Abstract
This research evaluates the environmental and health risks linked to potentially toxic elements (PTEs) and PAHs along the western coast of the Gulf of Suez, Egypt. This study investigated the concentration of 16 PAH compounds in the Suez Gulf, revealing significantly higher levels than the EU (0.20 µg/L) and US (0.030 µg/L) standards. The average total PAH concentration across eight locations was significantly higher, with the Suez area having the highest concentration at 479 µg/L. Pyrene (Pyr) was the dominant PAH with a concentration of 443 µg/L in Suez, while acenaphthylene (Ace) had the lowest concentration at 0.120 µg/L in Northern Zaafarana. Carcinogenic PAHs (CAR) ranged from 8.67 µg/L at Ras Gharib to 29.62 µg/L at Suez, highlighting the urgent need for regulatory measures. Confirmatory ratios pointed to industrial and shipping influences as petrogenic sources. Elevated total organic carbon (TOC) levels in Suez Bay indicated aggravated organic pollution, exacerbated by oil rigs and refineries. The ecological risk assessment highlighted substantial risks, particularly in Suez, necessitating immediate interventions to combat PAH contamination and preserve the environmental balance of the Red Sea. The dominant metals in water samples were arranged in descending order as follows: Pb > Fe > Cr > Cu > Zn > Mn > Cd > Ni. The study evaluated environmental and human health risks using a multifaceted approach, including cluster analysis, principal component analysis, and various indices (HPI, RI, MI, HQ, HI, and CR). Most water samples exhibited high pollution risks, surpassing permissible limits for HPI (> 100) and MI (> 6). Notably, HI oral values indicated significant non-carcinogenic risks for adults and children. While HI values for adults suggested low-risk dermal contact, those for children showed a substantial proportion in the high-risk category. Most water samples displayed CR values exceeding 1 × 10–4 for Cd, Cr, and Pb, indicating vulnerability to carcinogenic effects in both age groups. Monte Carlo simulations reinforced these findings, revealing a significant carcinogenic impact on children and adults. The identified clusters, reflective of industrial, petroleum-related, and urban runoff contamination sources, were consistently validated and clarified through PCA, enhancing the reliability of the findings. In light of these results, urgent and comprehensive water treatment measures are imperative to mitigate carcinogenic and non-carcinogenic health risks. These insights provide a foundation for implementing targeted management strategies to effectively address the challenges of heavy metal contamination in the Red Sea.
Similar content being viewed by others
Introduction
Environmental pollution from petroleum hydrocarbons, particularly in maritime habitats and inland aquatic breeding environments, poses a significant contemporary challenge. Approximately 5 million tons of crude oil enter the marine environment annually from diverse sources, constituting oil seepage. Various factors contribute to ocean oil spills, including intentional or unintentional ship discharges, tanker collisions, pipeline leaks, well explosions, offshore oil exploration, and onshore refinery leaks. Discharged fuels account for 48% of reported oil pollution, followed by crude oils at 29% and yearly tanker accidents at 5%. Despite a recent decline, the ecological threat from offshore oil spills persists1. The consequences of oil spills in estuarine ecosystems attract considerable scientific attention due to intrinsic toxicity to biological components. Approximately five million tons of oil containing harmful compounds enter the marine environment annually through spills worldwide. The process of oil spilling into saltwater involves weathering, including evaporation, natural dispersion, and emulsification. This severely impacts human health and biotic components, such as marine populations, estuaries, coral reefs, and mangroves. Oil contamination poses developmental limitations and socioeconomic risks, endangering marine ecology, the economy, and the human food chain2.
Oil components, including water-soluble substances like phenyls, directly harm aquatic life. Fish may absorb hydrocarbons, leading to physiological damage and tainted meat unsafe for human consumption3. Insoluble in water, petroleum hydrocarbons create surface films affecting gas exchange and oxygen transport. Polycyclic aromatic hydrocarbons (PAHs), classified as dangerous pollutants, result from oil spills and incomplete fuel combustion. PAHs, known for their toxicity, mutagenicity, and carcinogenicity, can harm aquatic animals4. Crude oil, rich in organic molecules, PTEs, and PAHs, poses environmental challenges, impacting marine creatures and ecosystems. The pollutants may enter higher trophic organisms through food chains, affecting coral reefs and mangroves globally. The decline of mangroves is partly attributed to heavy metals and persistent organic compounds acting as natural traps for pollutants5,6.
The global concern regarding the presence of heavy metals in aquatic habitats is rooted in the potential harm it poses to human health7,8. PTEs are recognized as systemic toxins capable of causing damage to multiple organs, as well as inducing teratogenic and carcinogenic effects9,9,10,11,12. Prolonged exposure to these metals has been linked to permanent intellectual and developmental impairments, behavioral issues, learning and attention difficulties, auditory challenges, and disruptions of visual abilities2. Furthermore, human exposure to PTEs from water bodies occurs through the bioaccumulation of metals in food sources1,13. Therefore, individuals can be exposed to high levels of PTEs by consuming plant and aquatic food sources irrigated with polluted waters, even if they do not directly drink water contaminated with PTEs8,14,15. These contaminants can originate from natural sources, such as geological erosion, weathering, and precipitation, as well as anthropogenic activities, such as mining, metal processing, industrial wastewater discharge, and applying pesticides and fertilizers16,17.
Indices like the heavy metal pollution index (HPI), metal index (MI), and potential ecological risk index (RI) have been utilized to assess the potential ecological risks to the environment8,18,19,20,21,22,23,24,25,26. Additionally, various complementary methods have enhanced analysis efficiency, including multivariate statistical analyses to identify heavy metal sources8,9,27,28. The use of the Monte Carlo method in assessing potential risks to human health has proven effective in addressing the challenge of obtaining precise results regarding parameter uncertainty. In recent years, Monte Carlo simulation has been popular in evaluating health hazards related to toxic elements in surface water, groundwater, and soil8,19,29.
Responding to water scarcity in Egypt, the increased desalination of Red Sea saltwater serves as an additional drinking water source alongside the Nile River. However, escalating concentrations of PTEs and polycyclic aromatic hydrocarbons (PAHs) in the coastal waters result from diverse pollution sources. Challenges arise in desalination techniques to effectively treat water with high contaminant levels, raising concerns about incomplete removal of heavy metals and pollutants. The local population in the study area directly faces Red Sea water exposure through dermal and oral contact. This emphasizes the urgency of assessing environmental and human health risks associated with pre-desalination heavy metal levels in Red Sea saltwater. Such insights are crucial for implementing effective water treatment strategies, ensuring public health safety, and promoting sustainable water practices in the region30,31,32.
The aims of this study were (1) to evaluate the accumulation and ecological risk of heavy metals, total organic carbon, and polycyclic aromatic hydrocarbons in collected samples from scattered points along the western shore of the Red Sea (2) to carry out a thorough analysis of the potential health risks posed by PTEs (non-carcinogenic and carcinogenic) through oral and dermal exposure for adult and children in the coastal water of the red sea in Egypt; (3) to identify potential sources of the heavy metals by employing cluster analysis and principal component analysis, (4) applying Monte Carlo method to decrease uncertainty and give more realistic approach insight of human health risks from heavy metals that should be considered during desalination of salt water to be utilized safely for drinking and domestic purposes.
Material and methods
Site description
The Gulf of Suez (GOS), a semi-enclosed shallow basin stretching 300 km from Port Suez to Shadwan Island, is linked to the northern Red Sea by the Straits of Gubal and the Mediterranean Sea by the Suez Canal. It represents the Red Sea's left arm, which separates Egypt's Sinai Peninsula from Saudi Arabia's Arabian Desert. The Gulf's surface area is around 10,510 km2, with sea depths varying from 16 m south of Suez Bay to 50–85 m near the open Gulf2,17. Egypt's main supply of crude oil is the GOS. The GOS oil production, comprising 26 offshore fields, 136 production platforms, and 570 oil wells connected by a submerged pipeline network spanning 450 nautical miles, accounts for 85% of Egypt's crude oil output. The daily canal traffic grew by more than 56 boats, from 18,830 in 2020 to 20,694 in 2021, when the new Suez Canal project was finished in 2015. Thus, the GOS's influence on the Egyptian economy increased considerably2.
As a result, there have already been several oil spills in the Gulf that have significantly impacted the marine and coastal ecosystems. There have previously been significant oil leak incidents in the Red Sea/Gulf of Suez (GOS) and the Suez Canal, the worst of which was in 1982. During the loading of a ship in Ras Shukheir, tens of thousands of tons of crude oil were spilled into the sea within 4–6 h. Based on research, the spread of oil pollution was 250 km south and 200 km north of the oil spill. A tanker grounded in the Suez Canal in 2004 spilled 9000 tons of crude oil, and the same thing happened again in 2006 when a tanker sank on the canal bank, spilling 5000 tons of crude oil. The most significant oil leak in Egyptian history, the Gabal El Zeit disaster, happened in June 2010, damaging nearly 160 km of North Red Sea shoreline. Furthermore, it has been stated that the traffic crossroads offshore the Sumed oil pipeline terminal near Ain Sukhna port are the places with the highest risk of tanker accidents2,33.
