Abstract
Cotton fiber fabric with practicability and functionality attracts much attention and plays an important role in many occasions. However, its surface contains many hydroxyl groups to show a hydrophilicity, leading to easy adhesion to stains to limit the application. In this work, polyglycidyl methacrylate (PGMA) was used as an oily and flexible matrix and graphene oxide (GO) particles were used as a filler. PGMA/GO composite modified fabrics with hydrophobicity, self-cleaning feature and wear resistance were prepared by constructing surface coatings. Compared with original fabric, PGMA/GO composite modified fabric has a surface hydrophobicity attributed to organic oily characteristic of PGMA, and high surface roughness from GO surface enrichment. The reasonable GO content (1 wt%) in composite coating makes the modified fabric exhibit the best overall performance (surface water contact angle of ~ 151.7°; chromatism of ~ 2.4; large water contact angle of ~ 140.2° after 200 surface wear cycles). This work provides an effective method for an industrial production of high-performance waterproof cotton garment fabrics.
Similar content being viewed by others
Introduction
Warm and soft cotton cloth is very popular1. Cotton fabric is woven from natural polymer fibers with surface hydroxyls to lead to high hydrophilicity. Fabric surface cannot show a self-cleaning property to mean a limited outdoor application2. Therefore, the fabric surface should be chemically or physically modified to show a superhydrophobicity3. Superhydrophobic and self-cleaning traits of a lotus leaf surface were studied4. A superhydrophobic surface should have a water contact angle of over 150°. The Wenzel-Cassie model indicated that a superhydrophobic surface has a dual micro-nano rough structure and low surface energy5.
A highly rough surface of clothing fabric can be obtained by surface deposition techniques such as immersion, spray and hydrothermal reaction. For example, Zou et al.6 prepared diblock copolymer coatings to acquire durable superhydrophobic cotton fabrics. Besides, Chao et al.7 used silica particles to construct a rough fabric surface followed by using a stearic acid to treat the surface to prepare superhydrophobic cotton textiles. Moreover, Ge et al.8 prepared a chemically stable superhydrophobic cotton fabric by aqueous polydimethylsiloxane emulsion. In addition, Yang et al.9 prepared a superhydrophobic cotton fabric coated with titanium fluoride and TiO2. Furthermore, the sol–gel method was used to prepare superhydrophobic fabrics by ZnO/SiO210 and TiO211 particles. Apart from this, silica particles were combined with graphene sponge surface followed by tetraethoxysilane modification to achieve a superhydrophobic sponge for clothing application12. Additionally, Xu et al.13 used silica and polyacrylate to modify a cotton fabric surface to obtain a water contact angle of ~ 152.2°. In the end, by grafting cetyltrimethoxysilane onto polyurethane foam surface, a superhydrophobic foam for clothing study was gained14.
However, the above preparation methods of hydrophobic fabrics can embody the complexity in polymer synthesis, particle synergy and sponge structure. To simplify the preparation, the other strategies should be exploited. Ring-opened epoxy groups in polymer coating can react with hydroxyls of cotton fabric surface to lead to a strong interface adhesion15. Compared with graphene16, graphene oxide (GO) has an improved surface reactivity to favor a chemical bonding between GO and polymer to improve interface stability17. In this work, the poly(glycidyl methacrylate) (PGMA)/GO composite coating modified cotton fabrics were fabricated. By alkaline tetrahydrofuran (THF) and ammonia water, the chemical bonding between PGMA and fabric surface was realized using solution immersion. Evenly dispersed GO particles were chemically bonded with PGMA matrix. The optimal composite coating modified fabric with a rough surface can have a superhydrophobicity, self-cleaning feature and strong wear resistance. The synergy between PGMA and GO favors a long-term hydrophobic protection of cotton fabric surface.
Compared with the work reported by other researchers, the advantages of using GO in hydrophobic application are as follows. For the innovation of this work, the GO introduction into PGMA can greatly improve the coating performances. First, GO addition can result in an adjustable coating structure. Lamellar structure and small size of GO can improve the micro-nano roughness of coating surface. The GO/PGMA chemical bonding can depress the phase separation. Then, lamellar GO can be uniformly dispersed in PGMA to enable a high light transmittance of the coating. Finally, lamellar GO as a physical barrier inside the coating can increase the winding degree of the molecular diffusion path, favoring strong weather resistances of coating to acid, alkali and salt.
