Abstract
The suprachiasmatic nucleus (SCN) contains a population of cell-autonomous circadian oscillators essential for entrainment to daily light–dark (LD) cycles. Synchrony among SCN oscillators is modified by photoperiod and determines functional properties of SCN clock cycling, including its amplitude, phase angle of entrainment, and free running periodicity (τ). For many species, encoding of daylength in SCN output is critical for seasonal regulation of metabolism and reproduction. C57BL/6 mice do not show seasonality in these functions, yet do show photoperiodic modulation of SCN clock output. The significance of this for brain systems and functions downstream from the SCN in these species is largely unexplored. C57BL/6 mice housed in a long-day photoperiod have been reported to perform better on tests of object, spatial and fear memory compared to mice in a standard 12 h photoperiod. We previously reported that encoding of photoperiod in SCN output, evident in τ in constant dark (DD), can be blocked by limiting food access to a 4 h mealtime in the light period. To determine whether this might also block the effect of long days on memory, mice entrained to 18 h:6 h (L18) or 6 h:18 h (L6) LD cycles were tested for 24 h object memory (novel object preference, NOP) and spatial working memory (Y-maze spontaneous alternation, SA), at 4 times of day, first with food available ad libitum and then during weeks 5–8 of daytime restricted feeding. Photoperiod modified τ as expected, but did not affect performance on the NOP and SA tests, either before or during restricted feeding. NOP performance did improve in the restricted feeding condition in both photoperiods, eliminating a weak time of day effect evident with food available ad-libitum. These results highlight benefits of restricted feeding on cognitive function, and suggest a dose–response relationship between photoperiod and memory, with no benefits at daylengths up to 18 h.
Similar content being viewed by others
Introduction
Circadian rhythms in mammals are regulated by a distributed system of cell-autonomous circadian oscillators (‘clock cells’) in the brain and most other body tissues1,2,3. A population of coupled clock cells in the retinorecipient suprachiasmatic nucleus (SCN) function as a central pacemaker essential for entrainment to daily light–dark (LD) cycles3,4. Synchrony within the population varies with photoperiod, and determines functional properties of SCN clock cycling, evident in the free-running periodicity (τ), phase of LD entrainment (ΦLD), amplitude and other parameters of clock-controlled rhythms5,6,7. These effects are readily observable by comparing locomotor activity rhythms of nocturnal mice housed under long-day or short-day photoperiods and then for a week or more in constant dark (DD). The longer the daylength, the shorter the active period (‘biological night’, to some minimum tolerable limit) and the shorter the circadian cycle in subsequent DD, an ‘aftereffect’ on τ that dissipates gradually over weeks to months8,9.
For many species, encoding of daylength in SCN output is critical for seasonal regulation of metabolism and reproduction10,11. House mice (Mus musculus), including the widely used C57BL/6 laboratory strain, do not show seasonality in these functions12, yet do show photoperiodic modulation of SCN clock output5,6,7. The significance of this for brain functions downstream from the SCN in these species is largely unexplored. One study reported that C57BL/6 mice housed under LD 20:4 (20 h light: 4 h dark) performed better on tests of object, spatial and fear memory compared to mice in LD 12:1213. This was associated with altered expression of circadian clock genes and growth factor genes in the hippocampus. Whether these effects depend on the SCN response to photoperiod, or are mediated by retinal pathways to other brain areas, was not tested directly.
Circadian rhythms can also be entrained by daily feeding schedules14, via phase-resetting effects of feeding-related stimuli on circadian clock cells in many brain areas and most body tissues1,15,16. Behaviorally, entrainment is evident in the form of a daily rhythm of locomotion and food seeking activity that anticipates a predictable daily mealtime (hereafter, food anticipatory activity, FAA)14,17,18. Notably, circadian oscillators in the SCN are not required for entrainment to feeding cycles, and remain synchronized to LD even when food access is restricted to the light period, a feeding schedule that induces robust FAA and markedly shifts circadian clocks in most other tissues and brain regions15,16.