Anthropogenic oil spills from oil rigs and ships have badly impacted the intertidal zone in the middle and southern Gulf of Suez. Many rocky coasts are covered with oil pavements, and in certain coastal regions, oil is discovered buried below a thin veneer of wind-blown sand. The expected distribution of oil contamination sources along the research region is diverse. To explain, although Suez City is prone to oil pollution due to navigation, industrial, manufacturing, and other human activities, Ain Sukhna is rarely contaminated as the area is dominated by tourism and fishing activities33,34.
Additionally, the coastal Red Sea and Gulf of Suez region is distinguished by vast areas of mangrove swamps and has a diversified and almost unique coral reef community that serves as a habitat for various marine life, including fish species with significant economic value. Potential toxic elements, petroleum compounds, and herbicides pose the greatest dangers to the Red Sea's coral reefs and mangrove ecosystems. Pollutants can potentially harm coral species, the accompanying creatures, and biodiversity in general. Therefore, to understand the potential dangers, it is crucial to measure the concentration of hydrocarbons, particularly PAHs, and heavy metals in aquatic habitats related to coral reefs and mangroves35.
Sampling and analysis
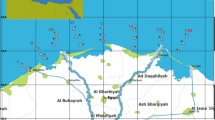
Water samples were gathered from 18 spots along the coastline of the Gulf of Suez (GOS), as shown in Fig. 1. The map for sampling was made using ArcGIS Pro 2.8.8 software. Sampling of surface water was done on March 17, 2023. In total, 30 samples were collected, with PAHs found in 10 samples and heavy metals analyzed in 18 samples to ensure area coverage at intervals. To guarantee the reliability and accuracy of data, duplicate water samples were taken at each sampling location. Surface water samples were drawn from a depth of half a meter and preserved in four-liter brown glass bottles to shield them from exposure that could degrade certain compounds. Upon arrival at the lab, all samples underwent filtration using filters with a size of 0.45 μm, under vacuum conditions, to separate suspended particles and obtain dissolved samples.
The concentrations of metals were assessed utilizing a coupled plasma optical emission spectrometer (ICP OES), specifically the iCAP 6500 Duo (Thermo Fisher Scientific, England) equipped with a charged injection device (CID) detector. The instrument was calibrated using a certified solution containing elements at a concentration of 1000 mg/L (Merck, Germany). Calibration was done at a concentration of 0.05 parts per million in line with the instructions provided by the manufacturer and following the USEPA Method 6010b (1996). Each sample was tested twice to verify the accuracy of the findings. Additionally, blank samples were examined to determine any background pollution. The detection limits for the heavy metals were as follows: Cadmium (Cd): 0.0001 mg/L, Chromium (Cr VI): 0.001 mg/L, Copper (Cu): 0.001 mg/L, Iron (Fe): 0.001 mg/L, Manganese (Mn): 0.0005 mg/L, Nickel (Ni): 0.001 mg/L, Lead (Pb): 0.001 mg/L, Zinc (Zn): 0.0005 mg/L. The external standards showed recovery rates between 74 and 123% for all elements tested, demonstrating the method's ability to measure metal concentrations accurately. Duplicate samples from the location had standard deviations (RSDs) below 10% for all metals, indicating high precision.
To analyze PAHs, 3 g of each water sample underwent extraction using 5 mL of acetonitrile and sonication for 15 min. Ensuring reliability extractions were performed in triplicate. Following extraction, the samples underwent centrifugation at 3000 rpm for five minutes. The resulting supernatant was filtered through PTFE 0.45 μm filters before undergoing high-performance liquid chromatography (HPLC) analysis. The HPLC system employed was an Agilent 1260 series equipped with a ZORBAX Eclipse PAH column (4.6 mm × 150 mm i.d. five μm). The mobile phase comprised water (A) and acetonitrile (B) with a flow rate set at 2.0 ml/min. The gradient programming was as follows: starting at time point zero with 40% B; from zero to 0.66 min maintaining at 40% B; from 0.66 to 20.0 min increasing from 40 to 100% B; holding at 100% B from minute20 to minute25; decreasing back to 40% B from minute25 to minute27; finally reaching again40% B from minute27 to minute30.Detection occurred at 220 nm utilizing an array detector with an injection volume of 5 μL. The detection limits for various PAHs were: Naphthalene (Naph): 0.05 μg/L, Acenaphthylene (Acthy): 0.10 μg/L, Acenaphthene (Ace): 0.10 μg/L, Fluorene (Fl): 0.05 μg/L, Phenanthrene (Phe): 0.05 μg/L, Anthracene (Ant): 0.05 μg/L, Fluoranthene (Flu): 0.05 μg/L, Pyrene (Pyr): 0.05 μg/L, Benzo (a) anthracene (BaA): 0.05 μg/L, Chrysene (Chry): 0.05 μg/L, Benzo (b) fluoranthene (BbF): 0.10 μg/L, Benzo (k) fluoranthene (BkF): 0.10 μg/L, Benzo (a) pyrene (BaP): 0.05 μg/L, Dibenzo (a,h) anthracene (DBA): 0.10 μg/L, Benzo (g,h, i) perylene (BghiP): 0.10 μg/L, Indeno (1,2,3-cd) pyrene (InP): 0.10 μg/L. Stringent quality control measures were implemented, which used standards to adjust for losses during extraction and preparation. Accelerated solvent extraction was utilized for PAHs with the inclusion of six standards to guarantee compensation. The recovery rates of these standards varied from 76 ± 9% to 118 ± 8%, highlighting the efficiency of the approach. The methods for identifying metals and PAHs in water samples underwent validation to guarantee precise and accurate results. The detection thresholds, recovery rates, and measurement consistency demonstrate the reliability and effectiveness of these techniques for surveillance.
Environmental risk
The environmental risks in the current study include Polycyclic aromatic hydrocarbon Risk, Ecological risk, and Heavy metals pollution index and metal index.
Polycyclic aromatic hydrocarbons risk
The ecological risk of PAHs on aquatic biota was evaluated by risk quotients (RQ). The following Eq. (1) calculated the RQ:
The CPAHs represented the PAH concentrations in the seawater, and CQV represented the quality value of PAHs in the seawater. The negligible concentrations (NCs) and the maximum permissible concentrations (MPCs) of individual PAHs in water represent the quality values, respectively. Hence, the RQNCs and RQMPCs were calculated according to Eqs. (2) and (3), respectively36:
Ecological risk
The application of the potential ecological risk index (RI) for PTEs (Eq. 4) proves to be a valuable methodology for assessing the potential environmental risks associated with PTEs37 In the surface water. Considering factors like concentrations, types, sensitivity, toxicity, and background levels of heavy metals, this approach allows for a comprehensive evaluation of ecological risks. The utilization of this method sheds light on the potential impact of heavy metal contamination on the Red Sea's marine ecosystem and aquatic life. The significance of monitoring and evaluating these risks cannot be overstated, especially in human activities such as desalinating Red Sea water for various purposes. This method offers a practical and insightful tool for understanding the marine environment's overall health and informs decisions to preserve the Red Sea's ecosystem.
where Tr means heavy metal toxic response factor, Cf is the contamination factor; \({C}_{ave}^{i}\) is the concentration of heavy metal; \({C}_{bg}^{i}\) are the background values. RI refers to contamination's potential ecological risk.
Heavy metals pollution index and metal index
The Heavy Metal Pollution Index (HPI), as explained is considered an effective tool for assessing water contamination levels in the presence of heavy metals (HMs) in water resources8,38. This method is valuable for decision-makers, providing a composite measure of the impact of dissolved heavy metals in water. The HPI evaluates water quality suitability for human consumption based on metal contamination, using a weighted arithmetic mean mechanism and assigning weights to each pollution parameter. The HPI is calculated based on the concentrations of eight heavy metals and one metalloid, considering their toxic characteristics. A three-class scale categorizes heavy metal pollution as low, medium, or high. Another metric, the Metal Index (MI), evaluates the overall quality of drinking water by considering the cumulative potential impact of PTEs on human health8,39. The MI assumes a linear relationship between the toxicity of HMs and their concentration, with concentrations exceeding allowable limits indicating degraded water quality. The MI, introduced by Tamasai and Cini40, is calculated through a comprehensive assessment of the current situation. The following equations were utilized for the calculation of HPI (Eqs. 4 and 5) and MI (Eq. 6)
where Qi stands for the sub-index parameter; n is the number of parameters taken for analysis; wi depicts the weight of each parameter, evaluated as 1/Si; Si symbolize the standard value of each parameter; Qi represents the sub-index of the boundary. Cave signifies the average concentration of each studied HMs; UALi stands for the upper allowable limit of the ith metal in the sample.
