Abstract
In Europe mainly at winter season the PM levels exceed air quality limits, which correlated with the operation of solid-fired boilers. More and more people are returning to using these devices due to energy shortage caused by the pandemic and regional conflicts. In addition, the phenomena of co-burning fuels and municipal waste in residential boilers in primarily fuel poverty households increases further the amount of pollutants in the atmosphere. This study aims to correlate the quantity and quality of air pollutants with the type of fuel (wood and wastes) burned. Combustion experiments were conducted using oak fuel mixed with three waste groups: (1) plastics (PP, HDPE, PET); (2) textiles (polyester—PES, cotton—COT); and (3) papers (cardboard—CARD, glossy coated paper—GCP, 84C/PAP). The addition of waste to wood fuel altered the morphology of emitted particles. While waste burning doesn't always increase particle quantity, it significantly raises PAH concentrations. A strong relationship exists between waste type, particle morphology, and PAH quality, where with lower molecular weight PAHs linked to tar agglomerates and higher ones to soot agglomerates with inorganic crystals.
Similar content being viewed by others
Introduction
At certain times in Europe (typically in winter), atmospheric levels of particulate matter (PM) exceed air quality limit values, posing a serious environmental and health risk1. The main contributor to health damage from air pollution is particulate matter in the air2. The smaller the diameter of the particulate matter, the easier it is to enter the human body, where it can have negative effects2,3.
Although biomass is frequently cited as a clean energy source, urban air quality data indicates that the combustion of wood for residential heating and energy generation4,5,6 is a significant contributor to carbon air pollutant emissions in the EU, creating new challenges for air quality, human health, and global and regional climate7.
Due to inflation and increasing fuel prices caused by the energy crisis, more households are turning to solid fuel burning devices. Multiple studies have reported that wood burning for residential heating during the winter season is a significant contributor to particulate matter emissions8,9,10. In Europe, emissions from biomass burning in residential boilers during the winter season can account for up to 70% of total organic particulate matter emissions11. According to data from the European Environment Agency, households are responsible for over 68% of PM2.5 emissions in Poland and the Czech Republic, and over 80% in Slovakia, Croatia, Romania, and Hungary12. Therefore, households are considered the primary source of emitted PM and PAH13. Additionally, an increase in PAH levels in the atmosphere is associated with an increase in PM2.5 emissions14. Despite these findings, the Hungarian government launched a wood-burning program due to skyrocketing gas prices, which has further encouraged the use of the solid-fired boilers.
Moreover, the practice of co-burning fuels and municipal waste in residential boilers, primarily in households experiencing fuel poverty, remains a persistent problem worldwide, despite being prohibited. This issue is prevalent in several European countries, including the Czech Republic, Poland, Lithuania, Finland, Estonia, Hungary, and Romania15,16,17,18,19,20,21. In Poland, at least 10% of the population burns various plastics and waste in their boilers, while in Hungary 2–10% of households do the same17,22. In the Czech Republic almost 20% of households (approximately 620,000) use solid fuels for heating, with 534,000 households still relying on outdated manually operated boilers. In suburban and rural areas with fuel-poverty households, the amount of waste collected from households decreases by 15–25% during the winter season, and in the landfilled waste increases significantly the percentage of ash and dust20. This further supports the fact that waste incineration occurs in these households.
Exposure to combustion products (PM, CH4, CO, PAH, VOC) emitted from the burning of solid fuels contributes to chronic illnesses and acute health effects23, as well as indirectly causing death in the form of stroke, heart disease, lung cancer, and chronic respiratory diseases24. Depending on their source, solid particles contain organic (VOC, PAH, BC) and inorganic components25. Yadav et al.26 stated that during biomass burning, organic carbon (OC) and black carbon (BC) make up 50–70% of all PM emissions. Inorganic elements such as potassium, chlorine, and calcium account for about 10% of total PM emissions. In terms of BC and OC from biomass burning, Jayarathne et al.27 obtained lower values compared to total PM2.5 emissions. However, burning plastic and foil packaging separately resulted in an increase of OC and BC emissions up to 60% of total PM2.5 emissions. Wu et al.28 suggest that carbon-containing materials, especially PAHs, dominate PM2.5 emissions from residential burning, while metal emissions are mainly associated with coal-fired power plants. Therefore, PM2.5 from residential burning is more toxic due to the associated PAHs. As the main contributors to negative health effects among the PM emission fractions are the PAHs and metals29,30. Moreover, several studies have shown that 95% of emitted solid PAH containing particles have a diameter smaller than 3 μm31,32,33,34.