Methods
Materials
Analytically pure azobisisobutyronitrile (AIBN, 99.0%), glycidyl methacrylate (GMA, 97.0%), anhydrous dibutyl ketone (99.5%), methanol (99.5%), KMnO4 (99.5%), NaNO3 (99.0%), and THF (99.0%) were purchased from Aladdin (Shanghai, China). Graphite powder (99.0%, AR grade) was purchased from Wuhan Mengqi Technology Corporation (Wuhan, China). Concentrated H2SO4 (98.0%), H2O2 (30.0%), diluted hydrochloric acid, and ammonia water were used as received. Cotton fiber fabrics (5 cm × 5 cm) by plain weave were purchased from a local garment factory.
Synthesis of PGMA
PGMA was synthesized by a free radical polymerization reaction18. Firstly, AIBN (10 mg) and GMA (1 g) were fully dissolved in anhydrous dibutyl ketone (2 g) with magnetic stirring at 70 ℃ for 5 h. Afterwards, a portion of anhydrous dibutyl ketone was removed by rotation evaporation. Finally, the solution was reprecipitated in methanol. An overnight drying in a vacuum drying box was finished to achieve PGMA powder.
Fabrication of PGMA/GO composite modified fabrics
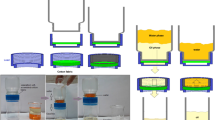
From graphite powder, GO particles were synthesized by a modified Hummers method19. The PGMA/GO composite coatings were constructed on the surfaces of cotton fabrics by solution immersion20. Weight fractions of GO in composites were 0 wt%, 0.5 wt%, 1 wt% and 2 wt% respectively, and the corresponding composite coatings were labeled with PGMA, PGMA/GO1, PGMA/GO2 and PGMA/GO3 respectively. First, PGMA and GO (according to a set proportion,1 g total weight) were added into THF (10 mL) followed by magnetic stirring at 25 ℃ for 30 min to achieve Mixture A. Then, ammonia water (0.5 mL/L) was gradually dripped into Mixture A for another 60 min to achieve Mixture B, in which the alkaline environment favored a ring opening of epoxy groups. After that, the entire fabric was immersed in Mixture B at 25 ℃ for 5 min, in which the dehydration-condensation reactions21 (between PGMA and fabric surface,between GO and PGMA) were finished. Eventually, the modified fabric was fully dried on a constant temperature heating table at 60 ℃ for 30 min. In Fig. 1, a schematic diagram of fabric surface treatment is given to exhibit an ease of operation. In Fig. 2, the desired performances of coatings are displayed, in which the nondestructive protection and strong adhesion are connected with PGMA while the high weather resistance and mechanical stability are related to GO. In addition, PGMA/GO composite coatings on the other substrates (glass slide and silicon wafer) were achieved by casting Mixture B followed by full drying at 60 ℃ for 30 min. Characterization methods of materials are given in Supporting Information.
Results and discussion
Characterization of PGMA and GO
Figure 3a shows a Fourier-transform infrared (FT-IR) result of PGMA. The absorption peaks (2965 cm−1 and 2890 cm−1) are attributed to methyl and methylene structures, the absorption peak (1720 cm−1) is caused by carbonyl structure, and the absorption peak (1150 cm−1) is triggered by C–O structure. The chemical structure of PGMA can be confirmed, and two characteristic peaks were marked with asterisk. In addition, Fig. 3b shows a transmission electron microscope (TEM) photograph of GO. The micron planar size (~ 6 μm), nanoscale thickness and irregularly flake-shaped morphology of GO can be verified. The GO particles can have a multilayer structure to suggest a self-lubricating property under surface friction. Finally, Fig. 3c shows a photograph of different mixtures consisting of the dissolved PGMA, dispersed GO and THF. Regardless of THF, the GO contents are 0 wt%, 0.5 wt%, 1 wt% and 2 wt% respectively from left to right. An increased GO content can favor a yellow change of the mixture due to the surface oxidation of GO, but all mixtures show an acceptable good transparency owing to good PGMA dissolving. To sum up, the chemical groups on PGMA, as well as GO micro-structure were confirmed, and the good transparency of PGMA/GO/THF mixture was verified.