A three-class modified scale is frequently used to depict moderate levels of heavy metal pollution accurately. This scale classifies heavy metal pollution as very low (HPI < 25), low (26 ≤ HPI ≤ 50), medium (51 ≤ HPI ≤ 75), High (76 ≤ HPI ≤ 100), unsuitable (HPI > 100)41.
Metal index (MI) has six classes: very clean (MI < 0.3); clean (0.3 < MI < 1); partly affected (1 < MI < 2); moderately affected (2 < MI < 4); heavily affected (4 < MI < 6); and severely affected (MI > 6)42.
Human health risk
The health risk assessment in this study adheres to the model recommended by the United States Environmental Protection Agency. This analysis explores the impact of environmental pollutants on health, categorizing risks into two main types: Carcinogenic (CR index) and non-carcinogenic (HQ and HI indices)43. Carcinogenic risks focus on the probability of developing cancer due to prolonged exposure to pollutants or a combination thereof. On the other hand, non-carcinogenic risks encompass various exposure-related effects, including genetic and teratogenic outcomes. Heavy metals (HMs) in drinking water enter the body primarily through ingestion and skin contact44. Consequently, this study's health risk assessment considers risks associated with direct drinking and skin contact, which are quantified through Eqs. (7) and (8).
In the given equations, CDI oral represents the average daily direct intake dose, while CDI Dermal corresponds to the average daily dose absorbed by the skin. Cw signifies the concentration of heavy metals (HMs) in the water sample (mg/L), IR denotes the daily ingestion rate (L/d), EF stands for the exposure frequency, ED represents the exposure duration, BW indicates the body weight, SA is the exposed skin area, Kp is the skin permeability coefficient, CF is the conversion factor, and ET signifies the exposure time. For detailed values of these exposure parameters, refer to Table 1s. The calculation Hazard quotient (HQ), reference dose (RFD), Health risk index (HI), and Carcinogenic risk (CR) are reported in Eqs. (9), (10), (11) and (12), while the used parameters for calculation of health risk indices based on USEPA were added Table 1s.
The reference dose is denoted as RfD, and ABS signifies the digestive coefficient of the gastrointestinal tracking (as outlined in Table 1). The carcinogenic risk (CR) resulting from both direct ingestion and skin contact can be formulated as follows:
CSF is a conversion slope factor of metals, as detailed in Table 1.
Monte Carlo simulation
Monte Carlo simulation is a strategy utilized in risk assessment to minimize uncertainties linked to heavy metal (HM) concentrations and exposure parameters, allowing for predicting both carcinogenic and non-carcinogenic risks. This method enables researchers to generate estimates of health risk values8,29. Typically, Python software, version 3.9.7, executes Monte Carlo simulations. In this study, the Python code underwent 10,000 iterations, facilitating Monte Carlo simulations to determine the probability risks related to carcinogenic and non-carcinogenic effects of heavy metals for both adults and children.
Statistical analysis and pollution source identification
Cluster analysis
Cluster analysis (CA) is a valuable technique employed for pattern recognition in datasets from diverse sources without supervision. It identifies distinctive features that differentiate groups within the dataset and clusters them accordingly. R mode and Q mode approaches are used to execute and construct CA, aiding in creating clusters of water samples based on their chemical properties. CA is particularly useful in surface water studies, helping group collected water samples into meaningful geological and hydrogeological categories. The clustering process is often represented using a cluster dendrogram, simplifying the complexity of the data and providing a clear depiction of groupings and their relationships8,23,27,45.
Principal component analysis
Principal component analysis (PCA) is a linear technique that handles complex multivariate datasets. Its objective is to analyze data without losing information, summarize the dataset, and estimate the number of variables required to explain the variance. By reducing data dimensionality, PCA reveals hidden patterns and relationships between variables that may not be immediately apparent. The Kaiser Criterion uses eigenvalues from the scree plot to assess surface water contamination to extract principal components. Tests like Kaiser Meyer Olkin (KMO) and Bartlett's tests are employed to evaluate the suitability of data for factor analysis, indicating whether variables are adequate or inadequate within the model. KMO values falling within specific ranges indicate the appropriateness of data, ensuring that researchers gain meaningful insights from complex datasets. This comprehensive approach enhances understanding while providing the data accurately representing underlying relationships9,23,24,25,26,27,28.
Results and discussion
Water quality associated with PTEs
The concentrations of potential toxic elements (heavy metals) in the water samples reported in Table 1 were, on average, as follows: Cd (0.011 mg/L), Cr (0.061 mg/L), Cu (1.051 mg/L), Fe (0.006 mg/L), Mn (0.012 mg/L), Ni (0.009 mg/L), Pb (0.158 mg/L), and Zn (0.038 mg/L). These levels are arranged in descending order: Pb > Fe > Cr (VI) > Cu > Zn > Mn > Cd > Ni. Notably, the average concentrations of Cd, Cr (VI), and Pb exceeded WHO's standard limits for drinking purposes48, while the concentrations of the other heavy metals remained within acceptable limits. This highlights a concerning contamination, especially for Cd, Cr, and Pb. Effective management strategies are crucial to address this issue, ensuring the quality of water resources and protecting public health. Increased monitoring and regulatory measures should be implemented, specifically focusing on the heavy metals that surpass recommended standards, to guarantee the safety and sustainability of water sources in the studied area.
Environmental and ecological risk
Pollution risk of PAHs on aquatic life and biodiversity
The studied PAHs were 16 compounds (Table 2): naphthalene (Naph), acenaphthylene (Acthy), acenaphthalene (Ace), fluorene (Fl), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), pyrene (Pyr), benzo(a)anthracene (BaA), chrysene (Chry), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenzo(a,h)anthracene (DBA), benzo(g,h, i)perylene (BghiP) and indo(1,2,3-cd)pyrene (InP). The distribution of the 16 PAHs in the water was inconsistent, with pyrene (Pyr) having the most significant concentration (443 µg/L) in site No.1 (Suez) and acenaphthylene (Ace) having the lowest concentration (0.120 µg/L) at site No.3 (Northern Zaafarana). Pyr may be created when low aromatic PAH rings condense at high temperatures17.
The Suez area has the greatest concentration of PAHs in the Suez Gulf (479 µg/L), primarily due to the refineries and oil production areas that are close. Following, the concentrations in the northern part of Zaafarana (about 26.73 µg/L), Qusier area in the South (22.537 µg/L), Ras Shukeir (20.45 µg/L) and near Ain Sukhna Resort (19.685 µg/L) are approximately the same. The low concentrations at some southern locations might be attributed to the extended distance from the point source15. Generally, the total PAH concentrations at the eight locations are far greater than the maximum permissible concentrations of the European Union, which is 0.20 µg/L, and the Environmental Quality Criteria of the United States, which is 0.030 µg/L for the protection of human consumers of aquatic life49. The United States Environmental Protection Agency (USEPA) and the World Health Organization (WHO) classified the PAHs Naph, Acthy, Fl, Phe, Ant, Pyr, Flu, and BaP as dangerous pollutants (CAR) among the other PAHs15,49,50. CAR ranged from 8.67 µg/L at Ras Gharib to 29.62 µg/L at Suez.
Source identification of PAHs in GOS
The marine environment primarily encounters two significant sources of polycyclic aromatic hydrocarbons (PAHs): petrogenic and pyrogenic sources. Pyrogenic sources stem from the incomplete combustion of fossil fuels, while petrogenic sources originate from the natural release of petroleum or its derivatives into the environment. While the high molecular weight (HPAHs) PAHs have four to six rings and are highly carcinogenic, the low molecular weight (LPAHs) only have two to three rings. With LPAHs/HPAHs > 1 indicates petrogenic, while less than one indicates pyrogenic15,17. The ratios in the current seawater data for all 8 locations were less than 1, which suggests a pyrogenic origin for the majority of the PAHs.
In pyrogenic assemblages, the Flu/Pyr ratio often reaches or rises above a value of one, whereas in petrogenic PAH assemblages, it usually drops below one. APhen/Ant varied from 5.406 in site No. 2 (Ain Sukhna) to 2.76 in site No.8 (Qusier). This refers to a pyrogenic nature for both (< 10), which agrees with the conclusion taken from the above LPAHs/HPAHs ratios. Meanwhile, site No.8 (Qusier) was supposed to come from a pyrogenic source (< 5), and site No. 2 (Ain Sukhna) was petrogenic in origin (> 5). Site No. 8 (Qusier) showed a BaA/(BaA + Chr) ratio that was slightly less than 0.35 and more than 0.2 (0.333), suggesting different sources because of industrial and shipping activity. Conversely, all locations displayed Flu/Pyr ratios of less than one and Flu/(Flu + Pyr) ratios of less than 0.4, indicating the presence of petroleum sources there15,17.