Despite the limited response of SCN clock phase to scheduled feeding in LD, we have observed in C57BL/6 mice that daytime feeding schedules prevent the aftereffect of photoperiod on τ, measured in DD with food available ad libitum19. This provides an opportunity to test whether encoding of photoperiod in SCN output is required for long-day enhancement of memory; if it is, then feeding schedules that block the aftereffect of photoperiod on τ may also block effects on memory. We tested object and spatial memory, at 4 times of day, in mice entrained to short-day (LD 6:18; L6) or long-day (LD 18:6; L18) photoperiods, with or without free access to a running disc, first with food available ad libitum, and then during the second month of a daytime restricted feeding schedule. We found a significant performance benefit of daytime restricted feeding, a limited benefit of test time (when food was available ad libitum), but no benefit of the long-day photoperiod or of access to a running disc. In mice housed with a running disc, τ in DD was significantly shorter in the L18 group compared to the L6 group prior to restricted feeding, but not after. These results indicate that encoding of photoperiod in SCN output, at daylengths up to 18 h, is not sufficient to affect performance on tests of object and spatial memory. Benefits may accrue only at more extreme daylengths or light intensities.
Methods
General housing
Male C57BL/6N mice (n = 40, 4 weeks age, Charles River Quebec) were individually housed in standard clear plastic cages, with corncob bedding and a transparent, amber-tinted plastic igloo house. Room temperature was ~ 22 °C. White LED lights provided ~ 15 lx of illumination at the cage floor for either 6 h/day (L6, short-day photoperiod, N = 20 mice) or 18 h/day (L18, long-day photoperiod, N = 20). We chose 15 lx of light to be within the range of intensities recommended for long-term housing of nocturnal rodents20. We chose L18 to avoid unstable entrainment that may occur at longer daylengths (preliminary observations)8. Locomotor activity was detected by infrared motion sensors mounted above each cage and continuously monitored using Clocklab Data Acquisition Software (Actimetrics, IL USA). Half of the mice in each lighting condition had access to a horizontal running disc (15 cm diameter) mounted on top of the igloo house. Cages and litter were changed every two weeks to minimize disruption to circadian rhythms. All procedures were approved by the University Animal Care Committee at Simon Fraser University (protocol # 1208-P-16). All experiments and methods were performed in accordance with relevant guidelines and regulations. The manuscript adheres to the ARRIVE guidelines for reporting of animal research.
Lighting, feeding and testing schedules
After 6 weeks exposure to L6 or L18, the mice were recorded in DD for 2 weeks to assess τ of free-running activity rhythms. The mice were then re-entrained to the original LD cycle for 8 weeks. During weeks 7–8, the mice were habituated to gentle handling for 3–5 min/day.
Object recognition memory was assessed using a 3-day Novel Object Preference (NOP) test21,22,23. Spatial working memory was assessed using a one-trial Spontaneous Alternation (SA) test24. To account for potential diurnal variation in performance each mouse was tested at 4 times of day. Using External Time (ExT) notation created for asymmetrical photoperiods25, with hour 0 (ExT0) set to middle of the light period by convention, test sessions were centered at ExT0, 6, 12 and 18 (Fig. 1). Using Zeitgeber Time (ZT) notation, with ZT12 anchored to lights-off by convention (for nocturnal species), test session times were centered at ZT15, 21, 3 and 9. Sessions spanned one hour before and after the target time (e.g., ZT15 testing would start at ZT14 and end by ZT16). A maximum of 20 mice were tested per day at each timepoint. Before the first training session, the mice were habituated to the NOP apparatus on consecutive days. The mice then received 3 consecutive days of NOP testing at one of the 4 test times, followed by a 3–7 day break. This sequence was repeated until each mouse had been tested at all 4 timepoints, using different sets of objects previously screened to elicit equivalent attention in naïve mice. The mice were then tested for SA once every other day, until all 4 test times were sampled. Lighting during the NOP and SA test sessions was ~ 20 lx (soft white incandescent) for tests in the light period, and < 2 lx (red incandescent) for tests in the dark period.
