Abstract
Neoadjuvant endocrine therapy (NET) for hormone receptor-positive (HR+) breast cancer might be as effective as chemotherapy, with a better toxicity profile. Blocking a crucial process such as angiogenesis with sunitinib may have a synergistic effect with NET. We aimed to assess the efficacy and safety of neoadjuvant sunitinib plus exemestane in early-stage HR+/HER2-negative breast cancer. In this phase I/II study, postmenopausal women with HR+/HER2− stage II-III breast cancer received neoadjuvant exemestane at conventional dose of 25mg plus sunitinib in a 3 + 3 design at 25mg (3/1weeks scheme) or 37.5mg continuous dose, for 6 months. Coprimary endpoints were the recommended dose of sunitinib combined with exemestane and objective response. Secondary endpoints included safety and biomarkers of early response. For 15 months, 18 patients were enrolled, 15 at sunitinib 25mg and 3 at 37.5mg. Median age was 73, 77% of patients had T2 tumors and 67% node-positive disease. The most common grade 2 toxicity was asthenia (44%), as was hypertension (22%) for grade 3. No grade 4–5 were reported. Twelve patients (66%) achieved an objective response. VEGFR-2 levels significantly decreased after one month of treatment. Differential gene expression analysis showed downregulation of ESR1, PGR and NAT1 in post-treatment samples and upregulation of EGFR, MYC, SFRP1, and FOXC1. PAM50 analysis on 83% of patients showed a prevalence of luminal A subtype, both in pre-treatment (63.6%) and post-treatment tumors (54.5%). Sunitinib plus exemestane was associated with substantial yet reversible toxicities, providing safety, efficacy and biological impact insights of combining an antiangiogenic drug with hormone therapy in early-stage breast cancer.
Trial registration: Registered with ClinicalTrials.gov, NCT00931450. 02/07/2009
Similar content being viewed by others
Introduction
In postmenopausal women with hormone receptor-positive, HER2-negative (HR+/HER2−) breast cancer, neoadjuvant treatment with aromatase inhibitors (AI) might be a valid alternative to chemotherapy due to a better toxicity profile and a similar response rate1,2. In addition, the neoadjuvant setting provides a unique opportunity to identify potential biomarkers of response/resistance to novel therapeutic agents.
Angiogenesis is one of the hallmarks of cancer, being a mandatory step for tumor growth, survival, and development of metastases. Angiogenesis involves many bioactive molecules, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF). VEGF receptor (R) and PDGFR are often overexpressed in breast cancer and associated with poor response to treatment and survival outcomes3,4. In mice models of breast cancer, estrogens seem to induce VEGF expression, whereas treatment with AI has the opposite effect. In addition, numerous studies indicate that hormone-dependent tumors secrete VEGF in response to estrogens or progestogens and that exposure of breast cancer cells to VEGF can override the effects of endocrine therapy5. Sunitinib is an oral multitargeted tyrosine kinase inhibitor of VEGFR-1, -2 and -3, PDGFR-α and –β, stem cell factor R (KIT), FMS-like tyrosine kinase 3 (FLT 3), colony-stimulating factor R 1 (CSF-1R), and glial cell line-derived neurotrophic factor R (RET). In human breast cancer xenografts, the combination of sunitinib and chemotherapy led to a reduction in tumor growth, and a survival benefit. Sunitinib as single agent was well tolerated and showed antitumor activity in heavily pretreated patients with metastatic HR+ breast cancer6.
Clinical trials with sunitinib usually involve 50 mg 4/2 dosing regimen where sunitinib is dosed continuously for 4 weeks followed by a 2-week drug-free period. However, superficial lesions may re-grow during the 2-week rest period as observed in patients with advanced gastrointestinal stromal tumor (GIST) or metastatic renal cell carcinoma7,8. In early breast cancer, low dose sunitinib showed a significant increase in vascular normalization index, in a phase Ib/II trial when given prior to chemotherapy9. Continuous dosing of sunitinib at lower doses and without treatment rests might result in improved efficacy while maintaining good tolerability. Moreover, the use of antiangiogenic agents at initial stages of the disease, when fewer proangiogenic factors are present, may hypothetically result in significantly greater efficacy than using these agents at a later stage. Therefore, we hypothesized that both drugs taken in combination could have a synergistic antitumor effect in early HR+/HER2− breast cancer.