The comparison of PAH levels along the GOS (Red Sea) with those reported worldwide (Table 4) provides valuable insights into the current pollution status. Efforts were made to select references similar to our study for a meaningful comparison. Total PAH levels (μg/L) in various locations, including the Mississippi River, Susquehanna River in the USA, Seine River in France, Surface waters, Hangzhou, Rivers in Tianjin, The Yellow River in China Giresun Coast, Samsun Coast in Turkey were observed to be lower than those recorded in the present study. This indicates relatively lower PAH pollution levels in these areas compared to the Red Sea in Egypt (GOS) (Table 3). The comparison of PAH levels along the GOS (Red Sea) with those reported worldwide (Table 4) offers critical insights into the current pollution status. It underscores the need for comprehensive risk assessment and mitigation strategies. A meaningful comparison was made possible by carefully selecting references akin to our study. It is noteworthy that total PAH levels (μg/L) in various locations, including the Mississippi River, Susquehanna River in the USA, Seine River in France, Surface waters in Hangzhou, Rivers in Tianjin, The Yellow River in China, as well as the Giresun and Samsun Coasts in Turkey, were observed to be lower than those recorded in the present study along the GOS. This disparity suggests a heightened level of PAH pollution in the GOS region, warranting urgent attention and proactive measures to mitigate environmental and public health risks. In interpreting these findings, it becomes evident that the elevated PAH levels in the GOS could have significant ecological ramifications, including adverse effects on marine biodiversity and ecosystem functioning. Moreover, considering the potential bioaccumulation of PAHs in aquatic organisms and their subsequent transfer along the food chain, there may be implications for human health, particularly for communities reliant on marine resources for sustenance. To address these concerns, it is imperative to conduct robust risk assessments that encompass both ecological and human health dimensions. Such assessments should evaluate the sources, pathways, and fate of PAHs in the marine environment and their potential impacts on vulnerable species and human populations. Additionally, targeted monitoring programs should be established to track PAH levels over time and assess the effectiveness of pollution control measures. Based on the outcomes of these risk assessments, tailored mitigation strategies should be developed and implemented, focusing on reducing PAH emissions from industrial and anthropogenic sources, enhancing wastewater treatment infrastructure, and promoting sustainable land use practices. Public awareness campaigns and stakeholder engagement initiatives can also be crucial in fostering a culture of environmental stewardship and community resilience to PAH pollution.
TOC (total organic carbon)
The mean TOC concentration was 1.671 mg/L, ranging from 1.52 to 2.31 mg/L. Because of the decreased frequency of tidal floods in this semi-enclosed basin and the higher deposition of debris on the surface of the sediments, the Suez Bay region had the highest value for TOC. Additionally, oil rigs and refineries significantly impact this area. They lead to oil spills in a constrained environment and deteriorate water quality by a high percentage of organics.
Potential ecological risks of PAHs
The value of RQ was used to classify the risk grade (Table 2s) of individual and total PAHs. For total PAHs, the ecological risk of surface seawater (Table 5) was at a high-risk level in sites NO.1, 3, and 8 with RQNCs ≥ 800 and RQMPCs ≥ 1.0 and medium risk 2 with RQNCs < 800 and RQMPCs ≥ 1.0 in site No.2, 4, 6 and 7. Furthermore, total PAHs were more predictable to pose a concern to the environment in Suez than in the other sites. Except for one or two sites, the RQNCs of Acthy and Ace were < 1.0, indicating that the ecological risk of Acthy and Ace was insignificant in the surface saltwater of the GOS coast. Apart from Acthy and Ace, all other PAH compounds had RQMPCs greater than 1.0, indicating that 11 of the 16 PAHs posed a significant danger (Table 3). Furthermore, all 16 PAHs were most likely to cause harmful ecological effects with extremely high RQNCs. In summary, PAHs in surface seawater from the Red Sea's uppermost portion and the coastal shores of the GOS pose significant ecological harm to aquatic life49.
Possible ecological risks of PAHs on biodiversity
The detrimental effects of PAHs on aquatic creatures in natural habitats have been documented in earlier studies. The biomagnification of PAHs may adversely impact the productivity and health of the marine environment through the food chain (PAH troph), which may also harm the health of people who eat these aquatic species. PAH consumption is linked to an increased risk of cancer, birth abnormalities, and mutations. Even though HMW-PAHs have more significant carcinogenic risks than PAHs with smaller ring systems, fluoranthene is light-induced toxicity to coral. In fact, BaP toxicity is used in human health risk assessment to show the carcinogenic potential risk of PAH contamination with the intake of coral reef fish. Since BaP is the only PAH for which toxicological data network (TOXNET) are sufficient for the generation of a cancer potency factor (CPF) among all known other PAHs that may cause cancer, certain regulatory authorities have established a standard for quantitative risk assessment of PAHs (particularly for BaP) in food60,61. High levels of PAHs in fish livers posed a concern to the health of coral fish; they also disrupted the energy metabolism of Mytilus galloprovincialis60. Additionally, it has been suggested that PAH may interact with DNA through the BaPDE's binding to the nucleophilic centers of exocyclic amino groups of purines to form DNA adducts or other genotoxic effects when it is converted to the diol epoxides of BaPDE (benzo[a] pyrene-7,8-diol-9,10-epoxide)61. Coral reefs, in particular, are sensitive to oil spills because of the rugosity and porosity of their structures, which allow oil to be stored for extended periods. As a result, coral populations suffer from acute to chronic impacts, affecting reproduction and maintenance throughout time62. Significant amounts of organic substances or mixtures (for example, crude oil) can poison corals by altering their development, recruitment, and reproduction, resulting in substantial mortality rates for colonies. Regrettably, even modest quantities of PAHs in the environment can be rapidly absorbed and stored in corals63. Furthermore, the negative impacts of these PAHs on corals have been demonstrated. For example, benzo[a]pyrene (BaP) was found to decrease antioxidant enzyme activity in coral embryos, generating significant alterations in gene-expression pathways. It was shown that the PAH anthracene (Ant), which accumulates readily in cell membranes and alters cellular metabolism, may impede coral larval settling and survival. The combined interaction of UV light and fluoranthene (Flu) in coral reefs may result in 78% mortality and bleaching64.
Oil pollution also has an impact on mangrove trees, which continue to play an important role in maintaining ecological balance by giving nutrients to the maritime environment and forming forests of salt-tolerant plant species that contain a large number of marine microorganisms. Organic pollutants in mangroves, like as polycyclic aromatic hydrocarbons, have been a major source of worry due to their persistence, bioaccumulation, toxicity, and long-range transmission. This kind of pollution prevents growth, lessens photosynthesis, causes leaf necrosis, and eventually causes death. Mangrove roots have a larger abundance of HMW PAHs than LMW PAHs. This is because LMW PAHs can move from roots to shoots, but HMW PAHs are only weakly translocatable and firmly adsorbed in the roots. Through slow diffusion from the root surface via plasmodesmata, PAHs enter the tissues. Being hydrophobic, PAHs are bound to the lipid portions of the organelles and cell membranes after they have entered the tissues. By increasing the permeability of lipidic molecules, PAHs harm cell membranes. Additionally, the chloroplasts, mitochondria, and nuclei of cells are disrupted and fragmented by these organic contaminants, which can result in cell death. PAHs disrupt the regulation of the trans membrane electrical potential, which governs metal absorption and bioavailability, by harming the cell membrane. PAHs appear to impede metallothioneins, which play an important role in metal detoxification. Contamination of PAHs in mangrove sediments may potentially encourage unfavorable genetic changes in mangrove plants6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65.
Ecological risk of PTEs in coastal water
The ecological risk index (RI) is a crucial tool incorporating diverse factors like heavy metal concentration, ecological impact, toxicology, and environmental consequences. This enables the assessment of ecological risks linked to heavy metal pollution in both sediment and water. In this study, the investigation into the potential environmental risk index of PTEs in the Red Sea, as depicted in Fig. 2a, revealed an average RI of 3.38 and a maximum value of 10.06 for the collected samples. This suggests a relatively low ecological risk (RI < 30). The visualization in Fig. 2a establishes a connection between pollution levels and ecological risk across different locations in the study area. Notably situated near residential areas and petroleum extraction sites, the study area may contribute to heightened concentrations of PTEs in the Red Sea. Thus, a comprehensive assessment of the ecological risk associated with heavy metal contamination in aquatic ecosystems becomes imperative. This research plays a vital role in guiding effective management strategies and mitigating potential risks these trace pollutants pose.
Heavy metals pollution index and metal index
The HPI for studied heavy metal elements in the Red Sea by 2023 has been recorded, with a mean HPI value of 537.8 and a range of 38.02 to 2097.8 (Fig. 2b). According to Ref.41, almost 5.55%, 5.55%, 5.55%, and 83.33% of samples fall within the Good, poor, very poor, and unsuitable class, respectively, based on the HPI rating, indicating minimal contamination in surface water only in sample 1 while the other location is highly contaminated. To understand the impact of heavy metals on water quality, the MI method, alongside the HPI index, assessed the level of contamination based on the upper allowable limit values of the WHO guideline48. The average MI values were 20.5 and ranged from 1.5 to 101.2, indicating variation in the contamination degree with different locations (Fig. 2c). It was found that 11.11%, 22.22%, 11.11%, and 55.55% of samples fall within the partly affected, moderately affected, heavily affected, and severely affected class based on the MI rating, indicating minimal contamination in surface water only in sample 1 and sample 3 while the other location is highly contaminated.