Properties of composite coatings on non-fabric substrates
Figure 4a exhibits an ultraviolet–visible (UV–vis) absorption spectra of PGMA/GO composite films on glass slides. With an increase in GO content, the light transmittance of PGMA/GO composite film gradually decreases. Non-transparent GO can absorb light22 to reduce the transparency. The maximum light transmittance with the increased GO content is 96.1%, 95.0%, 92.8% and 90.3%, respectively. The transparency of these composites is good, resulting from high content of amorphous PGMA with optical isotropy. Additionally, Fig. 4a manifests a photo of these films with good transparency (GO content increases from left to right). High light transmittance of film is mainly decided by a reasonable proportion of PGMA and GO.
Figure 4b shows static surface water contact angles (WCAs) of composites on glass slides. Because organic PGMA is oily, the WCA on the surface of pure PGMA film reaches 88.5 ± 1.3° close to the threshold of hydrophobicity (90°)23. With an increase in GO content, the WCA of film surface can increase. When the GO content increases to 2 wt%, the WCA reaches 121.2 ± 3.1° to display a hydrophobicity due to an enrichment of GO particles on the film surface. Two-dimensional structure of GO24, as well as discontinuous uniform distribution of GO on the film surface, can increase the surface roughness to result in an increase of WCA25. If more GO particles are initially added, more GO particles are enriched on the film surface to enhance the surface rough structure to hold more air cushions. Air cushions fight against a wetting of water droplet to the coating surface. The increased WCA above can be understood.
To study the difference of surface roughness of composite films on silicon wafers, Fig. 5 shows scanning electron microscopy (SEM) pictures of film surfaces. In Fig. 5a, the surface of pure PGMA film is flat and smooth, because the entire PGMA homopolymer film has a homogeneous structure. In Fig. 5b,c, with an increase of GO dose, the film surfaces can exhibit a wrinkled structure. In Fig. 5d, by 2 wt% of GO introduction, the wrinkled feature of film surface becomes very obvious, and the surface roughness is greatly improved. GO particles can be enriched on the film surface to form a stable rough structure26. The increased GO dose can effectively improve the roughness and WCA of the surface of PGMA/GO composite film. In the progress of drying composite film, THF with a low boiling point can be quickly evaporated. PGMA can be quickly precipitated on the substrate surface, enabling the upward movement of GO particles (surface enrichment) to increase the surface roughness. The gap in solvent sensitivity of PGMA and GO should be emphasized. Furthermore, in Fig. S1 in Supporting Information, high-resolution X-ray photoelectron spectroscopy (XPS) results of O element in two samples (PGMA/GO2 film without ring opening of epoxy groups,PGMA/GO2 film with ring opening of epoxy groups) are given. To summarize, layered structure and small size of GO particles enriched on the coating surface can increase surface roughness to induce a high hydrophobicity of transparent coating.
Surface micro-structures of composite modified fabrics
Figure 6 manifests surface SEM pictures of the untreated and treated fabrics at different magnifications (treated fabric: 1 wt% GO in the coating). Figure 6a,c show the surface morphology of untreated fabric. The surface of each fiber is relatively smooth, explaining the fact that the original fabric cannot be hydrophobic27. After surface treatment of fabric in Fig. 6b,d, the surface of each fiber has an uniform polymer layer and many small protuberances (non-smooth characteristic). In Fig. 6d, the surfaces of fibers can show the obvious rough morphology, attributed to GO enrichment on the surface of composite coating bonded with fibers. The basic reason for GO surface enrichment here was stated in discussing the results in Fig. 5. However, the fabric substrate here has a pristine high surface roughness of micron grade from fiber woven structure, and thus the overall surface roughness of composite coating on the fabric can surpass that of the coating on the surface-smooth substrate (glass slide or silicon wafer). This gap in overall surface roughness can result in various surface hydrophobic properties and WCAs. Compared with untreated fabric, the treated fabric can have a significantly improved surface roughness to improve the surface hydrophobic property. To sum up, the surface modification of fibers on the fabric surface by PGMA/GO composite coating can lead to an improved surface roughness of fibers (ascribed to GO surface enrichment on the coating surface), and the fiber woven structure of fabric substrate can increase the surface roughness of treated fabric to some extent.