As this combination had not been previously assessed, this phase I/II neoadjuvant multicenter study SUT-EXE-08 (NCT00931450, date of first registration: 02/07/2009) was conducted to establish the dosage of sunitinib, which could be safely given with exemestane, together with objective clinical response of the combination in postmenopausal women with HR+/HER2− localized breast cancer. In addition, we aimed to assess biological effects of the combination specifically in terms of angiogenesis, proliferation, and the expression of biomarkers both before and during therapy.
Material and methods
Study design and participants
SUT-EXE-08 trial was a multicenter (Institut Català d’Oncologia l’Hospitalet and Hospital Universitari Arnau de Vilanova, Spain) phase I/II study (NCT00931450) to determine the safe dose level of sunitinib that could be given at a continuous dose in combination with exemestane at conventional dose (25 mg/d) in cycles of 4 weeks for 6 months as neoadjuvant treatment for postmenopausal women with Estrogen Receptor (ER)+/HER2− early breast cancer. Initially, it was designed as separate phase I and II trials, but due external reasons (explained later), it was ultimately conducted as a combined phase I/II trial. Two doses of sunitinib as a continuous regimen were defined: dose 0 as starting level dose at 25 mg QD, and dose 1 at 37.5 mg QD. Initially, two patients would receive daily treatment with exemestane plus 25 mg of sunitinib for 4 weeks. If no dose-limiting toxicity (DLT) would be observed, a third patient would receive treatment at the same dose level. In case no DLT observed either, the next patient would receive the next dose level: daily 25 mg of exemestane plus 37.5 mg of sunitinib. Otherwise, the dose level 0 cohort was expanded sequentially up to a maximum of 6 patients, and if there were not any DLTs, then escalated to the next dose level. Eligible patients were postmenopausal women (by bilateral oophorectomy, ≥ 60 years, or younger than 60 but amenorrhea for at least 12 months in the absence of chemotherapy, tamoxifen, and follicle-stimulating hormone and estradiol levels in the postmenopausal range) with histologically confirmed, stage II-III invasive breast cancer and ER ≥ 50% or Alfred score ≥ 6, and HER-2 negative by local assessment. Patients with confirmed lymph node involvement could be enrolled regardless of tumor size. Additional criteria were palpable lesion measuring more than 3 cm in at least one dimension, in case of node negative, Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, and adequate hematological, renal, and liver functions. Adequate left ventricular ejection function at baseline by either echocardiogram or MUGA and adequate organ function were also required. Patients were ineligible if they presented inflammatory (T4d) or metastatic disease, uncontrolled hypertension (systolic > 150 mmHg and/or diastolic > 100 mmHg), symptomatic heart disease, or history of recent hemorrhagic or thrombotic events. Concurrent use of drugs that are known CYP3A4 inhibitors or inducers or CYP1A2 inducers were not permitted during the study period.
All patients provided written informed consent before any study-specific procedures were done. The trial protocol and all amendments were approved by Hospital Universitari de Bellvitge (HUB) and Hospital Arnau de Vilanova Research Ethics Committees (AC 063/08). The trial was conducted in accordance with Good Clinical Practice guidelines, the provisions of the Declaration of Helsinki.
Objectives
The coprimary endpoints were clinical response using WHO criteria and the recommended dose of sunitinib combined with exemestane, while the secondary endpoints were the safety and viability of the study combination. The recommended dose of sunitinib was defined as the highest dose of sunitinib at which less than 2 patients out 6 experience a DLT. Adverse effects were classified according to NCI Common Terminology Criteria for Adverse Events (CTCAEv3.0). DLT was defined as toxicity ≥ grade 3 during the first cycle (4 weeks), with the following exceptions: febrile neutropenia, grade 4 neutropenia lasting less than 7 days, grade 3 thrombopenia without concurrent bleeding, and grade 3 nausea/vomits controlled with appropriate concomitant medication were not considered DLT. Other secondary endpoints encompassed relationship between study biomarkers expression before and during therapy and clinical response. Here we focused on angiogenesis, proliferation, and apoptosis biomarkers. PAM50 was also assessed in paired tumor samples, this is pre-treatment and at surgery.