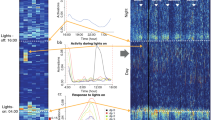
Circadian activity rhythms in long-day photoperiods (LD18:6, blue symbols, bars and lines) and short-day photoperiods (LD 6:18, orange), with food available ad-libitum or restricted to a 4 h daily meal in the light period. (A–D) Activity data in double-plotted actogram format. Each line represents 2 days, in 10 min time bins plotted left to right. Consecutive days are also aligned vertically. Black bars indicate time bins when activity counts were registered (bar height in quantiles). Green shading denotes mealtime during restricted feeding. Abbreviations: DD, constant dark; H, daily handling for habituation; NOP, days when mice were tested on the Novel Object Preference test; SA, days when mice were tested on for spontaneous alternation; RF, restricted feeding. (E,F) Group mean average waveforms of activity in mice housed with or without a running disc, in either L6 or L18 photoperiods, with food available ad-libitum. (H,I) Average waveforms during restricted feeding. Horizontal red bars denote NOP and SA test times. (G,J) Food anticipatory activity (FAA) expressed as a ratio of counts during the 3 h before mealtime relative to total daily activity (excluding mealtime) with food available ad-libitum or restricted. (K,L) The time of day of nocturnal activity onset (a measure of phase of entrainment, ФLD) on the first day of constant dark (DD), expressed in hours relative to lights-off time(ZT12), where positive values denote onsets before lights-off, and negative values onsets after lights-off. (M,N) Periodicity (τ) of activity rhythms free-running in DD before and after the restricted feeding schedule. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Following NOP and SA testing, the mice were food deprived overnight and then provided food for 10 h, beginning 4 h before lights-off (ZT8). Food was removed 2 h earlier every other day until a meal duration of 4 h was reached on day 7. After 4 weeks of restricted feeding, the NOP and SA tests were repeated as above. New sets of objects were used in the NOP tests. One day after the final SA test, food was provided ad libitum and the LD cycles replaced by DD for two weeks.
Novel object preference (NOP)
Mice were first habituated to the test apparatus by placing them in the opaque white, rectangular open field (55 × 37 × 33 cm) for 10 min on two consecutive days. The 3-day NOP task consisted of two 5-min sample trials and one 5-min choice trial separated by 24-h intervals. For the sample trials, two identical objects were placed 9 cm from the side and back walls. For the choice trial, one of the objects was replaced with a novel object. The mice were always placed in the open field facing away from the objects. A USB web camera was mounted above each open field to record all trials. Objects and open fields were cleaned with 50% ethanol and dried with paper towel between mice.
NOP data analysis
Object exploration was scored when a mouse directed its nose toward an object at a distance of 2 cm or less26. Climbing, sitting, or chewing on the object were not scored as exploration. Scoring was done manually by an experienced coder, blinded to group assignment, using JWatcher (v1.0, JWatcher, USA) (https://www.jwatcher.ucla.edu). Scoring drift checks were conducted by a second experienced coder, also blinded to groups. Mice that explored for < 1 s during the choice phase were excluded from the analysis. Preference for the novel object was quantified as a Discrimination Index (DI):
DI scores > 0.5 represent a preference for the novel object, from which is inferred memory of the familiar object.
To exploit the power advantage of a within-subjects design, each mouse was tested at all four timepoints. In the ad libitum food access phase of testing, some mice did not meet the inclusion criterion for total exploration. A second exclusion criterion was implemented such that mice were excluded from statistical analyses if they failed to perform on 2 out of 4 timepoints. This resulted in a final sample size of 13 mice in the L18 photoperiod group and 14 mice in the L6 photoperiod group. In the restricted feeding phase of testing, all mice met the exploration criterion.
Spontaneous alternation (SA)
Performance on the NOP test is thought to rely on the perirhinal cortex rather than the hippocampus21,22,23. To assess hippocampus-dependent memory, the mice were also tested for spontaneous alternation24. During each trial, mice were first confined for 1 min to the start arm of an opaque white 3-arm Y-maze (arm length 16 cm, arm width 8 cm, wall height 30 cm). A guillotine door was then lifted allowing mice to freely explore all 3 arms for 7 min. Spatial working memory is inferred by an exploration pattern characterized by alternating between arms, rather than by entering arms in a random sequence. Alternation was scored when mice entered each of the three arms of the maze consecutively. For example, the sequence ABBACCABACBA contains 5 alternations (BAC, CAB, BAC, ACB, CBA). Total alternations are divided by total number of alternation opportunities (total arm visits minus one) to yield an alternation percent score.
