Abstract
The dose–response of intravenous lidocaine in preventing postoperative vomiting (POV) in children remains unclear. This study investigated whether intravenous lidocaine dose-dependently decreased POV risk within 24 h postoperatively in children undergoing tonsillectomy (with or without adenoidectomy) without severe complications. Patients aged 3–12 years (American Society of Anesthesiologists grade I–II) scheduled for elective tonsillectomy (with or without adenoidectomy) were enroled from December 2021 to March 2022. They were randomly grouped according to the lidocaine dose (A [0 mg kg−1], B [1 mg kg−1], C [1.5 mg kg−1], and D [2 mg kg−1]) and were administered the same induction protocol (sufentanil, propofol, and suxamethonium chloride). Anaesthesia was maintained with sevoflurane. The incidence of POV within 24 h postoperatively was 46, 40, 36, and 20% in groups A, B, C, and D, respectively, with significant differences between groups D and A. Postoperative analgesic rescues in groups A, B, C, and D were 62, 36, 34, and 16%, respectively, with significant differences between groups D and B, C and A, and D and A. No severe adverse events were reported. Intravenous lidocaine has a dose-dependent effect on reducing the risk of POV in children undergoing tonsillectomy (with or without adenoidectomy) without serious adverse events.
Trial registration: Chinese Clinical Trial Registry, ChiCTR2100053006.
Similar content being viewed by others
Introduction
Tonsillectomy is the most common procedure for the treatment of paediatric recurrent acute tonsillitis and tonsillar enlargement1. Even though the surgery is safe, common complications include postoperative nausea and vomiting (PONV), throat pain, and bleeding2. Children (≥ 3 years) are at a high risk of PONV, which is the most common cause of dissatisfaction in children and their parents and prolongs hospitalisation3,4. Numerous interventions have been recommended to decrease the incidence of PONV, especially dexamethasone and 5-HT3 receptor antagonist4. However, the optimal management of PONV remains a challenge for clinical anaesthesiologists owing to the need to balance the benefit of PONV intervention with the risk of adverse effects.
Lidocaine has analgesic, antihyperalgesic, and anti-inflammatory properties, making it a general anaesthetic adjuvant5. Intravenous lidocaine has antiemetic properties in paediatric patients in combination with other antiemetics6,7. The mechanism by which lidocaine reduces PONV is unclear, but may be due to its opioid-sparing, anti-inflammatory, and central antihyperalgesic effects. However, the effect of intravenous lidocaine solely for the prevention of PONV in paediatric tonsillectomy remains unclear. Therefore, we hypothesised that intravenous lidocaine solely may decrease the risk of PONV in a dose-dependent manner in children undergoing tonsillectomy (with or without adenoidectomy) without severe side effects. Owing to the difficulty in assessing nausea in paediatric patients, our trial focused solely on evaluating postoperative vomiting (POV). The selection of POV as the primary outcome was guided by the need for clinically meaningful outcomes with potential benefits.
Methods
Study design
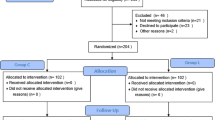
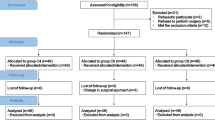
This was a single-centre, parallel-group, randomised, double-blind controlled trial. Ethical approvance was obtained from the Institutional Review Board of Yichang Central People’s Hospital (HEC-KYJJ-2020-038-02) and registered at www.chictr.org.cn (ChiCTR2100053006). The trial was conducted in compliance with the tenets of the Declaration of Helsinki and was registered on 8 November 2021. The study was conducted from 1 December 2021 to 1 March 2022 at Yichang Central People’s Hospital. Written informed consent was obtained from the parents of each child.
The patients were randomised in a 1:1 ratio to each group. Randomisation was computer-generated, and each patient was assigned a code.
Patients
Patients aged 3–12 years (American Society of Anesthesiologists grade I–II) scheduled for elective tonsillectomy (with or without adenoidectomy) were enrolled. The children were divided into four groups: A (0 mg kg−1 lidocaine), B (1 mg kg−1 lidocaine), C (1.5 mg kg−1 lidocaine), and D (2 mg kg−1 lidocaine) (Anhui Changjiang Pharmaceutical Co. Ltd, Wuhu, China). The exclusion criteria were as follows: chronic cough, history of steroid or bronchodilator treatment, respiratory tract reactive disease, upper airway infection in the previous 2 weeks, therapy with angiotensin-converting enzyme inhibitors, gastroesophageal reflux, morbid obesity, known allergy to any of the study drugs, and use of medications and nutraceuticals known to affect blood pressure (BP) and heart rate (HR).