Study assessments
During the treatment period, patients were evaluated every 2 weeks during the first two months and monthly thereafter. The periodical visits included vital signs, physical examination with breast exploration, tumor evaluation with caliper, and blood tests. Heart assessment (electrocardiogram and ventricular function) and radiologic evaluation by ultrasound (US) and mammography were carried out every 12 weeks. Breast magnetic resonance (MRI) was done at baseline, after 4 weeks of study treatment and prior to surgery (week 22). Patients who presented disease progression during the treatment were taken out from the study and treated according to investigator criteria. Following the 6 months treatment, surgery was performed between 7 and 15 days after the last sunitinib administration. Exemestane was prescribed until the day before surgery. Adjuvant chemotherapy was administered, when possible, in cases with baseline lymph node involvement, T4, and/or Ki-67 levels > 20%.
Regarding biomarkers, tissue samples were obtained by fine-needle aspiration or core needle biopsy (preferably) before starting the treatment, one month after and at surgery. All samples were formalin-fixed paraffin-embedded and immunostaining was performed to analyze Ki67 and phospho-ERK expression, and mean vessel density (by CD34). At the same time points, plasma levels of angiopoietin 2 (ANG 2), soluble VEGFR-2 and VEGF were analyzed by ELISA at Catalan Institute of Oncology (ICO)/Bellvitge Biomedical Research Institute (IDIBELL) by the Angiogenesis Group. PAM50 assessment was performed centrally at Translational Genomics and Targeted Therapies in Solid Tumors Lab at August Pi i Sunyer Biomedical Research Institute (IDIBAPS).
Sample size and statistical analysis
Due to the scarce clinical data on the combination of exemestane and sunitinib, we conducted a phase I study to confirm the safety of this combination. The number of patients to be enrolled in the study depended on the safety profile observed, at the two dose levels studied. A total of 3 to 12 patients were planned to be included in the study. The population for statistical analysis included the patients meeting all the inclusion criteria and none of the exclusion criteria, and receiving at least one dose of study treatment. All analyses were performed with the overall number of patients on an intention-to-treat basis.
Descriptive statistics were performed for all parameters, which included measures of central tendency and dispersion for quantitative variables, as well as absolute and relative frequencies for qualitative variables with their 95% confidence interval in both cases. Nanostring nCounter expression data was normalized with RUV-seq10. Then differences between pre- and post-treatment samples were assessed adjusting for patient using a likelihood ratio test. Multiple testing correction was applied using false discovery rate. Survival was assessed using Cox-Proportional Hazards models and associations with clinicopathological variables like ER or Progesterone Receptor (PgR) immunohistochemistry were assessed using Mann–Whitney U test (small sample size). All analyses were performed in R language v4.0.411.
Results
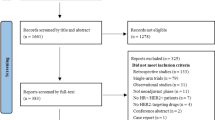
From July 2009 to September 2010, 18 patients with clinical stage II or III ER + breast cancer were enrolled in the study. Table 1 shows baseline characteristics. Median age was 73 (range 57–84) years, and most patients had T2 tumors (77%) and lymph node-positive disease (67%) at diagnosis. At the time of inclusion, most patients (62%) were ineligible for conservative surgery, according to the multidisciplinary team’s criteria, and 33% of patients had locally advanced disease. All patients presented positivity for ER expression, while 67% presented positively for PgR. Median Ki67 level were 16.4% (range 4–25). Half of participants had baseline-controlled hypertension taking at least one antihypertensive therapy at the beginning of the study.
All patients received at least one dose of study treatment. Three participants received dose level 0 of sunitinib (25 mg QD); among these, there were no DLTs. Therefore, next 3 participants received the treatment at dose level 1 (37.5 mg QD). At dose level 1, there was one DLT (grade 3 mucositis), so the cohort was expanded with 6 additional patients at dose level 0. Although it was not contemplated in the definition of DLT, due to persistent grade 2 asthenia in these additional patients by the third week of treatment that led to temporary sunitinib’s interruption, it was accorded to include 6 more patients at dose level 0 with a 3 weeks ON/1 OFF regimen (not predefined in the protocol).