Data visualization and inferential statistics
Activity data were visualized in actogram format using Clocklab 6 (Actimetrics, Wilmette Illinois). Data were exported to GraphPad Prism 9.0 (GraphPad Software, Inc., La Jolla, CA) (https://actimetrics.com/products/clocklab/) to generate bivariate plots (average waveforms) and scatterplots. To quantify τ in DD, Clocklab algorithms were used to identify the onset of the daily activity period on days 4–11 of DD and fit a regression line to those onsets, the slope of which represents the average daily deviation of the rest-activity cycle from 24 h. Food anticipatory activity (FAA) was quantified as the number of activity counts during the 3 h immediately preceding mealtime, expressed as a ratio relative to total daily activity (excluding the 4 h mealtime).
Effects of photoperiod, time of day and feeding condition on NOP and SA performance were evaluated using two-way repeated measures or mixed model ANOVA as appropriate, with Šídák's multiple comparisons post hoc tests (GraphPad Prism 9.0) (https://www.graphpad.com). Effects of photoperiod and disc access on FAA and τ in DD were also evaluated by ANOVA. One sample t-tests were used to assess the significance of group DI scores relative to chance. Group averages in the text and figures are reported ± S.E.M. unless otherwise indicated.
Results
Activity rhythms with food available-ad-libitum
With food available ad libitum, the timing and waveform of the daily activity rhythm varied with both photoperiod and running disc availability (Fig. 1). In the short-day (L6) group, nocturnal activity (α, defining the biological night) began at lights-off and declined monotonically from an early peak to low levels by the end of the 18 h night. In the long-day (L18) group, activity began ~ 1 h before lights-off and ended ~ 2 h after lights-off, but was largely compressed within the 6 h night, with peaks of activity early and late in the night. This ‘dawn/dusk’ bimodal distribution was accentuated in mice housed without a running disc (Fig. 1e,f).
To quantify group differences in the phase of LD entrainment (ΦLD, the time of activity onset relative to lights-off), independent of masking effects of LD (light suppresses activity), activity onset on the first day of DD was derived by extrapolating a regression line fit to activity onsets on days 4–14 of DD. ANOVA confirmed that ΦLD varied significantly with photoperiod (F1,32 = 131.9, p < 0.0001) and disc access (F1,32 = 9.30, p = 0.005), with a significant interaction (F1,32 = 7.56, p = 0.009). Mice previously entrained to L18 had an advanced ΦLD compared to the mice in L6. Mice with discs had an advanced ΦLD compared to mice without discs (Fig. 1k,l). The main effect of disc access was driven largely by the L18 group, which on the first night of DD became active 2.71 ± 0.44 h prior to the time of lights-off, compared to 1.41 ± 0.15 h in L18 mice with no running disc. In L6, ΦLD was modestly delayed relative to lights-off in both the disc (− 0.48 ± 0.07 h) and no-disc (− 0.54 ± 0.13 h) groups.
ANOVA also confirmed a main effect of photoperiod on α duration (F1,35 = 232.9, p < 0.0001), which was significantly shorter in L18 compared to L6 mice. There was no significant main effect of disc access (F1,35 = 2.10, p = 0.16) and no significant interaction (F1,35 = 0.50, p = 0.48).
Activity rhythms were recorded for two weeks in DD prior to food restriction and the first round of memory tests. ANOVA of τ prior to restricted feeding confirmed the main effect of photoperiod (F1,32 = 55.72, p < 0.0001), with τ significantly shorter in the L18 mice (Fig. 1m,n). Consistent with prior reports [e.g.,27], there was also a main effect of disc access (F1,32 = 17.96, p = 0.0002), with τ significantly shorter in mice with running discs compared to mice without a disc. The interaction was not significant (F1,32 = 3.56, p = 0.07).
Activity rhythms with food restricted to a 4 h daily meal in the light period
When food was restricted to the last 4 h of the light period, all mice became active prior to mealtime within a few days (Fig. 1a–d,h, i). Consistent with previous findings19, FAA was enhanced in long-day mice compared to short-day mice, in the groups with a running disc (t15 = 2.657, p = 0.0173), but not in the groups without a disc (t16 = 0.557, p = 0.585, Fig. 1j).