Perioperative anaesthetic care
Preoperatively, all children fasted for 6 h and were restricted from oral intake of clear fluids for 1 h. The children entered the operating room with their parents to curb their separation anxiety. Noninvasive BP, HR, electrocardiography, and pulse oxygen saturation were measured using a multifunction monitor (GE Healthcare, Helsinki, Finland). The width of the BP cuff for each patient was approximately two-thirds of the upper arm length. After 5 min of stabilisation, the baseline HR, systolic BP, diastolic BP, and mean arterial pressure values were obtained from the average of three measurements taken 2 min apart. A 22-gauge intravenous catheter was subsequently inserted into the veins at the back of the hand.
After preoxygenation, the respective treatments were injected over a 3-s period. An anaesthetic nurse who had prepared the study treatments was blinded to the study and administered the injection with pump activation. Two minutes after the injection, general anaesthesia was induced following the induction protocol: sufentanil (Yichang Renfu Pharmaceutical Co. Ltd., Yichang, China) 0.25 μg kg−1, propofol (Fresenius Kabi Deutschland GmbH, Homburg, Germany) 2.0 mg kg−1, and suxamethonium chloride (Xi’an Hanfeng Pharmaceutical Co. Ltd., Xi’an, China) 1 mg kg1. When eyelash reflexes were absent, the patient was ventilated via a facemask with 100% oxygen. A cuffed endotracheal tube was used, the size of which was selected based on a widely used formula (3.5 + age in years/4). Patients were excluded from the study if any difficulty was encountered during facemask ventilation. After intubation, rocuronium (Zhejiang Xianju Pharmaceutical Co. Ltd., Taizhou, China) 0.3 mg kg−1 was injected to maintain muscle relaxation. Anaesthesia was maintained with 2%–3% sevoflurane (Maruishi Pharmaceutical Co., Ltd., Osaka, Japan) and 50% medical air in oxygen. All children were administered ibuprofen (10 mg/kg), acetaminophen (15 mg/kg), and tramadol (1 mg/kg) during the surgical procedure.
The surgery was performed by an experienced surgeon. At the end of the operation, sevoflurane was discontinued, and neostigmine (Zhejiang Xianju Pharmaceutical Co. Ltd., Taizhou, China) 0.04 mg kg−1 and atropine (Suicheng Pharmaceutical Co. Ltd., Xinzheng, China) 0.02 mg kg−1 were administered to antagonise any residual neuromuscular blockade. After extubation, an anaesthesiologist who was not involved in the study performed anaesthesia emergence and subsequently graded cough response. Following the completion of the surgery, oral suction was performed immediately after removing the endotracheal tube. Extubation was performed by confirming adequate tidal volume, regular spontaneous respiratory pattern, and purposeful behaviour (eyes open upon request). After extubation, the children were monitored for at least 5 min to resume regular spontaneous respiration and subsequently transferred to the postanaesthesia care unit (PACU). Electrocardiography, peripheral pulse oximetry, and noninvasive BP measurements were performed.
In the PACU, the anaesthetic nurse who was blinded to the study recorded the pain scores every 15 min, and the patient was kept in the PACU for 1 h. The patients needed a Steward recovery score of > 4 to be discharged to the ward, where they stayed overnight. Pain levels were assessed two times daily when patients were discharged from the PACU. If the pain score (using the Face, Legs, Activity, Cry, and Consolability scale for children aged 3–4 years8, the Wong–Baker scale for those aged 4–7 years9, or the visual analogue scale for those aged ≥ 8 years) was ≥ 3 at rest10, the attending PACU nurse administered intravenous propacetamol (30 mg kg−1) as rescue analgesia during the trial.
To ensure that these side effects did not complicate the children's postoperative experience, only acetaminophen 15 mg/kg was administered every 6 h for pain management during the observation period of the study. After the trial, postoperative analgesia was administered following the protocols of the local clinician, who suggested that morphine be used for pain treatment with continued acetaminophen administration.
Primary outcomes
The primary outcome was the incidence of POV within 24 h postoperatively. Rescue methods for POV were intravenous ondansetron 50 μg kg−1 and dexamethasone 0.1 mg kg−1.
Secondary outcomes
Secondary outcomes were evaluated to determine the analgesic efficacy of lidocaine. The incidence of children requiring analgesic rescue within 24 h was recorded. Adverse events included laryngospasm, oxygen desaturation, upper airway obstruction, and arrhythmia.