Safety and sunitinib recommended dose
Main toxicities (all grades) were asthenia (n = 16, 88.9%), leucopenia (n = 10, 55.6%), mucositis (n = 8, 44.4%), diarrhea (n = 8, 44.4%) and high blood pressure (n = 6, 33.3%). Seventy-eight percent of patients presented grade 2 toxicities, mainly asthenia (n = 8), mucositis (n = 3), and diarrhea (n = 5) and 22% had grade 3, being hypertension (n = 4) the most frequently observed. There were no grade 4–5 adverse events or serious adverse events reported. Table 2 outlines grade 3 adverse events. Summary of the side effects are described in Supplementary Table 1. All patients received six cycles (24 weeks) of exemestane, however only 72% completed the 6 cycles of sunitinib. Median time on treatment with the combination was 21.7 weeks in the dose level 0 arm and 14.7 weeks at level 1. Only one patient (7%) temporarily held exemestane, while 44% of patients held sunitinib. Two patients needed one dose reduction of sunitinib, both in dose level 1.
Efficacy
Prior to surgery, one third of patients had achieved a clinical complete response and one third a partial response, all of them in level 0 dose. The proportion of patients achieving an objective response measured by US and MRI was 44% and 50%, respectively. Supplementary Table 2 summarizes the clinical and radiological response before surgery (week 22). No patient experienced progressive disease. All patients underwent surgery after 24 weeks of treatment. Seven patients (39%) were treated with breast conserving surgery (BCS), and the rest with mastectomy. In 13 patients (72.2%) the axillary approach was lymphadenectomy. No patient achieved a pathological complete response (pCR). Following surgery, 7 (39%) received adjuvant chemotherapy. Subsequently, 12 patients (67%) received adjuvant radiotherapy as indicated, which was given concurrently with adjuvant endocrine therapy. All patients received adjuvant endocrine therapy. With a median of follow up of 11.4 years (range 8.3–12.2; with a cut-off on November 28th, 2022), relapse occurred in 7 out of 18 participants. Of these, 1 experienced local relapse, while the other six encountered metastatic disease, primarily affecting the bone (n = 4, 66.7%). Median overall survival (OS) was 11.4 years (95% CI 9.6-NA) for all patients. Median OS difference depending on basal Ki67 levels between low (≤ 20%) vs high (> 20%) was statistically significative (p = 0.026) with median OS not achieved vs 5.8 years (95% CI 4-NA), respectively.
Translational analysis
In order to study possible biomarkers of response to angiogenic therapy we determined the levels of several angiogenesis-related molecules in plasma in all eighteen patients included in the trial. Interestingly, a 33% reduction in mean levels of VEGFR-2 was observed after one month of treatment (p = 0.0046), corresponding to 12,284 ± 3449 ng/ml at baseline; 8148 ± 3216 ng/ml at one month and 7732 ± 3052 ng/ml at 6 months. No differences were seen between 1 and 6 months, showing a relevant early decrease of VEGFR-2 plasma levels. In contrast, levels of VEGF did not change significantly over time. No correlation between basal plasma levels of VEGFR-2, VEGF and ANG-2, and radiological/pathological response was observed. Nevertheless, differences that are not statistically significant were observed between basal levels of ANG-2 and radiological response (2396 ± 650 ng/mL in stable disease, 3455 ± 1394 ng/mL in partial response; p = 0.08). Conversely, the assessment of mean vessel density assessed by CD34 immunostaining revealed no significant alterations within this timeframe. On the other hand, an absolute decrease of 3.7% in median Ki67 levels (p = 0.062) was observed between all pre- and post-treatment samples. Assessed by immunohistochemistry at the same time point, the combination of exemestane and sunitinib was able to significantly downregulate ER (p = 0.037) as well as PgR (p = 0.01).
PAM50 analysis was assessed in 15 out of 18 patients (83%) in pre-treatment and post-treatment tumor samples, but only seven cases were paired samples. In three patients was not assessed due to insufficient tumor tissue. In the pre-treatment tumor samples, 7 (63.6%) were luminal A, 2 (18.2%) were HER2 enriched, 1 (9.1%) was luminal B and 1 (9.1%) normal. In the post-treatment ones, the majority (6 cases, 54.5%) were luminal A, while the rest of them were normal subtype (5 patients, 45.5%). Figure 1 shows PAM50 changes between pre-treatment and post-treatment biopsies. In the comprehensive analysis of differential gene expression patterns, comparing genetic activity in samples collected before treatment with that in samples obtained after treatment, significant down-regulated genes were identified. These include PgR (PGR), MKI67, exonuclease 1 (EXO1), Ubiquitin-Conjugating Enzyme E2C (UBE2C), and Arylamine N-acetyltransferase 1 (NAT1). Conversely, among the up-regulated genes that stand out, we find Secreted Frizzled-Related Protein 1 (SFRP1), EGFR, MYC, and Forkhead Box C1 (FOXC1). Differential gene expression analysis between pre-treatment and post-treatment samples is represented in Fig. 2.