Human health risk
This section focuses on evaluating health risks associated with exposure to various heavy metals through both ingestion and dermal absorption pathways. The assessment involves calculating hazard quotients (HQ) for non-carcinogenic and carcinogenic risks using hazard indices (HI). The results provide insights into the overall potential health risks for individuals, considering both children and adults, who may come into contact with these heavy metals. The emphasis is on understanding the combined impact of different exposure routes on human health.
Non-carcinogenic risk
The toxic elements cadmium, chromium, copper, iron, manganese, nickel, lead, and zinc were evaluated to determine the non-carcinogenic risk in children and adults (Fig. 3a–d). It's crucial to emphasize that the HQ oral values for both children and adults fall within the acceptable limit (below 1) for copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), and zinc (Zn). However, in specific instances, the HQ oral values for cadmium (Cd), chromium (Cr), and lead (Pb) surpass 1, indicating an elevated risk. Specifically, the HQ oral value for adults exceeded 1 in 16.7%, 22.22%, and 22.22% of the water samples for Cd, Cr, and Pb, respectively. Similarly, the HQ oral value for children exceeded 1 in 61.11%, 50%, and 22.22% of the water samples for Cd, Cr, and Pb, respectively. These percentages are tied to the location and timeframe of the study, underscoring the necessity of considering diverse factors like exposure duration and frequency, individual susceptibility, and environmental conditions in evaluating the actual human health risks. The hazard quotient (HQ) dermal values indicate that the potential health risks associated with dermal exposure to heavy metals are generally within acceptable limits for adults, staying below the threshold of 1 for all parameters. Conversely, the HQ dermal value for children exceeded 1 in 33.33% of the water samples for chromium (Cr) while remaining within acceptable limits for the other heavy metals. This highlights a concern for children exposed to Cr through dermal contact in a third of the studied water samples. As with oral exposure, these findings emphasize the importance of location-specific considerations and the need to account for various factors such as individual susceptibility, environmental conditions, and the duration and frequency of exposure when assessing actual human health risks. The results suggest that Cd, Cr, and Pb are the primary contributors to human health risks for both adults and children, whether through oral or dermal contact. Notably, in the case of dermal exposure, children exhibit increased vulnerability to chromium (Cr) from the coastal waters of the Red Sea. At the same time, adults face no significant risk through this route.
The hazard index (HI) is a vital tool for gauging the overall health risks linked to heavy metals in Siwa Oasis's surface water. It considers various exposure routes, including ingestion and dermal pathways, offering a comprehensive view of combined health hazards from heavy metal contamination. Calculated by summing Hazard Quotients (HQs) for each heavy metal and exposure route, HI values (Fig. 4) ranged from 0.23 to 12.94 for adults and 0.89 to 49.39 for children through oral exposure. HI values ranged from 0.02 to 0.81 for adults and 0.05 to 2.4 for children for dermal exposure. Analysis revealed that HI oral values for adults and children exceeded safe levels (HI > 1) in 61.11% and 88.88% of water samples, respectively, indicating a high-risk category for non-carcinogenic impact. Conversely, HI values for adults indicated that 100% of water samples fell within the low-risk category for dermal contact. However, HI values for children showed 61.11% in the low-risk class and 38.88% in the high-risk category for dermal contact. This study underscores children's vulnerability to oral and dermal exposure to heavy metals, stressing the need to monitor these metals in the untreated water of the Red Sea. With the high cost of treating and desalinating saltwater, a primary water resource due to the study area's distance from primary freshwater sources like the Nile River, understanding and mitigating potential health risks is crucial. Specifically, samples 10, 11, 12, 13, 15, 16, and 18 were identified as the most contaminated, posing notable non-carcinogenic risks based on the HI (dermal and oral).
Carcinogenic risk
Carcinogenic risks involve assessing the likelihood of developing cancer due to prolonged exposure to pollutants. Our study used the traditional Carcinogenic Risk (CR) calculation as a baseline and compared it with CR values obtained through Monte Carlo simulation. For adults, the CR oral values for cadmium (Cd), chromium (Cr), and lead (Pb) ranged between 0.0002 to 0.006, 0.00002 to 0.003, and 0.00003 to 0.02, respectively (Fig. 5a). For children, these values ranged from 0.0007 to 0.02, 0.00006 to 0.01, and 0.0001 to 0.06 for Cd, Cr, and Pb, respectively (Fig. 5a). Analyzing the oral contact with heavy metals from the Red Sea water, we found a high carcinogenic risk (CR > 1 × 10–4) in 100%, 66.66%, and 66.66% of water samples for Cd, Cr, and Pb, respectively, in adults. For children, this risk was identified in 100%, 83.33%, and 100% of water samples for Cd, Cr, and Pb, respectively. Moving on to dermal exposure, the CR dermal values for adults were between 0.0009 to 0.03, 0.0001 to 0.03, and 0.00001 to 0.007 for Cd, Cr, and Pb, respectively (Fig. 5b). For children, these values ranged from 0.003 to 0.08, 0.0004 to 0.08, and 0.00004 to 0.02 for Cd, Cr, and Pb, respectively (Fig. 5b).
Examining dermal contact with heavy metals from the water samples revealed a high carcinogenic risk (CR > 1 × 10–4) in 100%, 100%, and 44.44% of water samples for Cd, Cr, and Pb, respectively, in adults. For children, this risk was found in 100%, 100%, and 83.33% of water samples for Cd, Cr, and Pb, respectively. These results underscore the urgent need for an effective treatment technique for the coastal water of the Red Sea before using it for any purpose. Regular monitoring of heavy metal concentrations after desalination is crucial, given the substantial carcinogenic risk to the health of both children and adults.
Monte Carlo simulation
The Monte Carlo simulation, a statistical tool, anticipates health risks from eight heavy metals (cadmium, chromium, copper, iron, manganese, nickel, lead, and zinc). It estimates HQ (Fig. 6) for non-carcinogenic risks through oral and dermal exposure and CR for cancer-related concerns in cadmium, chromium, and lead (Fig. 7). HQ reveals potential health impacts, while CR assesses cancer risks. Tailored for adults and children, considering age-specific susceptibilities, this simulation enhances our understanding of heavy metal health risks. The predictions contribute vital insights for practical risk assessment and management strategies, which are crucial in safeguarding human health from environmental threats.
Non-carcinogenic risk
The Monte Carlo simulation results provide valuable insights into the non-carcinogenic health risks associated with heavy metal exposure through oral and dermal pathways. For adults (Fig. 6a), the HQ oral values were generally below 1, indicating low non-carcinogenic risk for most metals except for Pb, which showed a higher risk. In contrast, children exhibited higher non-carcinogenic risks (HQ oral > 1) for Cd, Cr, and Pb (Fig. 6b), highlighting potential health concerns in this age group. Interestingly, the predicted HQ dermal values for adults and children were consistently below 1 for all eight metals, suggesting dermal exposure poses a lower non-carcinogenic risk. This finding is reassuring, indicating that when in contact with the skin, the water samples do not pose a significant threat regarding non-carcinogenic health risks for adults and children (Fig. 6c,d),.
Comparing the Monte Carlo results with traditional calculations, the simulation method proved effective in reducing uncertainty and providing realistic estimates. The confirmation of previous calculations adds robustness to the study's findings, emphasizing the reliability of the Monte Carlo simulation in assessing non-carcinogenic health risks associated with heavy metal contamination in water samples. This contributes to understanding potential health risks and supports informed decision-making for water quality management.
Carcinogenic risk
Oral exposure routes
The assessment of carcinogenic risk (CR) from oral exposure to heavy metals in Red Sea water samples reveals distinct patterns between children and adults. Across all parameters, CR values consistently indicate a higher likelihood of cancer risk in children compared to adults. Adults demonstrated relatively lower estimated cancer risks. The 5th percentile CR oral values for adults were 0.0012, 0.00056, and 0.0014 for Cd, Cr, and Pb, respectively, with 95th percentile values of 0.0029, 0.0013, and 0.0035, indicating reduced potential risks compared to children (Fig. 7a). In contrast, children exhibited 5th percentile CR oral values of 0.005, 0.0022, and 0.0056 for Cd, Cr, and Pb, respectively, with elevated risks at the 95th percentile (0.011, 0.0052, and 0.0134) (Fig. 7b). Nevertheless, predicted CR through oral exposure suggests a high cancer risk in many Red Sea water samples for both children and adults (CR > 1 × 10–4). The comparison of calculated and predicted CR underscores the effectiveness of Monte Carlo simulation in providing accurate estimations of cancer risk, emphasizing the substantial carcinogenic impact of specific metals on both age groups. This highlights the urgency of implementing measures to mitigate heavy metal exposure in the coastal waters of the Red Sea, particularly in regions where risks are most pronounced.