Statistical analysis
Based on the results of the preliminary experiment, groups A, B, C, and D had 55, 35, 30, and 14%, respectively. We found that a sample size of 179 patients would be required to achieve 95% power at a two-sided α level of 0.05 to demonstrate a relative between-group difference. Considering a 10% leak rate, a total of 200 patients were finally required, with 50 in each group. Patient characteristics (including age, height, and weight), time of surgery, and time to extubation were expressed as means and standard deviations and were analysed using analysis of variance. The incidence of PONV and consumption of analgesics were expressed as a ratio and analysed using χ2, with a P value < 0.05 indicating a difference. Statistical significance was set at P < 0.01 for all analyses. GraphPad Prism 8.0.2 (GraphPad Software Inc., San Diego, CA, USA) was used for all analyses.
Results
Patients
Between 1 December 2021 and 1 March 2022, 280 patients were enroled in the study, of whom 200 were included in the analysis and divided into four groups; the demographic characteristics and operative data did not differ between the groups (Table 1).
Primary outcomes
The incidence of POV was 46% (95% confidence interval [CI] 32–61%), 40% (95% CI 26–55%), 36% (95% CI 23–51%), and 20% (95% CI 10–34%) in groups A, B, C, and D, respectively. There was a significant difference between groups D and A (P ≤ 0.01), with a dose-dependent decrease in POV (Fig. 1).
Secondary outcomes
The incidence of postoperative analgesic rescue in groups A, B, C, and D was 62% (95% CI 47–75%), 36% (95% CI 23–51%), 34% (95% CI 21–49%), and 16% (95% CI 7–29%), respectively. Significant differences were observed between groups D and B and groups C and A (P ≤ 0.01) and between groups D and A (P ≤ 0.001) (Fig. 2). There were no significant differences among the groups in the time to extubation, even when this difference was extended to 2 min longer than that in group A (Table 1). Moreover, no severe complications, such as postoperative hypoxaemia, laryngospasm, or arrhythmia, were observed in any group.
Discussion
The 2020 consensus guidelines for the management of PONV recommend 5-HT3 antagonists and dexamethasone (0.15 mg kg−1) as the standard prophylaxis4. Dexamethasone is an efficacious antiemetic but is associated with a risk of postoperative bleeding, which is sometimes fatal11. 5-HT3 receptor antagonists are commonly used in PONV management and may cause adverse cardiac events, such as arrhythmia12. Intravenous lidocaine is considered inexpensive and safe and has been commonly used as an adjuvant for paediatric general anaesthesia. Our results showed that intravenous lidocaine effectively and dose-dependently reduced the incidence of POV in children undergoing tonsillectomy (with or without adenoidectomy) without severe side effects. Only 2 mg kg−1 lidocaine achieved a significantly better clinical effect (20%).
Significantly fewer children received rescue analgesics in each group during the first 24 h. Significant differences were observed between groups D and B, groups C and A (P ≤ 0.01), and groups D and A (P ≤ 0.001). The reduction in rescue analgesic requirement may not be explained by the analgesic effect of lidocaine because the half-life of lidocaine is 2 h13. Other trials have demonstrated that the clinical effect of lidocaine exceeds its half-life and that the extent of the effect is suited for preventive analgesia14. The mechanism underlying this phenomenon remains unclear and may be associated with the anti-inflammatory effects of intravenous lidocaine.
Our trial first found that the single dose of lidocaine needed to achieve an antiemetic effect was higher than that needed to achieve an analgesic effect within 24 h postoperatively. This is due to the multimodal anaesthesia and opioid-sparing effect of lidocaine. There were no significant differences in the clinical characteristics and clinical data between the groups. No severe side effects were observed in this trial. However, in our study, the time of extubation in the groups administered intravenous lidocaine was prolonged by nearly 2 min, which differs from previous studies. This difference may be attributed to the effect of the analgesic combination, which increased tolerance to endotracheal tube placement.
The most common method of lidocaine administration is intravenous infusion, which is attributed to its tendency to accumulate and cause toxicity. We believe that administering a single dose of lidocaine would be more suitable and convenient because of the short duration of the surgery (approximately 30 min). Moreover, no severe complication was observed during the trial. This observation indicates that a single-dose injection may be appropriate for surgical procedures of this nature. Concerning the effects of lidocaine on the reduction of nociception and/or cardiovascular responses to surgical stress, we believe that pre-induction lidocaine administration has some advantages over intra- and post-operation administration.
Our study has certain limitations. First, we chose a single dose of lidocaine for the advent of lidocaine accumulation. Second, this was a single-centre study, which reduced the power and reliability of the results. Third, we used the concentration of sevoflurane in the end-expiratory gas to monitor the depth of anaesthesia, potentially leading to an increased incidence of POV. Fourth, we should record the number of POV cases in the early, late, and next-day periods, as well as the need for rescue antiemetics during these same periods.