Discussion
The SUT_EXE-08 study met its primary endpoint by recommending a dose of sunitinib that could be combined with exemestane as preoperative ET in postmenopausal women with ER+, HER2− localized breast cancer. None of the patients at 37.5 mg dose level could tolerate full doses for the entire treatment period, and most participants needed an interruption at a continuous regimen; for this reason, the recommended dosage of the combination in our study was 25 mg exemestane plus 25 mg sunitinib in a 3 weeks ON/1 OFF regimen.
The recommended dose of sunitinib for gastrointestinal stromal tumor and advanced renal cell carcinoma is 50 mg taken once daily12,13, on a schedule of 4 weeks on treatment followed by 2 weeks off. In neuroendocrine tumors, a continuous regimen of 37.5 mg without interruption is approved14. Given the previous documentation of hyper-progression during the scheduled break9, our study was designed to adopt a continuous regimen. However, it’s worth noting that in our study, continuous regimens of either 37.5 mg QD or 25 mg QD were not well tolerated. Consequently, our recommended dose is an interrupted regimen.
Notably, hypertension was observed as one of the main toxicities (Grade 3, 22%) associated with the combination therapy, aligning with findings from other studies exploring antiangiogenic agents in breast cancer treatment. For instance, the phase III LEA study15, which compared the combination of endocrine therapy plus bevacizumab against endocrine therapy alone in advanced breast cancer, demonstrated a higher incidence of hypertension in the combination arm. Interestingly, this trial16 suggested a potential association between the development of hypertension during antiangiogenic treatment and improved clinical outcomes. However, in our study, we did not assess the relationship between hypertension and treatment response due to the limited number of patients enrolled.
Regarding efficacy, 66.6% of patients achieved a clinical objective response with the combination, and 50% presented a partial radiological response measured by MRI. Several phase II trials have evaluated the efficacy of neoadjuvant exemestane as single agent in early breast cancer, with an overall response rate between 51 and 63%17,18,19. In this trial, all patients underwent surgery, with 40% of patients being able to benefit from BCS, which is similar to the rate observed in other studies assessing neoadjuvant exemestane alone17. No patient achieved a pCR in our study. In neoadjuvant studies, pCR, a surrogate marker for prognosis, is considered a validated endpoint of long-term outcomes, especially in more biologically aggressive subtypes such as triple negative and HER2-positive breast cancers. However, since pCR is rarely achieved with neoadjuvant endocrine therapy (NET) in HR + breast cancer (< 5%) the primary endpoint of this study (clinical response) was chosen based on the long-term follow-up data of several NET trials. Importantly, in our study the median age was 74 years, and a significant number presented with comorbidities as well as locally advanced disease.
An extensive translational research to provide comprehensive biological information and to identify potential biomarkers that might predict benefit to the combination of exemestane and sunitinib was performed. Paired tumor biopsies before and after the combination were collected in all patients, assessing cellular and molecular changes. The only biologically significant marker identified was a marked reduction in VEGFR-2 plasma levels after four weeks of treatment, which was statistically significant. There was also an observable trend indicating a correlation between baseline ANG-2 levels and radiological response, although this did not reach statistical significance (p = 0.08). No significant associations were found when assessing mean vessel density through immunohistochemistry or with VEGF/VEGFR-2 plasma levels. Consequently, the early alterations in VEGFR-2 levels appear to hold potential as an early on-treatment predictive indicator of treatment response in our trial. Previous research has shown that VEGFR-2 expression in breast cancer is associated with poor overall survival and contributes to tumor progression and metastasis20,21. Therefore, targeting and inhibiting VEGFR-2 could be a viable strategy for intervention. In this context, sunitinib demonstrated to effectively and specifically decrease the levels of VEGFR-2 prematurely.