Chromatism and hydrophobic antifouling trait of treated fabric surfaces
Figure 7a shows surface chromatism (ΔE) values of fabrics treated with different composite coatings. Chromatism values were obtained by defining that the surface of original fabric has a zero chromatism. With an increase of GO content from 0 wt% to 2 wt%, the chromatism of fabric surface can be increased (1.1 ± 0.1, 1.8 ± 0.1, 2.4 ± 0.2 and 3.8 ± 0.3 in turn). With 2 wt% of GO dose, the surface chromatism exceeds 2.5 to fail to meet a requirement of practical application28. Low content of GO is beneficial to maintaining the initial color of fabric surface. When the GO dose is 1 wt%, the chromatism is acceptable. Highly transparent PGMA matrix (high light transmittance from amorphous property) in coating is responsible for the small chromatism of treated fabric.
(a) Surface chromatism values of fabrics treated with different composite coatings, (b) surface WCAs of fabrics treated with various composite coatings, (c) a photo of untreated fabric after water droplet contact, (d) a photo of treated fabric (GO dose: 1 wt%) after water droplet contact, and (e) photos of treated fabric (GO dose: 1 wt%) before and after a soaking in blue black ink.
Figure 7b manifests surface WCAs of fabrics treated with various composite coatings. The WCA of the fabric treated with neat PGMA is 125.5 ± 1.3° to suggest a hydrophobicity, because the fabric contains a large number of cotton fibers. The cotton fibers have a woven structure29 to lead to a micron-graded surface rough structure. Besides, oily PGMA has a low hydrophilicity. With an increase of GO dose, the WCA can be gradually improved. The maximum WCA is 154.2 ± 3.1° to suggest a superhydrophobicity30. On one hand, this surface superhydrophobicity results from a strong GO enrichment on the coating surface, increasing the nanoscale surface roughness. The improved surface roughness is conducive to realizing a high hydrophobicity and large WCA. On the other hand, the substrate of the coating is the fabric, and the surface of pristine fabric has a fiber woven structure to endow the surface with a micro-level rough structure. As a result, the WCAs herein are larger than the corresponding WCAs in Fig. 4b. The cotton fibers (on the fabric surface) and GO particles (being enriched on the coating surface) can contribute to a high surface roughness. The combination of both leads to the surface superhydrophobicity. As the GO dose is 1 wt%, the WCA can still reach 151.7 ± 2.8° to demonstrate a superhydrophobicity. Compared with the surface of a cotton fiber, the surface of the composite coating can have negligible hydrophilic hydroxyl groups and low surface energy, favoring a greatly depressed hydrogen bonding effect between water droplet and coating surface as well as an increased gap in the surface energy of water and coating. In terms of favorable surface chemical composition, the superhydrophobicity above is understood. Besides, a favorable surface micro-structure reflected by the high surface roughness for holding air cushions can notably contribute to the superhydrophobicity.
Figure 7c is a photo of untreated fabric after water droplet contact. The water droplets can quickly spread on the fabric surface, manifesting that this surface is rather hydrophilic. In Fig. 7d, a photo of treated fabric (GO dose is 1 wt%) after water droplet contact is given. The water droplets can maintain a droplet shape on the surface, illustrating that the treated fabric has a good hydrophobicity.
In Fig. 7e, the treated fabric (GO dose is 1 wt%) was soaked into water-based blue black ink for a certain period of time. There were no stains left on the surface after taking the fabric out, illustrating that the treated fabric has strong antifouling and self-cleaning traits ascribed to the surface hydrophobicity31. To sum up, the greatly improved surface hydrophobicity of treated fabric from the increased surface roughness can be confirmed, and the GO surface enrichment and fiber woven structure are important. Meanwhile, the good surface antifouling property and small color change of treated fabric can be obtained.
Friction resistance of treated fabric surfaces
In Fig. 8a, a photo of the fabric treated with the coating bearing 1 wt% GO before surface abrasion is given. The whole fabric is flat without deformation. In Fig. 8b, after 50 times of surface friction, the surface shows an obvious deformation. However, when the water droplets were dripped onto the surface of rubbed fabric in Fig. 8c, the surface still has a good hydrophobicity. Here, 50 times of surface friction cannot remarkably vary the chemical composition and micro-structure of the coating surface of this treated fabric.
(a) Photo of fabric treated with coating bearing 1 wt% GO before surface abrasion, (b) photo of the same fabric after 50 times of surface friction, (c) photo of this rubbed fabric after water droplet contact, (d) WCAs of the surfaces of fabrics treated with various coatings (0 wt% and 1wt% GO) under 200 times of surface friction, (e) SEM picture of the surface of fabric treated with coating containing 1 wt% GO after 50 times of friction, and (f, g) SEM images of fabric surface treated with pure PGMA coating under different magnifications after 50 times of friction.