Therefore, improving air quality can primarily be achieved through reducing residential waste burning and optimizing the operation of boilers. That is why this research focuses on the practice of residential solid fuel combustion, including the illegal burning of waste, and the mechanisms behind the generation of air pollutants that accompany it. The goal is to uncover the relationship between the type of fuel and the gas and solid air pollutants emitted during combustion.
Materials and methods
Materials
During the experiments, the combusted wastes were classified into three groups: (1) Plastics: PP, HDPE, PET; (2) Textiles: Polyester (PES), Cotton (COT); (3) Papers: Cardboard (CARD), Glossy Coated Paper (GCP), 84C/PAP.
The selected wastes were fed into the boiler during the combustion experiments at a weight ratio of 1 waste/9 oak.
Methods
Raw material analysis
For the raw material analysis, the following procedures were used:
-
(1)
Mettler Toledo HB43-S Halogen Moisture Analyzer is used for the determination of moisture content in a sample. The instrument uses the principle of loss on drying, where a sample is heated at a constant temperature while the weight loss due to the evaporation of moisture is recorded.
-
(2)
Ash content determination is carried out by heating a sample at 850 ± 15 °C until a constant mass is achieved, following the MSZ EN ISO 18122:2016 standard.
-
(3)
The analysis is focused on the determination of C-, H-, N-, S- components using a Carlo Erba EA1108 elemental analyzer, following the MSZ EN ISO 21644:2021 standard.
-
(4)
The calorific value of a sample is determined using a Parr 6200 isoperibol bomb calorimeter, following the MSZ EN ISO 18125:2017 standard.
Residential-scale combustion experiments
Several examples of similar studies can be found in the literature, for example, Tomsej et al.20 added plastic waste at a rate of 7% w/w to beech firewood, while Edo et al.35 burned heterogeneous municipal solid waste with firewood, where the waste quantity was 20% w/w during combustion. The combustion experiments were carried out in a TOTYA S18 residential boiler, which is easily available and widely used in Hungary. A fan was placed in the flue pipe, which drew clean air with a pressure of 28.5 Pa. This method made the combustion more stable.
Three measurement lines were included in the measurement circuit (Fig. 1):
-
1)
The first line is employed to measure the pressure in the flue pipe.
-
2)
The second line is used to measure the composition and temperature of the flue gas, and to separate solid pollutants for PAH analysis.
-
3)
The particle size distribution of solid particles was carried out by the third line.
The data from each measurement line was recorded and further analyzed.
The pressure of the flue pipe determination was carried out using a differential pressure probe for the TESTO 400 multifunctional base unit. The composition of the flue gas was determined using a Horiba PG 250 gas analyzer, with simultaneous measurement of the flue gas temperature during extraction. To examine the morphology of solid particles and to detect the solid-phase polycyclic aromatic compounds, a quartz fiber filter was placed in the extraction pipe. The particle size distribution of solid particles was determined using a Dekati PM10 impactor, which allowed for the classification of particles larger than 10 µm, between 2.5 and 10 µm, and less than 2.5 µm. The DEKATI PMP-500/450 pumping system assisted in the extraction, and the extraction rate was set using the MASS-VIEW sensor in the system.
The combustion experiments consisted of cycles, whose length was determined by the combustion time of the fuel mixture fed at once. Based on this, each combustion cycle lasted for 20–25 min. This method enabled the comparability of combustion experiments with different fuel mixtures.
Morphological analysis of solid particles
The morphology of solid particles trapped on a quartz fibre filter from combustion experiments were investigated using a Zeiss EVO MA10 scanning electron microscope equipped with an EDAX probe.