Regarding PAM50 assessment, the pre-treatment distribution of luminal A or B, HER2-enriched, and basal subtypes aligns with what is commonly reported in the literature19. Within our limited set of seven paired biopsies, we observed notable changes. These included instances where tumors potentially transformed to a luminal A subtype, and in some cases, shifted to a basal subtype, presumably as an indication of tumor response. These observations suggest that the combination of exemestane and sunitinib could significantly alter tumor biology. The combination of exemestane and sunitinib was able to significantly downregulate ER, as assessed by immunohistochemistry between pre- and post-treatment samples (p = 0.037) as well as PgR (p = 0.01). Conversely Arylamine N-acetyltransferase 1 (NAT1) was also downregulated. NAT1 expression has been associated with the ER and lowers levels been proposed as a bad prognostic marker for ER positive cancers22. Dismissively, some upregulated genes are also associated with tumor growth, as EGFR, MYC, SFRP1 and FOXC123,24,25,26. Following studies20,21 have proposed that antiangiogenic therapy could, under certain circumstances, lead to more invasive or metastatic behavior.
To our knowledge this trial represents the first one combining sunitinib with an aromatase inhibitor in neoadjuvant setting, marking a novel approach in ER+/HER2-negative breast cancer. This study however, had several limitations as it is a non-randomized study with small sample size, which was conducted several years ago, and the list of genes analyzed was limited, potentially overlooking other relevant genetic factors. And importantly, the development of sunitinib for breast cancer was discontinued by Pfizer in favor of other tumor settings (e.g. renal cell carcinoma, neuroendocrine and GIST). Nonetheless, the present findings improve insights into the safety, benefits and biological effects of combining an antiangiogenic drug with hormone therapy in early-stage breast cancer.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Beresford, M. J., Ravichandran, D. & Makris, A. Neoadjuvant endocrine therapy in breast cancer. Cancer Treat. Rev. 33(1), 48–57. https://doi.org/10.1016/j.ctrv.2006.10.003 (2007).
Ellis, M. J. et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: Evidence from a phase III randomized trial. J. Clin. Oncol. 19(18), 3808–3816. https://doi.org/10.1200/JCO.2001.19.18.3808 (2001).
Aalders, K. C., Tryfonidis, K., Senkus, E. & Cardoso, F. Anti-angiogenic treatment in breast cancer: Facts, successes, failures and future perspectives. Cancer Treat. Rev. 53, 98–110. https://doi.org/10.1016/j.ctrv.2016.12.009 (2017).
Ayoub, N. M., Jaradat, S. K., Al-Shami, K. M. & Alkhalifa, A. E. Targeting angiogenesis in breast cancer: Current evidence and future perspectives of novel anti-angiogenic approaches. Front. Pharmacol. https://doi.org/10.3389/fphar.2022.838133 (2022).
Dabrosin, C., Margetts, P. J. & Gauldie, J. Estradiol increases extracellular levels of vascular endothelial growth factor in vivo in murine mammary cancer. Int. J. Cancer 107(4), 535–540. https://doi.org/10.1002/ijc.11398 (2003).
Burstein, H. J. et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 26(11), 1810–1816. https://doi.org/10.1200/JCO.2007.14.5375 (2008).
George, S. et al. Phase II study of sunitinib administered in a continuous daily dosing regimen in patients (pts) with advanced GIST. J. Clin. Oncol. 24(18S), 9532 (2006).
Srinivas, S. et al. Continuous daily administration of sunitinib in patients (pts) with cytokine-refractory metastatic renal cell carcinoma (mRCC): Updated results. J. Clin. Oncol. 25(18S), 5040 (2007).
Wong, A. L. A. et al. Phase Ib/II randomized, open-label study of doxorubicin and cyclophosphamide with or without low-dose, short-course sunitinib in the pre-operative treatment of breast cancer. Oncotarget https://doi.org/10.18632/oncotarget.11596 (2016).
Bhattacharya, A. et al. An approach for normalization and quality control for NanoString RNA expression data. Brief. Bioinform. 22(3), 163. https://doi.org/10.1093/bib/bbaa163 (2021).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2021) https://www.R-project.org/.
Demetri, G. D. et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 368(9544), 1329–1338. https://doi.org/10.1016/S0140-6736(06)69446-4 (2006).
Méjean, A. et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N. Engl. J. Med. 379(5), 417–427. https://doi.org/10.1056/NEJMoa1803675 (2018).
Raymond, E., Dahan, L., Raoul, J. L., Bang, Y. J., Borbath, I., Lombard-Bohas, C., Valle, J., Metrakos, P., Smith, D., Vinik, A., Chen, J. S., Hörsch, D., Hammel, P., Wiedenmann, B., Van Cutsem, E., Patyna, S., Lu, D. R., Blanckmeister, C., Chao, R. & Ruszniewski, P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 364(6), 501–513. https://doi.org/10.1056/NEJMoa1003825. (2011). Erratum in: N Engl J Med. 2011 Mar 17;364(11):1082. PMID: 21306237.