Figure 8d shows the WCAs of the surfaces of the fabrics treated with two various composite coatings (0 wt% and 1wt% of GO respectively) under 200 times of surface friction. The fabric surface (1 wt% of GO dose) can have far larger WCAs than that (pure PGMA coating). With increasing the friction cycle number, the WCAs of two treated fabrics can be decreased, showing that the surface friction can have an impact on surface micro-structure. However, the affecting degrees for both are different. An improved GO dose can favor a strong surface friction resistance. A slow WCA reduction of treated fabric (1 wt% of GO) can be found. For this fabric, a strong hydrophobicity is well preserved with the WCAs altering from 151.7 ± 2.8° to 140.2 ± 1.1°. The WCA reduction of the fabric treated with pure PGMA coating is fast with an increase of friction times. This treated fabric has a significantly decreased surface hydrophobicity (WCAs: from 125.5 ± 1.3° to 73.2 ± 2.1°). The flexible amorphous PGMA film has a poor wear resistance, while the surface enriched GO particles are well wear-resistant32. About anti-wear trait, the layered structure of GO can favor a formation of the stable lubrication film between the mechanical contact surfaces, effectively reducing the friction coefficient to postpone the wear. The use of GO with surface enrichment effect can improve the friction resistance of coating surface, reduce the degree of friction damage to the coating surface, and maintain a large WCA after surface friction.
Figure 8e shows a SEM picture of the surface of the fabric treated with the composite coating containing 1 wt% GO after 50 times of surface friction. After 50 times of friction, the coating on the fiber surfaces cannot be damaged (without cracking). When the surface is subjected to friction, the lamellar structure of surface-enriched GO particles is conducive to layer-layer slippage33. Therefore, the composite coating on the fiber surfaces cannot be damaged. In addition, GO particles are hard to be wear-resistant. The GO particles may play a surface self-lubricating role34 in the friction process, ensuring that the micro-structure of the coating surface is well maintained.
Figures 8f,g manifest SEM images of the fabric surface treated with pure PGMA coating under different magnifications after 50 times of surface friction. The coating on the fiber surfaces can be greatly damaged with cracking, explaining the rapid decrease in the hydrophobicity of this fabric in Fig. 8d. Because PGMA is an organic amorphous polymer, its surface hardness is very limited, it is not well wear-resistant, and its surface is easy to be seriously damaged. This indicates that the GO surface enrichment is very important to a strong surface friction resistance. To summarize, the surface friction cannot severely damage the surface micro-structure of treated fabric. A high hydrophobicity is still maintained on the surface of treated fabric after surface friction. The use of GO can be in favor of a large WCA and strong friction resistance of treated fabric surface. The good durability of hydrophobic composite coating is confirmed.
Stress–strain curves of untreated fabric and PGMA/GO2 treated fabric are given in Fig. S2 in Supporting Information. The SEM images of PGMA/GO2 treated fabric before and after five laundering cycles by a soaping fastness tester are shown in Fig. S3 in Supporting Information. Compared with the similar fabrics reported by the other researchers35,36,37,38,39,40,41,42,43,44,45, the optimal composite coating treated cotton fabric prepared in this work can exhibit a better overall property.
Conclusion
PGMA powder and multilayer GO particles were synthesized. PGMA/GO composite coatings with various GO doses were fabricated on the surfaces of cotton fabrics. The increased GO dose can lower light transmittance of composite film due to light absorption of GO. The optimal PGMA/GO composite has a high light transmittance due to amorphous PGMA. GO particles can be surface enriched, and the increased GO dose improves the WCA of PGMA/GO composite film surface from the improved surface roughness. The high surface roughness from fiber woven structure and GO surface enrichment results in a superhydrophobicity. The surface of composite coating treated fabric has strong antifouling and self-cleaning properties due to surface hydrophobicity. The surface enrichment of GO with large hardness and self-lubricating property is responsible for the strong friction resistance of the surface of the composite treated fabric. This work favors an industrial fabrication of durable hydrophobic cotton garment fabrics with well-maintained color, good self-cleaning property and strong abrasion resistance.
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Mahbubul-Bashar, M. & Khan, M. A. An overview on surface modification of cotton fiber for apparel use. J. Polym. Environ. 21, 181–190. https://doi.org/10.1007/s10924-012-0476-8 (2013).