Dermal exposure routes
Evaluating carcinogenic health risks through skin contact in children and adults uncovers notable trends. Children consistently show higher probabilities of cancer risk than adults across various parameters. For adults demonstrate lower estimated CR levels for cancer risk through dermal exposure, with 5th percentile values of 0.0058, 0.0054, and 0.0007 for Cd, Cr, and Pb, and 95th percentile CR dermal values at 0.014, 0.013, and 0.0017, respectively (Fig. 7c).
In contrast, children, the estimated 5th percentile CR levels for cancer risk through skin contact were 0.016, 0.015, and 0.002 for Cd, Cr, and Pb, respectively, with 95th percentile CR levels at 0.041, 0.038, and 0.005 (Fig. 7d). Overall, these findings suggest that both children and adults are at a heightened risk of developing cancer due to exposure to Cd, Cr, and Pb in water resources within the Gulf of Suez. Predicted cancer risk levels from the Monte Carlo simulation exceed the acceptable threshold (CR > 1.0E-04) in most water samples, emphasizing the potential for cancer development with sustained exposure to these metals. These results underscore the need to minimize metal contamination in water sources to mitigate carcinogenic health risks for adults and children.
The comparison between the calculated CR (Fig. 5) and predicted CR (Fig. 7) through dermal contact with heavy metals reveals a consistently high carcinogenic impact of the three metals on children and adults across most water samples collected from the Gulf of Suez. The close alignment and calibration of the calculated and predicted CR values underscores the effectiveness of the Monte Carlo simulation in predicting CR dermal values. This successful simulation application emphasizes its reliability in assessing the carcinogenic impact of heavy metals through dermal exposure, providing valuable insights for understanding and managing health risks associated with metal contamination in water sources.
Statistical analysis and pollution source identification
Cluster analysis
The identification of three distinct groups in the analysis of coastal saltwater from the Red Sea (Fig. 8), achieved through a hierarchical cluster analysis employing the Wards linkage method and Euclidean distance, underscores the significance of using advanced analytical techniques for studying the chemical attributes of heavy metals in marine environments. The analysis of seawater samples from the Red Sea identified distinct chemical clusters, shedding light on potential contamination sources and guiding tailored management strategies. In Cluster 1 (Cadmium, Chromium, and Manganese), industrial discharges along the coastline are implicated, emphasizing the need for strict regulations, ongoing monitoring, and environmentally conscious industrial practices. Cluster 2 (Nickel, Zinc, and Iron) suggests a connection to petroleum-related activities, emphasizing the importance of thorough inspections, maintenance of offshore platforms, and robust response plans for potential oil spills. Cluster 3 (Copper and Lead) points to contamination sources in urban runoff from tourist areas and leaching from desalination station infrastructure. Effective management entails promoting responsible tourism, sustainable coastal development, and employing corrosion-resistant materials in desalination infrastructure. The presence of organic compounds, likely from anthropogenic activities, underscores the importance of identifying and controlling pollution sources and implementing advanced treatment methods, such as activated carbon filtration, before desalination. This site-specific approach, tailored to the characteristics of each cluster, aims to tackle heavy metal contamination challenges in the Red Sea proactively and efficiently.
Principal component analysis
The principal component analysis (PCA) proved to be an invaluable tool in simplifying the intricacies of the water chemistry dataset, revealing underlying patterns and providing a nuanced understanding. The decision to utilize PCA was supported by a favorable Kaiser Meyer Olkin (KMO) value of 0.6, surpassing the acceptable threshold of 0.5. Bartlett's test of sphericity, yielding a significant result (0.02 < 0.05), further validated the appropriateness of the data for PCA. The scree plot (Fig. 9a) identified three principal components (PC1, PC2, and PC3), each with eigenvalues exceeding 1 (Fig. 9a,b). Collectively, these components elucidated 74.26% of the data's variability, with PC1 contributing 35.05%, PC2 23.09%, and PC3 16.11% to the cumulative explained variance. Examining variable loadings highlighted the robust associations between principal components and original variables. PC1 showcased a strong link with Fe (0.9), Ni (0.82), and Mn (0.64) (Table 3s). PC2 revealed a notable positive relationship with Cd (0.77) and Cr (0.73), coupled with a significant negative correlation with Zn (-0.79). PC3 demonstrated a substantial positive correlation with Cu (0.86) and Pb (0.9) (Table 3s). These findings underscore the pivotal variables influencing each principal component, offering valuable insights into the dominant factors shaping water chemistry patterns in the Red Sea. The cumulative explained variance emphasizes PCA's efficacy in capturing a substantial portion of the dataset's variability, complementing the insights derived from the hierarchical cluster analysis. The outcomes derived from the principal component analysis (PCA) seamlessly align with and enhance the insights garnered from the hierarchical cluster analysis, offering a holistic comprehension of heavy metal chemical attributes in the coastal saltwater of the Red Sea. The hierarchical cluster analysis unveiled three unique groups, each representing a specific chemical cluster with distinct heavy metal characteristics, and these clusters find further affirmation and elucidation through the results obtained from PCA. In the hierarchical cluster analysis, the identification of Cluster 1 (Cadmium, Chromium, and Manganese) linked to industrial discharges along the coastline receives robust support from PCA. The pronounced association between PC1 and variables such as Fe, Ni, and Mn in PCA underlines the influence of industrial activities in this cluster. Likewise, Cluster 2 (Nickel, Zinc, and Iron) in the hierarchical cluster analysis, indicating ties to petroleum-related activities, aligns seamlessly with PCA. The positive correlation of PC2 with variables like Cd and Cr and the negative correlation with Zn resonates with the indications of petroleum-related activities. Cluster 3 (Copper and Lead) receives confirmation through PCA in the hierarchical cluster analysis, hinting at contamination from urban runoff and desalination station infrastructure. The robust positive correlation between PC3 and variables Cu and Pb solidifies the association with these specific heavy metals. The overall consistency between hierarchical cluster analysis and PCA underscores the reliability of the findings. Both methodologies converge on identifying unique chemical clusters, offering valuable insights into potential contamination sources and guiding targeted management strategies to address the challenges of heavy metal contamination in the Red Sea.
Reasearch work limitation and future work
This study underscores the importance of adopting a holistic approach to combat heavy metal pollution, considering the broader environmental context and advocating for proactive measures. However, there are certain limitations in the current research that could be addressed in future studies. One key aspect for further exploration involves measuring heavy metal and PAHs in the sediments and PTEs concentrations in water samples after the desalination process. This future work could offer more reliable insights into the health risks associated with drinking water. To enhance our understanding of heavy metal dynamics, future research should investigate how concentrations vary over time post-desalination, covering different seasons and years. This comprehensive approach could provide valuable insights into the long-term effects of metal pollution. Additionally, exploring various water treatment technologies and their effectiveness in reducing metal levels would contribute to the development of more efficient mitigation strategies. Understanding the social impacts of metal contamination on communities and industries is crucial for formulating holistic management strategies. It is essential to consider not only the environmental implications but also the broader socio-economic consequences. Lastly, further exploration into the feasibility and consequences of implementing suggested measures, such as desalination stations and salt extraction companies, would offer practical insights for effective environmental management in the Gulf of Suez.