Detection of polycyclic aromatic compounds
A gas chromatograph coupled with a mass selective detector (GC-MSD) was utilized to determine the PAHs. First, 10 μL deuterated PAH surrogate standard mix containing 20 μg/mL acenaphtene-d10, phenantrene-d10, fluoranthene-d10, benzo(a)anthracene-d12, benzo(a)pyrene-d12 and dibenzo(ah)antracene-d14 was added to the samples prior extraction. Ultrasonic agitation was used to extract each filter with 10 mL n-pentane at room temperature for 30 min. The solutions were then concentrated to 1 mL using a gentle stream of nitrogen, and 10 μL of benzophenone internal standard (20 μg/mL) was added to the resulting solutions before the gas chromatography measurement.
The GC–MSD system consisted of an Agilent 7890A GC and Agilent 5975C MSD. For the separation Restek Rxi-5MS (30 m × 0.25 mm × 0.25 μm) capillary column was used. The chromatographic conditions were as follows: injector temperature 300 °C, detector temperature 230 °C, quad temperature 150 °C. Helium was the carrier gas with 0.3 m/s linear velocity. 1 μL sample was injected with the use of pulsed splitless injection mode.
The recoveries of the surrogate dPAH ranged widely varied from 44% (acenaphtene-d10) to 88% (dibenzo(ah)antracene-d14). The recoveries were taken into account for each sample during the quantitative evaluation. It has to be noted, that the method is not necessarily suitable for analysing light PAHs such as naphtalene, acenaphthylene, acenaphtene, and fluorene.
The calculation of flue gas emission factors and PAH-emission factors were carried out via several calculation steps and using multiple equations. The corresponding details can be found in the Supplementary material.
Results and discussion
Evolution of emission factors from boiler combustion experiments
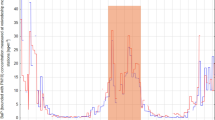
Flue gas emission factors were evaluated from the combustion of oak and different waste added in 10% w/w to the oak (Fig. 2). The co-combustion of PP and PE increases CO2 emissions, while the emissions of certain oxygen-containing compounds36, in this case, O2 and CO, decrease. Furthermore, it was found that materials (COT, CARD, GCP, 84C/PAP) with higher ash content than oak had higher CO emissions. The CO and O2 concentrations show a similar trend of variation. For example, with the addition of cotton and cardboard, not only CO but also O2 concentrations in the flue gas increased significantly. In practice, when the air factor is higher and the fuel–air mixing is better, the CO emissions will be lower37. This is counteracted by the introduction of too much air, which cools the firebox temperature significantly, thus favouring CO formation38. In poorer combustion conditions, the CO2 concentration should also decrease, but the addition of cotton and cardboard gave the highest CO2 concentrations.
This can be explained by the fact that materials with a high ash content, once burnt, spread across the woodpile, blocking the primary combustion air path. This leads to a deterioration in combustion quality, as indicated by increased CO. As the primary combustion air path is blocked, the flue gas fan draws in combustion air through the secondary vent, reducing the firebox temperature and increasing both the combustion air and the flue gas flow rate (Table S1). The CO2 value detected by the gas analyzer must also take into account the CO2 content of the air, which increases with the amount of air introduced.
The amount of CO2 generated is essentially determined by the temperature and design of the firebox and the C content of the fuel. Since plastics have a higher C content and at the same time less CO2 is produced during wood co-firing, it can be assumed that this parameter is not the determining factor in this investigation.
Emissions of solid-phase PAHs and PM during combustion
The co-incineration of wastes affected the amount of emitted PMs, although the percentage distribution of PMs by mass did not change for different sizes (Fig. 3). There is a close relationship between the emission factors of particulate matter, CO, and O2, as their trends are similar in change with waste materials. Indicators of incomplete combustion include not only CO but also PMs and PAHs39. However, the relationship between them is not clarified. Although some studies have found a strong correlation between PM and CO40, Carter et al.41 suggest that this close relationship cannot be described by a ratio or other measures.
Furthermore, it is clearly demonstrated that co-burning increased the amount of PAHs (polycyclic aromatic hydrocarbons) since burning waste generates more solid-phase PAHs than burning only wood (Fig. 3). In some cases, such as in the textile group, the volume of waste fed into the boiler was too high, blocking the flow of combustion air through the grate, which choked the combustion. In the case of the paper group, a large amount of residual ash left on the unburned wood caused the aforementioned fire suppression. These unfavourable conditions further increased the amount of PAHs produced.
