitamin D supplementation on viral upper respiratory tract infections in young healthy children. JAMA 318(3), 245–254 (2017).
Van Etten, E. & Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 97(1–2), 93–101 (2005).
Fernandes, A. L. et al. Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19. Am. J. Clin. Nutr. 115(3), 790–798 (2023).
Li, Y. et al. Saliva is a non-negligible factor in the spread of COVID-19. Mol. Oral Microbiol. 35, 141–145 (2020).
McCormick, W. L., Koster, M. P., Sood, G. N. & Mermel, L. A. Level of respiratory protection for healthcare workers caring for coronavirus disease 2019 (COVID-19) patients: A survey of hospital epidemiologists. Infect. Control Hosp. Epidemiol. 43(5), 681–683 (2022).
Singh, S. P., Singh, P., Gupta, O. P. & Gupta, S. Coronavirus disease: Dental review. J. Oral Maxillofac. Pathol. 26(1), 16–20 (2022).
Anderson, D. E. et al. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2 the virus causing COVID-19 disease. Infect. Dis. Ther. 9(3), 669–675 (2020).
Sbricoli, L. et al. Efficacy of different mouthwashes against COVID-19: A systematic review and network meta-analysis. Jpn. Dent. Sci. Rev. 59, 334–356 (2023).
Vergara-Buenaventura, A. & Castro-Ruiz, C. Use of mouthwashes against COVID-19 in dentistry. Br. J. Oral Maxillofac. Surg. 58(8), 924–927 (2020).
Buonavoglia, A. et al. In vitro virucidal activity of mouthwashes on SARS-CoV-2. Oral Dis. 25, 1 (2020).
Rahman, G. S., Alshetan, A. A. N., Alotaibi, S. S. O., Alaskar, B. M. I. & Baseer, M. A. Is chlorhexidine mouthwash effective in lowering COVID-19 viral load? A systematic review. Eur. Rev. Med. Pharmacol. Sci. 27(1), 366–377 (2023).
Herrera, D., Serrano, J., Roldan, S. & Sanz, M. Is the oral cavity relevant in SARS-CoV-2 pandemic?. Clin. Oral Investig. 24(8), 2925–2930 (2020).
Seneviratne, C. J. et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore. Infection 49, 305–311 (2021).
Falzone, L., Gattuso, G., Tsatsakis, A., Spandidos, D. A. & Libra, M. Current and innovative methods for the diagnosis of COVID-19 infection (Review). Int. J. Mol. Med. 47(6), 100 (2021).
Marty, F. M., Chen, K. & Verrill, K. A. How to obtain a nasopharyngeal swab specimen. N. Engl. J. Med. 382, e76 (2020).
Collecting and handling saliva for DNA analysis handbook. Salimetrics. Rev. 4, 28 (2023).
Malamud, D. et al. Antiviral activities in human saliva. Adv. Dent. Res. 23(1), 34–37 (2011).
Murai, I. H. et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19. JAMA 325(11), 1–9 (2021).
Acknowledgements
We thank Dr. Edward Cupler (Research and Innovation chair, KFSHRC- Riyadh, KSA) and Dr. Arnab Pain (King Abdullah University of Science and Technology KAUST, Jeddah, KSA) for their guidance and support during this study. We also thank the pharmacy staff (Zinab Rashed, Amnah Mukhtar, and AbdulMohsen Marghalani), nursing staff (Mohammad Al Hroub, Rainavel Romano, Mary Rescate, Afrah Alhalal, Lea Manreza, Sheila Rescate, Cecilia San Martin, Gina Barren, Hala Hassan, Jolly Borre, Lama Hefni, Mavanessa Cruz, Rosalinda Panagan, Walaa Mujalli, Maryam Alaqeel, Amnah Almagady, Carol Capones, Eman Albishi, Cheryl Clar, Menerva Vicente, Vimela Moodley, and Ellaine Joy Quadra. Khaled Abu Zer, Raksha Parsoo, Mary Joy Debalucos, Ali Alshehri, and Amirah Alamri) from the ER, family medicine, inpatient and OPD, and dental assistants (Hanan AlAnazi, Salha Labban, Ehsan Fakharani, Reem Ghazi, Areej Aljarad, Tarfah Alhajjar, Nada Alrheli, and Mildred Angelie Larosa) and dental PCA and research coordinators (Haneen Al-Omar, and Donya Bahussain) at KFSHRC-J, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All the authors have made substantial contributions to the conception and design of the study. SMNF, SZ, AD, DJ, and ELGH were involved in the data collection and data analysis. SMNF, AD, ELGH, and DJ were involved in the data interpretation. SMNF, AD, ELGH, and DJ were involved in drafting the manuscript. SMNF, AD, ELGH, and GW were helped in revising the manuscript critically and provided final approval for the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feteih, S.M.N., Dada, A., Heaphy, E.L.G. et al. The effect of novel vitamin D3 compounds on saliva samples from COVID-19 patients: a lab study. Sci Rep 14, 19415 (2024). https://doi.org/10.1038/s41598-024-70429-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70429-z