Conclusion
This in-depth study delves into the potential environmental and health hazards linked to PTEs and polycyclic aromatic hydrocarbons (PAHs) along the western coast of the Gulf of Suez. The investigation, centering on 16 PAHs, highlights concerning concentrations of pyrene (Pyr), particularly in the Suez area influenced by nearby refineries. Ratios confirming petrogenic sources point to the impact of industrial and shipping activities. Furthermore, heightened organic pollution in Suez Bay, exacerbated by oil rigs and refineries, is indicated by elevated levels of total organic carbon (TOC). The ecological risk assessment underscores significant risks, especially in Suez, emphasizing the urgent need for interventions to combat PAH contamination and protect aquatic ecosystems in the Red Sea. The identified clusters of industrial, petroleum-related, and urban runoff contamination are consistently validated by PCA. Notably, the Hazard Pollution Index (HPI) records a mean value of 537.8, exceeding permissible limits in 83.33% of samples, indicating significant contamination. The Metal Index (MI) ranges from 1.5 to 101.2, with 55.55% of samples categorized as severely affected. The Hazard Index (HI), calculated by summing hazard quotients (HQs) for each heavy metal and exposure route, ranges from 0.23 to 12.94 for adults and 0.89 to 49.39 for children through oral exposure. Through dermal exposure, HI values range from 0.02 to 0.81 for adults and 0.05 to 2.4 for children. Analysis reveals that HI oral values for adults and children exceeded safe levels (HI > 1) in 61.11% and 88.88% of water samples, respectively, indicating a high-risk category for non-carcinogenic impact. Conversely, HI values for adults indicated that 100% of water samples fell within the low-risk category for dermal contact. However, HI values for children showed 61.11% in the low-risk class and 38.88% in the high-risk category for dermal contact. The Monte Carlo simulation results provide valuable insights into the non-carcinogenic health risks associated with heavy metal exposure through oral and dermal pathways. For adults, the HQ oral values were generally below 1, indicating low non-carcinogenic risk for most metals except for Pb, which showed a higher risk. In contrast, children exhibited higher non-carcinogenic risks (HQ oral > 1) for Cd, Cr, and Pb, highlighting potential health concerns in this age group. Interestingly, the predicted HQ dermal values for adults and children were consistently below 1 for all eight metals, suggesting dermal exposure poses a lower non-carcinogenic risk. Addressing contamination before the desalination process is pivotal, as the identified pollutants not only impact desalination efficiency but also pose potential health risks if not mitigated. Immediate remediation efforts, stringent regulations, and enhanced monitoring are recommended, especially in high-risk areas like Suez. Implementing pre-desalination treatment methods, such as activated carbon filtration, is essential to ensure the quality of the produced water. Long-term monitoring, public awareness campaigns, and collaborative initiatives involving government, industries, and local communities are vital for sustained environmental health and the region's water sustainability. This study provides a roadmap for proactive measures, underlining the critical need to minimize contamination before embarking on the desalination process. The identified clusters and novel insights contribute to the development of targeted management strategies for effectively addressing heavy metal and PAH contamination challenges in the Red Sea. The future work can include monitoring the concentration of PTEs and PAHs in the sediments.
Data availability
The datasets utilized and/or analyzed during the current study are available upon request from the corresponding author.
References
Abou El-Magd, I., Zakzouk, M., Abdulaziz, A. M. & Ali, E. M. The Potentiality of operational mapping of oil pollution in the Mediterranean Sea near the entrance of the Suez canal using Sentinel-1 SAR data. Remote Sens. 12, 1352 (2020).
Abdallah, I. M. & Chantsev, V. Y. Modeling marine oil spill trajectory and fate off Hurghada, Red Sea coast, Egypt. Egypt. J. Aquat. Biol. Fish. 26, 41–61 (2022).
Mohsenpour, R. Investigation of oil pollution on aquatic animals and methods of its prevention. JAMB 9, 160–165 (2020).
Kelly, C. et al. Polycyclic aromatic hydrocarbons in oysters from coastal waters of the Lebanon 10 months after the Jiyeh oil spill in 2006. Mar. Pollut. Bull. 56, 1215–1218 (2008).
Jiang, R. et al. Heavy metal pollution and ecological risk assessment in the Maowei sea mangrove, China. Mar. Pollut. Bull. 161, 111816 (2020).
Zhang, Z.-W. et al. Heavy metal and organic contaminants in mangrove ecosystems of China: A review. Environ. Sci. Pollut. Res. 21, 11938–11950 (2014).
Jiang, D. et al. Multivariate analyses and human health assessments of heavy metals for surface water quality in the Xiangjiang River Basin, China. Environ. Toxicol. Chem. 38, 1645–1657 (2019).
Saeed, O. et al. Investigating the impacts of heavy metal (loid) on ecology and human health in the lower basin of Hungary’s danube river: A python and monte carlo simulation-based study. Environ. Geochem. Health https://doi.org/10.1007/s10653-023-01769-4 (2023).
Gad, M. et al. Groundwater quality and health risk assessment using indexing approaches, multivariate statistical analysis, artificial neural networks, and GIS techniques in El Kharga Oasis, Egypt. Water 15, 1216 (2023).
Mitra, S., Sarkar, S. K., Raja, P., Biswas, J. K. & Murugan, K. Dissolved trace elements in Hooghly (Ganges) River Estuary, India: Risk assessment and implications for management. Mar. Pollut. Bull. 133, 402–414 (2018).
Rajmohan, N. & Elango, L. Distribution of iron, manganese, zinc and atrazine in groundwater in parts of Palar and Cheyyar River Basins, South India. Environ. Monit. Assess. 107, 115–131 (2005).
Vineethkumar, V., Sayooj, V. V., Shimod, K. P. & Prakash, V. Estimation of pollution indices and hazard evaluation from trace elements concentration in coastal sediments of Kerala, Southwest Coast of India. Bull. Natl. Res. Cent. 44, 198 (2020).
Kapsalis, K., Kavvalou, M., Damikouka, I. & Cavoura, O. Investigation of petroleum hydrocarbon pollution along the coastline of South Attica, Greece, after the sinking of the Agia Zoni ΙΙ oil tanker. SN Appl. Sci. 3, 48 (2021).
Ahmed, O. E., Eldesoky, A. M. & El Nady, M. M. Evaluation of petroleum hydrocarbons and its impact on organic matters of living organisms in the northwestern Gulf of Suez, Egypt. Petrol. Sci. Technol. 37, 2441–2449 (2019).
Elmorsi, R. Distribution of Oil, Grease and Petroleum Hydrocarbons in Coastal Water and Sediments of Suez Bay. https://www.researchsquare.com/article/rs-367534/v1 (2021). https://doi.org/10.21203/rs.3.rs-367534/v1.
Mohebian, M., Sobhanardakani, S., Taghavi, L. & Ghoddousi, J. Analysis and potential ecological risk assessment of heavy metals in the surface soils collected from various land uses around Shazand oil refinery complex, Arak, Iran. Arab. J. Geosci. 14, 2019 (2021).
Soliman, Y. A. et al. Ecological assessment of polycyclic aromatic hydrocarbons in water, sediment, and fish in the Suez Bay, Egypt, and related human health risk assessment. Egypt. J. Bot. 63, 475–490 (2022).
Marufi, N., Oliveri Conti, G., Ahmadinejad, P., Ferrante, M. & Mohammadi, A. A. Carcinogenic and non-carcinogenic human health risk assessments of heavy metals contamination in drinking water supplies in Iran: A systematic review. Rev. Environ. Health https://doi.org/10.1515/reveh-2022-0060 (2022).
Mohammadi, A. et al. Probabilistic risk assessment of soil contamination related to agricultural and industrial activities. Environ. Res. 203, 111837 (2022).
Soleimani, A. et al. Health risk assessment and spatial trend of metals in settled dust of surrounding areas of Lake Urmia, NW Iran. Int. J. Environ. Anal. Chem. https://doi.org/10.1080/03067319.2022.2032013 (2022).
Eid, M. H. et al. New approach into human health risk assessment associated with heavy metals in surface water and groundwater using Monte Carlo Method. Sci. Rep. 14, 1008 (2024).
Eid, M. H. et al. Application of stable isotopes, mixing models, and K-means cluster analysis to detect recharge and salinity origins in Siwa Oasis, Egypt. Groundw. Sustain. Dev. 25, 101124 (2024).
Gaagai, A. et al. Application of water quality indices, machine learning approaches, and GIS to identify groundwater quality for irrigation purposes: A case study of Sahara Aquifer, Doucen Plain, Algeria. Water 15, 289 (2023).
Salem, S. et al. Applying multivariate analysis and machine learning approaches to evaluating groundwater quality on the Kairouan plain, Tunisia. Water 15, 3495 (2023).
Saeed, O. et al. Correction: Investigating the impacts of heavy metal(loid)s on ecology and human health in the lower basin of Hungary’s Danube River: A Python and Monte Carlo simulation-based study. Environ. Geochem. Health 45, 9785–9785 (2023).
Ibrahim, H. et al. Evaluation and prediction of groundwater quality for irrigation using an integrated water quality indices, machine learning models and GIS approaches: A representative case study. Water 15, 694 (2023).
Al-Mashreki, M. H. et al. Integration of geochemical modeling, multivariate analysis, and irrigation indices for assessing groundwater quality in the Al-Jawf Basin, Yemen. Water 15, 1496 (2023).
Eid, M. H. et al. Evaluation of groundwater quality for irrigation in deep aquifers using multiple graphical and indexing approaches supported with machine learning models and GIS techniques, Souf valley, Algeria. Water 15, 182 (2023).
Mohammadpour, A. et al. Trace elements human health risk assessment by Monte Carlo probabilistic method in drinking water of Shiraz, Iran. Int. J. Environ. Sci. Technol. 20, 3775–3788 (2023).
Batisha, A. Water desalination industry in Egypt. In Proc. of the Eleventh International Water Technology Conference (IWTC11 2007), Sharm El-Sheikh, Egypt, 15–18 (Citeseer, 2007).
El-Sadek, A. Water desalination: An imperative measure for water security in Egypt. Desalination 250, 876–884 (2010).
Elsaie, Y., Ismail, S., Soussa, H., Gado, M. & Balah, A. Water desalination in Egypt; literature review and assessment. Ain Shams Eng. J. 14, 101998 (2023).