In the case of PP and HDPE, although the amount added to wood does not appear significant (10% w/w), it still significantly affects the composition of the resulting PAHs. This is because the PAHs from plastics can be up to 4 orders of magnitude greater than the amount of PAHs from natural materials such as cardboard, glossy coated paper42, and presumably from wood. Additionally, the dominant compounds are those with 4–5 rings, which shifts the mechanism towards the formation of higher molecular weight compounds during co-burning with PP and HDPE.
Morphology of solid particles from combustion tests in boiler
Particulate matter emitted from residential combustion can take different morphologies, which consist of organic and/or inorganic. Atiku et al.43 investigated how the properties of fuels (different elemental composition, and physical properties) affect the composition of the resulting soot. During the flaming phase of combustion, black carbon emissions dominated. The black carbon consisted of spherical particles that were compressed into agglomerate chains and had a high C content. Inorganic compounds such as Na and K in the fuel contributed strongly to the formation of soot aggregates44. Scanning electron microscopy of wood-burning smoke particles from wood combustion by Hunter et al.45 observed soot agglomerates with similar chain structures. In this study, soot agglomerates with high C content are only detected during the co-combustion of PP and HDPE. These soot agglomerates were linked with large inorganic minerals (Fig. 4, Fig. S1).
Inorganic crystals are formed from inorganic compounds absorbed as nutrients by the plant and defined as ash. The inorganic content of wood is strongly influenced by its type, age and environment46,47. According to Torvela et al.48, the amount of component of inorganic ash emitted with solid particles from wood combustion increases with increasing burning temperature. This observation is strongly related to the fact that the highest flue gas temperatures were achieved by co-combustion of PP and HDPE waste, which can be attributed to their high C content (Supplementary Data). Torvela et al.48 further observed that primary ash particles (also known as fly ash agglomerate) were increased by sintering, deposition on larger particles, or condensation of gaseous hydrocarbons.
According to Bhandari49 and Luo et al.50, soot aggregates can mix with other chemical components, such as inorganic and organic materials, which can lead to more complex morphologies. These morphologies can take one of two forms: (1) the soot particles may be completely or partially coated by condensing inorganic materials; (2) or the soot particles may be attached to the coagulated particles which is also shown in Fig. 4. Freshly released soot particles are generally hydrophobic in nature, but the organic and inorganic substances coating their surface make them hydrophilic51,52. The chain fractal-like morphology of soot particles provides active sites for attachment to the surface, such as adsorption and deposition of other chemical species53,54.
Considering additional morphologies, irregular particles detached from the structure of burning material may also appear on the filter in individual cases (Fig. 5).
Lyubov et al.55, in experiments with emitted particles from a residential boiler, observed morphologies with sharp structures that were considered primary structures. Franus et al.56 discovered unburned carbonaceous matter in coal combustion particulate matter. According to Rabacal et al.57, irregular agglomerates are typically composed of unburnt carbon and non-volatile compounds. Some volatile compounds indicate that the ash volatiles in the solid product residue from biomass pyrolysis agglomerate in the fuel bed during combustion and then form alkali chlorides and sulphates on the surface by heterogeneous condensation, which are subsequently emitted with the flue gas. Yao et al.58 found that irregular fragments from coal combustion are mainly composed of unburnt coal, anhydrite, and calcite. At the relatively low temperatures (750–800 °C) available in a residential boiler, most of the minerals in coal do not melt but only soften.
Large spherical morphologies detected among the solid particles (Fig. 6). The first column shows Ca–Si mineral spheres, while the second column illustrates high-carbon tar spheres. The tar spheres are likely to contain an inorganic coating, as detectable amounts of Si, Na, K, and Ca are present. During the combustion of waste fuels with the same composition, both tar and mineral spheres can be found on the filters simultaneously (cotton, 84C/PAP).
By adding waste materials (except for PP and HDPE), larger-sized spherical structures were detected on the filters. Due to their relatively large size, these morphologies can primarily be identified as tarball agglomerates (Fig. 7, Fig. S2). According to Girotto et al.59, a particle can be considered an agglomerate if it consists of 8 or more spheres. Zangmeister et al.60 suggest that particles containing 2–7 spheres are in an intermediate growth phase before forming agglomerates.