Shateri-Khalilabad, M. & Yazdanshenas, M. E. One-pot sonochemical synthesis of superhydrophobic organic–inorganic hybrid coatings on cotton cellulose. Cellulose 20, 3039–3051. https://doi.org/10.1007/s10570-013-0040-2 (2013).
Przybylak, M., Maciejewski, H., Dutkiewicz, A., Dąbek, I. & Nowicki, M. Fabrication of superhydrophobic cotton fabrics by a simple chemical modification. Cellulose 23, 2185–2197. https://doi.org/10.1007/s10570-016-0940-z (2016).
Li, S., Huang, J., Chen, Z., Chen, G. & Lai, Y. A review on special wettability textiles: Theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. A 5, 31–55. https://doi.org/10.1039/C6TA07984A (2017).
Sarshar, M. A., Jiang, Y., Xu, W. & Choi, C. H. Depinning force of a receding droplet on pillared superhydrophobic surfaces: Analytical models. J. Colloid Interface Sci. 543, 122–129. https://doi.org/10.1016/j.jcis.2019.02.042 (2019).
Zou, H. et al. Simple approach towards fabrication of highly durable and robust superhydrophobic cotton fabric from functional diblock copolymer. J. Mater. Chem. A 1, 11246–11260. https://doi.org/10.1039/C3TA12224G (2013).
Wang, H., Kumar, R. & Memon, H. Strongly hydrophobic and superoleophilic PMMA based nanocoated cotton fabrics. Coatings 10, 943. https://doi.org/10.3390/coatings10100943 (2020).
Ge, M. et al. A “PDMS-in-water” emulsion enables mechanochemically robust superhydrophobic surfaces with self-healing nature. Nanoscale Horizons 5, 65–73. https://doi.org/10.1039/C9NH00519F (2020).
Yang, M. et al. Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol-gel process. Carbohyd. Polym. 197, 75–82. https://doi.org/10.1016/j.carbpol.2018.05.075 (2018).
Xue, C. H., Yin, W., Jia, S. T. & Ma, J. Z. UV-durable superhydrophobic textiles with UV-shielding properties by coating fibers with ZnO/SiO2 core/shell particles. Nanotechnology 22, 415603. https://doi.org/10.1088/0957-4484/22/41/415603 (2011).
Dejang, N., Watcharapasorn, A., Wirojupatump, S., Niranatlumpong, P. & Jiansirisomboon, S. Fabrication and properties of plasma-sprayed Al2O3/TiO2 composite coatings: A role of nano-sized TiO2 addition. Surf. Coat. Technol. 204, 1651–1657. https://doi.org/10.1016/j.surfcoat.2009.10.052 (2010).
Zhao, X. et al. Hydrophobic, blocky silica-reduced graphene oxide hybrid sponges as highly efficient and recyclable sorbents. Appl. Surf. Sci. 486, 303–311. https://doi.org/10.1016/j.apsusc.2019.05.017 (2019).
Xu, L. H., Pan, H., Wang, L. M., Shen, Y. & Ding, Y. Preparation of fluorine-free superhydrophobic cotton fabric with polyacrylate/SiO2 nanocomposite. J. Nanosci. Nanotechnol. 20, 2292–2300. https://doi.org/10.1166/jnn.2020.17317 (2020).
Wu, F., Pickett, K., Panchal, A., Liu, M. & Lvov, Y. Superhydrophobic polyurethane foam coated with polysiloxane-modified clay nanotubes for efficient and recyclable oil absorption. ACS Appl. Mater. Interfaces 11, 25445–25456. https://doi.org/10.1021/acsami.9b08023 (2019).
Gupta, S. et al. Gold nanoparticles immobilized on stimuli responsive polymer brushes as nanosensors. Macromolecules 41, 8152–8158. https://doi.org/10.1021/ma801557u (2008).
Liu, J., Chen, S., Liu, Y. & Zhao, B. Progress in preparation, characterization, surface functional modification of graphene oxide: A review. J. Saudi Chem. Soc. 26, 101560. https://doi.org/10.1016/j.jscs.2022.101560 (2022).
Fu, J. et al. Enhancing interfacial properties of carbon fibers reinforced epoxy composites via layer-by-layer self assembly GO/SiO2 multilayers films on carbon fibers surface. Appl. Surf. Sci. 470, 543–554. https://doi.org/10.1016/j.apsusc.2018.11.168 (2019).