Martín, M. et al. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the Letrozole/Fulvestrant and Avastin (LEA) study. J. Clin. Oncol. 33(9), 1045–1052. https://doi.org/10.1200/JCO.2014.57.2388 (2015).
De la Haba-Rodríguez, J. et al. Bevacizumab plus Letrozol (LEA clinical trial phase III). Using hypertension for finding biomarkers of efficacy. J. Clin. Oncol. https://doi.org/10.1200/jco.2015.33.15_suppl.2524.0 (2015).
Barnadas, A. et al. Exemestane as primary treatment of oestrogen receptor-positive breast cancer in postmenopausal women: A phase II trial. Br. J. Cancer 100(3), 442–449. https://doi.org/10.1038/sj.bjc.6604868 (2009).
Tubiana-Hulin, M. et al. Exemestane as neoadjuvant hormonotherapy for locally advanced breast cancer: Results of a phase II trial. Anticancer Res. 27(4C), 2689–2696 (2007).
Ellis MJ, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol. 2011 Jun 10;29(17):2342-9. doi: 10.1200/JCO.2010.31.6950. Epub 2011 May 9. PMID: 21555689; PMCID: PMC3107749.
Ebos, J. M., Lee, C. R. & Kerbel, R. S. Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin. Cancer Res. 15(16), 5020–5025. https://doi.org/10.1158/1078-0432.CCR-09-0095 (2009).
Ebos, J. M. & Kerbel, R. S. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat. Rev. Clin. Oncol. 8(4), 210–221. https://doi.org/10.1038/nrclinonc.2011.21. (2011). Erratum in: Nat Rev Clin Oncol. 2011 Jun;8(6):316. Erratum in: Nat Rev Clin Oncol. 2011;8(4):221. PMID: 21364524; PMCID: PMC4540336.
Carlisle, S. M. & Hein, D. W. Retrospective analysis of estrogen receptor 1 and N-acetyltransferase gene expression in normal breast tissue, primary breast tumors, and established breast cancer cell lines. Int. J. Oncol. 53(2), 694–702. https://doi.org/10.3892/ijo.2018.4436 (2018).
Sung, H. et al. Heterogeneity of luminal breast cancer characterised by immunohistochemical expression of basal markers. Br. J. Cancer 114, 298–304. https://doi.org/10.1038/bjc.2015.437 (2016).
Xu, J., Chen, Y. & Olopade, O. I. MYC and breast cancer. Genes Cancer 1(6), 629–640. https://doi.org/10.1177/1947601910378691 (2010).
Lo, P. K. et al. Epigenetic suppression of secreted frizzled related protein 1 (SFRP1) expression in human breast cancer. Cancer Biol. Ther. 5(3), 281–286. https://doi.org/10.4161/cbt.5.3.2384 (2006).
Ray, P. S. et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 70(10), 3870–3876. https://doi.org/10.1158/0008-5472.CAN-09-4120 (2010).
Acknowledgements
We thank all the patients for participating in this study, Biobank HUB-ICO-IDIBELL (PT20/00171) integrated in the ISCIII Biobanks and Biomodels Platform, and Xarxa Banc de Tumors de Catalunya (XBTC) for their collaboration. Primary funding was provided by Pfizer SA. This study was developed at the EORTC-ESMO-AACR Workshop on Methods in Clinical Cancer Research. We thank CERCA programme/Generalitat de Catalunya for institutional support.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.P., M.G.; A.L.; Data Curation: A.A., DC. Formal Analysis: A.A., D.C., O.C., A. P.; Funding Acquisition: S. P.; Investigation: S.M., A.P., H.V., M.G., S.P.; Methodology: V.N.; A.A., D.C.; Writing–Original Draft: B.F., S.P.; Writing–Review & Editing: all authors contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fullana, B., Morales, S., Petit, A. et al. Neoadjuvant sunitinib plus exemestane in post-menopausal women with hormone receptor-positive/HER2-negative early-stage breast cancer (SUT_EXE-08): a phase I/II trial. Sci Rep 14, 23626 (2024). https://doi.org/10.1038/s41598-024-72152-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72152-1