Although many people believe that tarballs cannot form block structures61,62,63, Yuan et al.64 and Corbin and Gysel-Beer65 have provided examples to the contrary, where tarballs appeared as agglomerates with other materials.
Due to the fact that tarballs are mainly composed of carbon and oxygen, with only traces of inorganic components such as nitrogen, potassium, silicon, and sulphur59,66, the tarballs are likely coated with an inorganic layer whose composition varies as waste fuels are burned. Except for glossy paper, where calcium strongly dominates, the main components of the coating are silicon and potassium, with small amounts of sodium and sulphur also present. As explained by Leskinen et al.67, particles generated from the efficient combustion of biomass are predominantly composed of different alkali salts that correspond to the ash properties of the fuel. As a result, the emitted solid particles are compacted into a highly sintered regular chain-like structure. In their analysis of solid particles generated from burning cardboard, Liu et al.68 found that 91% of the primary particles were irregularly shaped organic-containing particles containing carbon and oxygen, as well as small amounts of silicon. The degree of sintering depends on the ratio of sulphate, chloride, and carbonate compounds in the alkali salt mixture.
These tarball agglomerates may likely contain significant amounts of PAHs, as the lowest PAH emissions were obtained during wood burning (where this morphology type was not detectable).
Conclusion
The study identified several morphologies of solid particles emitted during combustion and deposited on filters, including
-
(1)
soot agglomerates associated with inorganic crystal particles,
-
(2)
unique spherical particles that may be organic or high-carbon content,
-
(3)
irregular particle shapes detached from the fuel structure, and
-
(4)
tarball conglomerates.
The addition of municipal waste to the wood fuel at 10% w/w was found to clearly alter the morphology of emitted solid particles increasing PAH emissions. A close relationship was observed between the appearance of tarball conglomerates during waste-wood combustion and the increased concentration of polycyclic aromatic hydrocarbons (PAH) observed on the filter. These detected morphologies are closely related not only to polycyclic aromatic compounds but also to the fact of waste combustion. Based on the results, recommendations can be developed for fuel-poverty households to mitigate the effect of waste incineration.
Data availability
Data is provided within the manuscript and supplementary information files.
Abbreviations
- BC:
-
Black carbon
- CARD:
-
Cardboard
- COT:
-
Cotton
- EC:
-
Elemental carbon
- GCP:
-
Glossy coated paper
- HDPE:
-
High-density polyethylene
- OC:
-
Organic carbon
- PAH:
-
Polycyclic aromatic hydrocarbon
- PET:
-
Polyethylene-terephthalate
- PES:
-
Polyester
- PM:
-
Particulate matter
- PP:
-
Polypropylene
- VOC:
-
Volatile organic compound
References
Uramné Lantai Katalin. Levegőminőség vizsgálata, szmoghelyzetek elemzése az Észak-Magyarorszég Régióban. Anyagmérnöki Tudományok 38, 309–318 (2013).
McDuffie, E. E. et al. Source sector and fuel contributions to ambient PM2.5 and attributable mortality across multiple spatial scales. Nat. Commun. 12, 3594 (2021).
Xing, Y. F., Xu, Y. H., Shi, M. H. & Lian, Y. X. The impact of PM2.5 on the human respiratory system. J. Thorac Dis. 8, 69–74 (2016).
Oughton, D., Hodkinson, S. & Brailsford, R. M. Faber & Kell’s Heating and Air-Conditioning of Buildings (Routledge, 2015).
Szul, T. Analysis of heat source selection for residential buildings in rural areas. In BIO Web of Conferences 10: Contemporary Research Trends in Agricultural Engineering 02034 (EDP Sciences, 2018). https://doi.org/10.1051/bioconf/20181002034.
Curd, E. F. & Howard, C. A. Domestic hot water generation and distribution. In Introduction to Building Services (ed. Macmillan Press, L. T. D.) 10–15 (1996).
Cincinelli, A., Guerranti, C., Martellini, T. & Scodellini, R. Residential wood combustion and its impact on urban air quality in Europe. Curr. Opin. Environ. Sci. Health 8, 10–14. https://doi.org/10.1016/j.coesh.2018.12.007 (2019).