Rananavare, A. P. & Lee, J. Hydrophobic cotton fabric synthesized via dispersion polymerization from poly(glycidyl methacrylate) nanoparticles for self-cleaning applications. Progress Org. Coat. 170, 107006. https://doi.org/10.1016/j.porgcoat.2022.107006 (2022).
Shojaeenezhad, S. S., Farbod, M. & Kazeminezhad, I. Effects of initial graphite particle size and shape on oxidation time in graphene oxide prepared by Hummers’ method. J. Sci. Adv. Mater. Device 2, 470–475. https://doi.org/10.1016/j.jsamd.2017.09.003 (2017).
Duan, C., Han, L., Yang, G., Schubert, D. W. & Zhang, T. Multi-functional PDMS/MMT coating on magnesium substrates: hydrophobicity, durability, and EMI shielding. Mater. Today Commun. 39, 108603. https://doi.org/10.1016/j.mtcomm.2024.108603 (2024).
Mokoena, L. S. & Mofokeng, J. P. Synthesis and characterization of graphene oxide (GO) for the removal of lead ions in water. Carbon Trends 15, 100339. https://doi.org/10.1016/j.cartre.2024.100339 (2024).
Kausor, M. A. & Chakrabortty, D. Syntax of referencing in Comprehensive Analytical Chemistry (ed. Kailasa, S.) 39–72 (Elsevier, 2024).
Nguyen-Tri, P. et al. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 132, 235–256. https://doi.org/10.1016/j.porgcoat.2019.03.042 (2019).
Tissera, N. D., Wijesena, R. N., Perera, J. R., de Silva, K. M. N. & Amaratunge, G. A. J. Hydrophobic cotton textile surfaces using an amphiphilic graphene oxide (GO) coating. Appl. Surf. Sci. 324, 455–463. https://doi.org/10.1016/j.apsusc.2014.10.148 (2015).
Yan, Y. Y., Gao, N. & Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Colloid Interface Sci. 169, 80–105. https://doi.org/10.1016/j.cis.2011.08.005 (2011).
Rananavare, A. P. & Lee, J. Hydrophobic, self-cleaning and ultraviolet protective fibers achieved by graphene oxide nanosheets functionalized via poly(glycidyl methacrylate) nanoparticles. Fiber Polym. 25, 155–167. https://doi.org/10.1007/s12221-023-00419-x (2024).
He, X. & Sui, D. Surface morphology analysis and hydrophobic property design for cotton clothing fabric based on vinyl-bearing silane coupling agent. Chem. Pap. 77, 5807–5815. https://doi.org/10.1007/s11696-023-02898-x (2023).
Sheehy, D. E. & Schmalian, J. Optical transparency of graphene as determined by the fine-structure constant. Phys. Rev. B 80, 193411. https://doi.org/10.1103/PhysRevB.80.193411 (2009).
Shi, Q., Fang, K., Chen, W., Tan, Y. & Zhang, C. Designing a superhydrophobic cotton fiber coating exploiting TiO2@g-C3N4 layered structure for augmented photocatalysis and efficient water-oil separation. Int. J. Biol. Macromol. 264, 130596. https://doi.org/10.1016/j.ijbiomac.2024.130596 (2024).
Brassard, J. D., Sarkar, D. K. & Perron, J. Studies of drag on the nanocomposite superhydrophobic surfaces. Appl. Surf. Sci. 324, 525–531. https://doi.org/10.1016/j.apsusc.2014.10.084 (2015).
Manatunga, D. C., de Silva, R. M. & de Silva, K. M. N. Double layer approach to create durable superhydrophobicity on cotton fabric using nano silica and auxiliary non fluorinated materials. Appl. Surf. Sci. 360, 777–788. https://doi.org/10.1016/j.apsusc.2015.11.068 (2016).
Mulone, A. et al. Synchrotron X-ray spectromicroscopy analysis of wear tested graphene-containing alumina coatings. Carbon 227, 119245. https://doi.org/10.1016/j.carbon.2024.119245 (2024).
Papageorgiou, D. G., Kinloch, I. A. & Young, R. J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 90, 75–127. https://doi.org/10.1016/j.pmatsci.2017.07.004 (2017).