In conclusion, our study revealed that intravenous lidocaine solely decreased the incidence of PONV in children undergoing tonsillectomy (with or without adenoidectomy) in a dose-dependent manner. No serious adverse events were observed after the administration of different doses of intravenous lidocaine.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Xu, B. et al. Primary and secondary postoperative hemorrhage in pediatric tonsillectomy. World J. Clin. Cases 9, 1543–1553. https://doi.org/10.12998/wjcc.v9.i7.1543 (2021).
Hawley, K. Tonsillectomy and adenoidectomy in children. JAMA Otolaryngol. Head Neck Surg. 145, 300. https://doi.org/10.1001/jamaoto.2018.3703 (2019).
Kermode, J., Walker, S. & Webb, I. Postoperative vomiting in children. Anaesth. Intensive Care 23, 196–199. https://doi.org/10.1177/0310057X9502300213 (1995).
Gan, T. J. et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth. Analg. 131, 411–448. https://doi.org/10.1213/ANE.0000000000004833 (2020).
Estebe, J. P. Intravenous lidocaine. Best Pract. Res. Clin. Anaesthesiol. 31, 513–521. https://doi.org/10.1016/j.bpa.2017.05.005 (2017).
Echevarría, G. C. et al. Intra-operative lidocaine in the prevention of vomiting after elective tonsillectomy in children: A randomised controlled trial. Eur. J. Anaesthesiol. 35, 343–348. https://doi.org/10.1097/EJA.0000000000000807 (2018).
Nakajima, D., Kawakami, H., Mihara, T., Sato, H. & Goto, T. Effectiveness of intravenous lidocaine in preventing postoperative nausea and vomiting in pediatric patients: A systematic review and meta-analysis. PLoS One 15, e0227904. https://doi.org/10.1371/journal.pone.0227904 (2020).
Redmann, A. J., Wang, Y., Furstein, J., Myer, C. M. 3rd. & de Alarcón, A. The use of the FLACC pain scale in pediatric patients undergoing adenotonsillectomy. Int. J. Pediatr. Otorhinolaryngol. 92, 115–118. https://doi.org/10.1016/j.ijporl.2016.11.016 (2017).
Garra, G. et al. Validation of the Wong-Baker FACES pain rating scale in pediatric emergency department patients. Acad. Emerg. Med. 17(1), 50–54. https://doi.org/10.1111/j.1553-2712.2009.00620.x (2010).
Bijur, P. E., Silver, W. & Gallagher, E. J. Reliability of the visual analog scale for measurement of acute pain. Acad. Emerg. Med. 8(12), 1153–1157. https://doi.org/10.1111/j.1553-2712.2001.tb01132.x (2001).
Czarnetzki, C. et al. Dexamethasone and risk of nausea and vomiting and postoperative bleeding after tonsillectomy in children: A randomized trial. JAMA 300, 2621–2630. https://doi.org/10.1001/jama.2008.794 (2008).
Tricco, A. C. et al. Interventions to decrease the risk of adverse cardiac events for patients receiving chemotherapy and serotonin (5-HT3) receptor antagonists: A systematic review. BMC Pharmacol. Toxicol. 16, 1. https://doi.org/10.1186/2050-6511-16-1 (2015).
Ochs, H. R., Knüchel, M., Abernethy, D. R. & Greenblatt, D. J. Dose-independent pharmacokinetics of intravenous lidocaine in humans. J. Clin. Pharmacol. 23, 186–188. https://doi.org/10.1002/j.1552-4604.1983.tb02723.x (1983).
Barreveld, A., Witte, J., Chahal, H., Durieux, M. E. & Strichartz, G. Preventive analgesia by local anesthetics: the reduction of postoperative pain by peripheral nerve blocks and intravenous drugs. Anesth. Analg. 116(5), 1141–1161. https://doi.org/10.1213/ANE.0b013e318277a270 (2013).
Author information
Authors and Affiliations
Contributions
Y.H. and M.D. helped with the data curation. Y.H. and Y.C. helped with the software and formal analysis. Y.H., M.D., and Y.C. helped with project administration. X.L. and J.J. helped with resources and investigation. Y.G. helped with the methodology, supervision, and validation. All authors have contributed equally to the manuscript and have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, Y., Du, Mc., Chen, Y. et al. Lidocaine and risk of postoperative vomiting in children undergoing tonsillectomy: a randomised clinical trial. Sci Rep 14, 19752 (2024). https://doi.org/10.1038/s41598-024-70804-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70804-w