Brandelet, B., Rose, C., Rogaume, C. & Rogaume, Y. Impact of ignition technique on total emissions of a firewood stove. Biomass Bioenergy 108, 15–24 (2018).
Denier van der Gon, H. A. C. et al. Particulate emissions from residential wood combustion in Europe: Revised estimates and an evaluation. Atmos. Chem. Phys. 15, 6503–6519 (2015).
Ozgen, S. et al. Analysis of the chemical composition of ultrafine particles from two domestic solid biomass fired room heaters under simulated real-world use. Atmos. Environ. 150, 87–97 (2017).
Nugraha, M. G., Saptoadi, H., Hidayat, M., Andersson, B. & Andersson, R. Particulate matter reduction in residual biomass combustion. Energies 14, 3341 (2021).
European Environment Agency. Air Pollution. https://www.eea.europa.eu/themes/air.
Guerreiro, C. B. B., Foltescu, V. & de Leeuw, F. Air quality status and trends in Europe. Atmos Environ 98, 376–384 (2014).
Liu, J. et al. Atmospheric levels and health risk of polycyclic aromatic hydrocarbons (PAHs) bound to PM2.5 in Guangzhou, China. Mar. Pollut. Bull. 100, 134–143 (2015).
Csontos, Cs. Belefulladunk a fűtési szezonba? Tudás.hu http://tudas.hu/belefulladunk-a-futesi-szezonba/ (2020).
Kauneliene, V. et al. PAHs in Indoor and Outdoor Air from Decentralized Heating Energy Production: Comparison of Active and Passive Sampling. Polycycl Aromat Compd 36, 410–428 (2016).
Hoffer, A. et al. Emission factors for PM 10 and polycyclic aromatic hydrocarbons (PAHs) from illegal burning of different types of municipal waste in households. Atmos. Chem. Phys 20, 16135–16144 (2020).
Hoffer, A., Eholade, N., Tóth, Á., Machon, A. & Gelencsér, A. Az illegális hulladék égetés hatása a levegőminőségre Budapesten. XIV. Magyar Aeroszol konferencia. https://www.szfki.hu/mak2019/abstracts/talks/Section/1.3HofferAndrás.pdf (2019).
Timonen, H. et al. Household solid waste combustion with wood increases particulate trace metal and lung deposited surface area emissions. J Environ Manage 293, 112793 (2021).
Tomsej, T. et al. The impact of co-combustion of polyethylene plastics and wood in a small residential boiler on emissions of gaseous pollutants, particulate matter, PAHs and 1,3,5- triphenylbenzene. Chemosphere 196, 18–24 (2018).
Levegő Munkacsoport. Illegális lakossági szemétégetés hazánkban. https://www.levego.hu/sites/default/files/Szemetegetes_tanulmany.pdf.
Muzyka, R., Chrubasik, M., Pogoda, M., Tarnowska, J. & Sajdak, M. Py–GC–MS and PCA analysis approach for the detection of illegal waste combustion processes in central heating furnaces. Chromatographia 82, 1101–1109 (2019).
Winijkul, E., Fierce, L. & Bond, T. C. Emissions from residential combustion considering end-uses and spatial constraints: Part I, methods and spatial distribution. Atmos. Environ. 125, 126–139 (2016).
World Health Organization. Air pollution. https://www.who.int/health-topics/air-pollution#tab=tab_2.
Fan, Z. & Lin, L. Exposure science: Contaminant mixtures. In Encyclopedia of Environmental Health 645–656 (Elsevier, 2011).
Yadav, I. C. & Devi, N. L. Biomass burning, regional air quality, and climate change. In Encyclopedia of Environmental Health 386–391 (Elsevier, 2019).
Jayarathne, T. et al. Nepal Ambient Monitoring and Source Testing Experiment (NAMaSTE): Emissions of particulate matter from wood-and dung-fueled cooking fires, garbage and crop residue burning, brick kilns, and other sources. Atmos. Chem. Phys. 18, 2259–2286 (2018).
Wu, D. et al. Toxic potency-adjusted control of air pollution for solid fuel combustion. Nat. Energy 7, 194–202 (2022).
Jin, L. et al. Contributions of city-specific fine particulate matter (PM2.5) to differential in vitro oxidative stress and toxicity implications between Beijing and Guangzhou of China. Environ. Sci. Technol. 53, 2881–2891 (2019).