Hussein, M. S. Assessment of the vulnerability of environmentally sensitive coasts to a large oil spill: The case of the northern part of the Gulf of Suez. Arab. J. Geosci. 14, 1899 (2021).
Dicks, B. Oil pollution in the Red Sea—Environmental monitoring of an oilfield in a coral area, Gulf of Suez. Deep Sea Res. A Oceanogr. Res. Pap. 31, 833–854 (1984).
Fine, M. et al. Coral reefs of the Red Sea—Challenges and potential solutions. Reg. Stud. Mar. Sci. 25, 100498 (2019).
Vodyanitskii, Y. N., Savichev, A. T., Trofimov, S. Y. & Shishkonakova, E. A. Accumulation of heavy metals in oil-contaminated peat soils. Euras. Soil Sci. 45, 977–982 (2012).
Zhu, H. et al. Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index. Trans. Nonferrous Metals Soc. China 22, 1470–1477 (2012).
Al-Hejuje, M. M. Application of Water Quality and Pollution Indices to Evaluate the Water and Sediments Status in the Middle Part of Shatt Al-Arab River (University of Basrah, 2014).
Ojekunle, O. Z. et al. Evaluation of surface water quality indices and ecological risk assessment for heavy metals in scrap yard neighbourhood. SpringerPlus 5, 560 (2016).
Tamasi, G. & Cini, R. Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy) possible risks from arsenic for public health in the Province of Siena. Sci. Total Environ. 327, 41–51 (2004).
Edet, A. E. & Offiong, O. E. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 57, 295–304 (2002).
Withanachchi, S., Ghambashidze, G., Kunchulia, I., Urushadze, T. & Ploeger, A. Water quality in surface water: A preliminary assessment of heavy metal contamination of the Mashavera River, Georgia. IJERPH 15, 621 (2018).
EPA, A. Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) (2004).
Jadoon, W. A. et al. Heavy metals in urban dusts from Alexandria and Kafr El-Sheikh, Egypt: Implications for human health. Environ. Sci. Pollut. Res. 28, 2007–2018 (2021).
Salem, S. B. H. et al. Applying multivariate analysis and machine learning approaches to evaluating groundwater quality on the Kairouan Plain, Tunisia. Water 15, 3495 (2023).
Flores, Y. G. et al. Integration of geological, geochemical modelling and hydrodynamic condition for understanding the geometry and flow pattern of the aquifer system, Southern Nyírség-Hajdúság, Hungary. Water 15, 2888 (2023).
Hamdy Eid, M., Szűcs, P. & Kovács, A. Problems threatening sustainability in Siwa Oasis and recommendations for understanding the sources of water quality deterioration. Geosci. Eng. 10, 138–153 (2022).
WHO. W. H. Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition (WHO, 2017).
Dotsika, E., Poutoukis, D., Michelot, J. L. & Kloppmann, W. Stable Isotope and chloride, boron study for tracing sources of boron contamination in groundwater: Boron contents in fresh and thermal water in different areas in Greece. Water Air Soil Pollut. 174, 19–32 (2006).
El Nemr, A., El-Sadaawy, M. M., Khaled, A. & Draz, S. O. Aliphatic and polycyclic aromatic hydrocarbons in the surface sediments of the Mediterranean: Assessment and source recognition of petroleum hydrocarbons. Environ. Monit. Assess. 185, 4571–4589 (2013).
Mitra, S. & Bianchi, T. S. A preliminary assessment of polycyclic aromatic hydrocarbon distributions in the lower Mississippi River and Gulf of Mexico. Mar. Chem. 82, 273–288 (2003).
Ko, F.-C., Baker, J., Fang, M.-D. & Lee, C.-L. Composition and distribution of polycyclic aromatic hydrocarbons in the surface sediments from the Susquehanna River. Chemosphere 66, 277–285 (2007).
Fernandes, M. B., Sicre, M.-A., Boireau, A. & Tronczynski, J. Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Mar. Pollut. Bull. 34, 857–867 (1997).
Chen, B. et al. Distributions of polycyclic aromatic hydrocarbons in surface waters, sediments and soils of Hangzhou City, China. Water Res. 38, 3558–3568 (2004).
Shi, Z. et al. Contamination of rivers in Tianjin, China by polycyclic aromatic hydrocarbons. Environ. Pollut. 134, 97–111 (2005).
Sun, J.-H. et al. Distribution of polycyclic aromatic hydrocarbons (PAHs) in Henan Reach of the Yellow River, Middle China. Ecotoxicol. Environ. Saf. 72, 1614–1624 (2009).
Aydın, H., Tepe, Y. & Ustaoğlu, F. Spatiotemporal PAH levels in surface water of the Southeastern Black Sea; baseline study from the Giresun shores. Mar. Pollut. Bull. 187, 114583 (2023).
Tepe, Y. & Taştekin, Ö. Spatiotemporal PAH levels in the coastal sediment of Samsun, a Metropolis between Turkey’s two largest deltas. Mar. Pollut. Bull. 181, 113907 (2022).
Tepe, Y., Aydın, H., Ustaoğlu, F. & Kaya, S. Seasonal distribution and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the Giresun coast of southeastern Black Sea. Mar. Pollut. Bull. 178, 113585 (2022).
Menezes, N., Cruz, I., Da Rocha, G. O., De Andrade, J. B. & Leão, Z. M. A. N. Polycyclic aromatic hydrocarbons in coral reefs with a focus on Scleractinian corals: A systematic overview. Sci. Total Environ. 877, 162868 (2023).
Yang, T. et al. Comparative study of polycyclic aromatic hydrocarbons (PAHs) and heavy metals (HMs) in corals, sediments and seawater from coral reefs of Hainan, China. Environ. Pollut. 264, 114719 (2020).
Basheer, M. A., El Kafrawy, S. B. & Mekawy, A. A. Identification of mangrove plant using hyperspectral remote sensing data along the Red Sea, Egypt. Egypt. J. Aquat. Biol. Fish. 23, 27–36 (2019).
Robin, S. L. & Marchand, C. Polycyclic aromatic hydrocarbons (PAHs) in mangrove ecosystems: A review. Environ. Pollut. 311, 119959 (2022).
Oladi, M. & Shokri, M. R. Multiple benthic indicators are efficient for health assessment of coral reefs subjected to petroleum hydrocarbons contamination: A case study in the Persian Gulf. J. Hazard. Mater. 409, 124993 (2021).
Aljahdali, M. O. & Alhassan, A. B. Ecological risk assessment of heavy metal contamination in mangrove habitats, using biochemical markers and pollution indices: A case study of Avicennia marina L. in the Rabigh lagoon, Red Sea. Saudi J. Biol. Sci. 27, 1174–1184 (2020).
Billah, M. M., Bhuiyan, M. K. A., Amran, M. I. U. A., Cabral, A. C. & Garcia, M. R. D. Polycyclic aromatic hydrocarbons (PAHs) pollution in mangrove ecosystems: Global synthesis and future research directions. Rev. Environ. Sci. Biotechnol. 21, 747–770 (2022).
Wu, J. et al. Pollution, sources, and risks of heavy metals in coastal waters of China. Hum. Ecol. Risk Assess. Int. J. 26, 2011–2026 (2020).
Acknowledgements
This publication is funded through the United States Agency for International Development (USAID). The contents are the responsibility of the Authors and do not necessarily reflect the views of USAID or the United States Government. The authors extend their appreciation to King Saud University for funding this work through Researchers Supporting Project number (RSP2025R133), King Saud University, Riyadh, Saudi Arabia.
Funding
Open access funding provided by University of Miskolc. This publication is funded through the United States Agency for International Development (USAID). The contents are the responsibility of the Authors and do not necessarily reflect the views of USAID or the United States Government. The authors extend their appreciation to King Saud University for funding this work through Researchers Supporting Project number (RSP2025R133), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
M.H.E., A.S.F., A.M.E & G.I.A. designed the study; A.S.F., E.A.M., and M.R.A. collected and prepared samples, performed field survey; A.S.F., E.A.M., and M.R.A. performed laboratory work; M.H.E., A.S.F., A.M.E & G.I.A prepared maps and graphs; M.H.E., A.S.F., A.M.E & G.I.A., E.A.M., and M.R.A. and M.R.A. wrote, reviewed, and edited the manuscript. All authors contributed extensively to the discussions about the work and in reviewing and revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sayed, F.A., Eid, M.H., El-Sherbeeny, A.M. et al. Environmental and health risk assessment of polycyclic aromatic hydrocarbons and toxic elements in the red sea using Monte Carlo simulation. Sci Rep 15, 4122 (2025). https://doi.org/10.1038/s41598-024-71547-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71547-4
Keywords
This article is cited by
-
Evaluation of the PAH content and VOCs emissions from waste wood from railroad ties
European Journal of Wood and Wood Products (2026)
-
Hydrogeochemical characterization and water quality evaluation associated with toxic elements using indexing approaches, multivariate analysis, and artificial neural networks in Morang, Tunisia
Environmental Earth Sciences (2025)