Markandan, K., Chin, J. K. & Tan, M. T. T. Recent progress in graphene based ceramic composites: A review. J. Mater. Res. 32, 84–106. https://doi.org/10.1557/jmr.2016.390 (2017).
Liu, H., Li, P., Zhu, P. & Liu, Y. Preparation and properties of flame retardant and hydrophobic cotton fabrics based on poly-(dimethylsiloxane-co-diphenylsiloxane, dihydroxy terminated)/ammonia phytate. Int. J. Biol. Macromol. 268, 131750. https://doi.org/10.1016/j.ijbiomac.2024.131750 (2024).
Sharif, R. et al. One pot application of a green chemistry-based finish for cotton fabric, providing hydrophobic, flame retardant, and antimicrobial properties. RSC Adv. 14, 6146–6155. https://doi.org/10.1039/d3ra07931g (2024).
Chen, X. Y., Ding, F., Hou, X. L. & Ren, X. H. Halloysite-based inorganic-organic hybrid coatings for durable flame retardant, hydrophobic and antibacterial properties of cotton fabrics. Int. J. Biol. Macromol. 277, 134357. https://doi.org/10.1016/j.ijbiomac.2024.134357 (2024).
Liu, I. C. et al. Fluorine-free nanoparticle coatings on cotton fabric: comparing the UV-protective and hydrophobic capabilities of silica vs silica-ZnO nanostructures. RSC Adv. 14, 4301–4314. https://doi.org/10.1039/d3ra08835a (2024).
Zhang, S. et al. Enhanced hydrophobicity and UV resistance of cotton fabrics through the synergistic effect of raspberry-shaped colored nanoparticles and polymethylhydrosiloxane. Appl. Surf. Sci. 637, 157849. https://doi.org/10.1016/j.apsusc.2023.157849 (2023).
Zheng, G. L. et al. Near infrared laser-mediated hydrophilicity-to-hydrophobicity switch of copper sulfide nanoflowers coating on cotton fabric. Prog. Org. Coat. 189, 108297. https://doi.org/10.1016/j.porgcoat.2024.108297 (2024).
Kong, Y. et al. Multifunctional flame-retardant cotton fabric with hydrophobicity and electrical conductivity for wearable smart textile and self-powered fire-alarm system. Chem. Eng. J. 487, 150677. https://doi.org/10.1016/j.cej.2024.150677 (2024).
Zhang, S. et al. Polymethylhydrosiloxane and ZIF-8/color nanoparticles enhanced the UV-resistance, antibacterial and hydrophobicity performance of cotton fabrics. Prog. Org. Coat. 182, 107702. https://doi.org/10.1016/j.porgcoat.2023.107702 (2023).
Liu, X. L. et al. Highly hydrophobic cotton fabric by in-situ co-deposition of lignin/metal particles for oil/water separation. Ind. Crops Prod. 204, 117393. https://doi.org/10.1016/j.indcrop.2023.117393 (2023).
He, J. L. et al. Hydrophobic and anti-fouling polyelectrolyte complex coating for durable flame-retardant cotton fabric. Cellulose 31, 5963–5977. https://doi.org/10.1007/s10570-024-05944-3 (2024).
Wang, P., Jin, Z. K., Mai, K., Zhu, L. & Lin, J. X. Wearability analysis of Ag nanoparticle-loaded cotton fabric modified by SPEEK/PVA blend polymer. Fiber. Polym. 25, 2131–2138. https://doi.org/10.1007/s12221-024-00588-3 (2024).
Acknowledgements
This work was supported by 2021 Science and Technology Project of Jiangxi Provincial Education Department of China (Grant No. GJJ212403). The authors express gratitude to Chinese Clothing Research Platform (Clothing Digital Sub-Platform) in Jiangxi Institute of Fashion Technology.
Author information
Authors and Affiliations
Contributions
X.Y. He wrote the main manuscript text and W.H. Zhou prepared all the figures. The authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, X., Zhou, W. Hydrophobic modification and durability protection of cotton garment fabric surfaces by graphene oxide/PGMA composite coatings. Sci Rep 14, 30174 (2024). https://doi.org/10.1038/s41598-024-71736-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71736-1
Keywords
This article is cited by
-
Green synthesis of superhydrophobic cotton filters using Pistacia atlantica gum for efficient oil and water separation
Scientific Reports (2025)
-
Enhancing textile properties using nanotechnology and 3D printing process
Journal of Sol-Gel Science and Technology (2025)