Shiraiwa, M., Selzle, K. & Pöschl, U. Hazardous components and health effects of atmospheric aerosol particles: Reactive oxygen species, soot, polycyclic aromatic compounds and allergenic proteins. Free Radic. Res. 46, 927–939 (2012).
Baek, S. O., Goldstone, M. E., Kirk, P. W. W., Lester, J. N. & Perry, R. Phase distribution and particle size dependency of polycyclic aromatic hydrocarbons in the urban atmosphere. Chemosphere 22, 503–520 (1991).
Shen, G. et al. Emission and size distribution of particle-bound polycyclic aromatic hydrocarbons from residential wood combustion. Biomass Bioenergy 55, 141–147 (2014).
Kakimoto, K. et al. Size distribution of chlorinated polycyclic aromatic hydrocarbons in atmospheric particles. Arch. Environ. Contam. Toxicol. 72, 58–64 (2017).
Jin, R. et al. Gas–particle phase partitioning and particle size distribution of chlorinated and brominated polycyclic aromatic hydrocarbons in haze. Environ. Pollut. 231, 1601–1608 (2017).
Edo, M., Ortuño, N., Persson, P.-E., Conesa, J. A. & Jansson, S. Emissions of toxic pollutants from co-combustion of demolition and construction wood and household waste fuel blends. Chemosphere 203, 506–513 (2018).
Ding, Z. et al. Microplastics as emerging contaminants in textile dyeing sludge: Their impacts on co-combustion/pyrolysis products, residual metals, and temperature dependency of emissions. J. Hazard. Mater. 466, 133465 (2024).
Vakkilainen, E. K. Solid biofuels and combustion. In Steam Generation from Biomass (ed. Vakkilainen, E. K.) 18–56 (Butterworth-Heinemann, 2017).
Johansson, L. S. et al. Emission characteristics of modern and old-type residential boilers fired with wood logs and wood pellets. Atmos. Environ. 38, 4183–4195 (2004).
Horak, J. et al. PAH emissions from old and new types of domestic hot water boilers. Environ. Pollut. 225, 31–39 (2017).
Pokhrel, R. P., Gordon, J., Fiddler, M. N. & Bililign, S. Determination of emission factors of pollutants from biomass burning of african fuels in laboratory measurements. J. Geophys. Res. 126, e2021JD034731 (2021).
Carter, E. et al. Assessing exposure to household air pollution: A systematic review and pooled analysis of carbon monoxide as a surrogate measure of particulate matter. Environ. Health Perspect. 125, 076002 (2017).
Mentes, D., Kováts, N., Muránszky, G., Hornyák-Mester, E. & Póliska, C. Evaluation of flue gas emission factor and toxicity of the PM-bounded PAH from lab-scale waste combustion. J. Environ. Manage. 324, 116371 (2022).
Atiku, F. A. et al. The impact of fuel properties on the composition of soot produced by the combustion of residential solid fuels in a domestic stove. Fuel Process. Technol. 151, 117–125 (2016).
Garcia-Maraver, A., Zamorano, M., Fernandes, U., Rabaçal, M. & Costa, M. Relationship between fuel quality and gaseous and particulate matter emissions in a domestic pellet-fired boiler. Fuel 119, 141–152 (2014).
Hunter, A. L. et al. Effect of wood smoke exposure on vascular function and thrombus formation in healthy fire fighters. Part Fibre Toxicol. 11, 62 (2014).
Fabio, E. S. et al. Contributions of environment and genotype to variation in shrub willow biomass composition. Ind. Crops Prod. 108, 149–161 (2017).
Hytönen, J. & Nurmi, J. Heating value and ash content of intensively managed stands. Wood Res. 60, 71–82 (2015).
Torvela, T. et al. Effect of wood combustion conditions on the morphology of freshly emitted fine particles. Atmos. Environ. 87, 65–76 (2014).
Bhandari, J. Morphology and Mixing State of Soot and Tar Balls: Implications for Optical Properties and Climate Implications for Optical Properties and Climate (Michigan Technological University, 2018).
Zhang, Y. et al. Sensitivity analysis of morphology on radiative properties of soot aerosols. Optics Express 26, A420–A432 (2018).
Zuberi, B. et al. Hydrophilic properties of aged soot. Geophys. Res. Lett. 32, L01807 (2005).
Zhang, R. et al. Variability in morphology, hygroscopicity, and optical properties of soot aerosols during atmospheric processing. Proc. Natl. Acad. Sci. USA 105, 10291–10296 (2008).
Popovicheva, O. et al. Water interaction with hydrophobic and hydrophilic soot particles. Phys. Chem. Chem. Phys. 10, 2332–2344 (2008).
Mikhailov, E. F., Vlasenko, S. S., Podgorny, I. A., Ramanathan, V. & Corrigan, C. E. Optical properties of soot–water drop agglomerates: An experimental study. J. Geophys. Res. 111, D07209 (2006).
Lyubov, V. K., Popov, A. N. & Popova, E. I. Emission of soot particles from the combustion of various fuels in boilers. IOP Conf. Ser. Earth Environ. Sci. 866, 012010 (2021).
Franus, W., Wiatros-Motyka, M. M. & Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 22, 9464–9474 (2015).
Rabaçal, M. & Costa, M. Particulate emissions from the combustion of biomass pellets. In Biomass pelletization: Standards and Production Vol. 85 (eds Garcia-Maraver, A. & Perez-Jimenez, J. A.) 101–135 (WIT Press, 2015).
Yao, Z. T. et al. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 141, 105–121 (2015).
Girotto, G. et al. Fractal-like tar ball aggregates from wildfire smoke. Environ. Sci. Technol. Lett. 5, 360–365 (2018).
Zangmeister, C. D. et al. Packing density of rigid aggregates is independent of scale. Proc. Natl. Acad. Sci. USA 111, 9037–9041 (2014).
Hand, J. L. et al. Optical, physical, and chemical properties of tar balls observed during the Yosemite Aerosol Characterization Study. J. Geophys. Res. 110, D21210 (2005).
Pósfai, M. et al. Atmospheric tar balls: Particles from biomass and biofuel burning. J. Geophys. Res. 109, D06213 (2004).
Wu, G. M. et al. Brown carbon in the cryosphere: Current knowledge and perspective. Adv. Clim. Change Res. 7, 82–89 (2016).
Yuan, Q. et al. Evidence for large amounts of brown carbonaceous tarballs in the Himalayan atmosphere. Environ. Sci. Technol. Lett. 8, 16–23 (2021).
Corbin, J. C. & Gysel-Beer, M. Detection of tar brown carbon with a single particle soot photometer (SP2). Atmos. Chem. Phys. 19, 15673–15690 (2019).
Tóth, A., Hoffer, A., Nyiro-Kósa, I., Pósfai, M. & Gelencsér, A. Atmospheric tar balls: Aged primary droplets from biomass burning?. Atmos. Chem. Phys. 14, 6669–6675 (2014).
Leskinen, J. et al. Effective density and morphology of particles emitted from small-scale combustion of various wood fuels. Environ. Sci. Technol. 48, 13298–13306 (2014).
Liu, L. et al. Morphology, composition, and mixing state of primary particles from combustion sources: Crop residue, wood, and solid waste. Sci. Rep. 7, 5047 (2017).
Acknowledgements
This research was supported by the National Research, Development, and Innovation Fund (Hungary), within the TKP2021‐NVA‐14 project. Financial support for the creation and complex development of National Laboratories within the RRF-2.3.1-21-2022-00014 Climate Change Multidisciplinary National Laboratory project is also acknowledged. DM thanks the support by the University Research Scholarship Program of the Ministry For Culture and Innovation from the Source of the National Research, Development and Innovation Fund.
Author information
Authors and Affiliations
Contributions
D.M. and C.P. conceived the scientific idea. D.M. carried out the experiments, analysed data and wrote the manuscript. B.F., B.V. carried out analysis and contributed to the manuscript text. A.J., L.F., and G.M. were carried out characterisation of the samples. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mentes, D., Jordán, A., Farkas, L. et al. Evaluating emissions and air quality implications of residential waste incineration. Sci Rep 14, 21314 (2024). https://doi.org/10.1038/s41598-024-72173-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72173-w