Previously, we reported that the aftereffect of photoperiod on τ in DD was absent in mice following a month of daytime restricted feeding19. This result was confirmed here in mice with access to a running disc, but only partially in mice without a disc (Fig. 1m,n). In mice with running discs, ANOVA of τ in DD before and after restricted feeding yielded significant main effects of photoperiod (F1,32 = 19.45, p = 0.0001) and feeding condition (F1,32 = 5.953, p = 0.0204) and a significant interaction (F1,32 = 12.16, p = 0.0014). The τ difference between L18 and L6 groups evident before restricted feeding was absent in DD after daytime feeding. In mice housed without a running disc, there were significant main effects of photoperiod (F1,32 = 133.9, p < 0.0001) and feeding condition (F1,32 = 9.115, p = 0.0049), and a significant interaction (F1,32 = 6.83, p = 0.0135). Unlike the disc groups, τ was significantly shorter in L18 mice compared to L6 mice both before and after restricted feeding. However, the magnitude of the group difference was reduced after restricted feeding, due to a significant shortening of τ in the L6 group (p < 0.001).
Novel object preference (NOP)
NOP (Fig. 2a) was quantified using the Discrimination Index (DI), where scores greater than 0.5 denote a preference for the novel object during the choice trial. Preliminary analysis indicated no effect of running disc access on DI scores. Therefore, the disc and no-disc groups were pooled to examine effects of photoperiod and time of day.
(A) Graphical representation of the Novel Object Preference test of long-term (24 h) object memory. Memory for the familiar of two objects is inferred from the Discrimination Index (DI, the fraction of total object exploration time spent exploring the novel object). Individual and group mean (± SEM) DI scores and exploration times are presented for each of 4 test times and averaged across test times (D,G), with food available ad-libitum (B,E) and restricted to a 4 h daily meal (C,F). In panels B and C, stars denote DI scores significantly greater than chance (1-sample t-tests, p < 0.05). In panels (D–G), stars denote differences between the long-day and short-day groups. Test times are denoted using Zeitgeber Time (ZT) and External Time (ExT) notation. Yellow and grey shading denotes lights-on or -off at each test time. Food restriction increased object exploration (G) but not the DI score (D).
With food available ad libitum performance significantly exceeded chance only at test times centered at ZT9 and ZT15 (2–4 h before and after lights off, respectively), and not at the other two test times, in both the L6 and L18 groups (one-sample t-tests, p < 0.05) (Fig. 2b). Despite this difference, ANOVA did not find a main effect of test time (F1,93 = 1.525, p = 0.213). There was also no main effect of photoperiod (F1,93 = 0.024, p = 0.878) and no interaction (F3,93 = 1.176, p = 0.323).
During weeks 5 to 8 of restricted feeding (6–9 weeks after the last NOP test), object memory was retested. Preference for the novel object was significantly greater than chance at all test times in both the L6 and L18 groups (one-sample t-tests, p < 0.05). ANOVA again did not find a significant main effect of test time (F3,125 = 0.126, p = 0.945) but did reveal a marginally significant effect of photoperiod (F1,125 = 3.916, p = 0.050), without a significant interaction (F3,125 = 1.634, p = 0.185) (Fig. 2c). DI scores were higher in the L6 group compared to the L18 group at each test time, but none of the differences were significant when corrected for multiple comparisons (p > 0.05). The difference was significant when DI scores were averaged across test times for each subject (7.1% higher in the L6 mice, p < 0.05; Fig. 2d).
Object exploration time (the number of seconds per 5 min test that mice investigated either object) did not differ between mice with and without running discs, in either the L6 or L18 groups, so the disc and no-disc groups were pooled for further analysis (Fig. 2e–g). ANOVA of exploration during the choice trial with food available ad-libitum indicated no main effect of photoperiod (F1,25 = 3.52, p = 0.072), a main effect of test time (F3,68 = 6.058, p = 0.001) and no interaction (F3,68 = 1.678, p = 0.179). Mice in L18 explored more at the ZT3 test time compared to the other test times (p < 0.05). During restricted feeding, there were main effects of photoperiod (F1,34 = 6.42, p = 0.016) and test time (F3,91 = 4.34, p = 0.006), and no interaction (F3,91 = 1.107, p = 0.35). Pairwise comparisons between test times within groups, and between groups at each test time, were not significant (p > 0.05). Exploration was greater during restricted feeding at most test times in both L6 and L18 groups, and averaging across test times, exploration was greater in the L6 group.
Spontaneous alternation (SA)
Preliminary analysis indicated no main effect of disc access on SA, so the disc and no-disc groups were pooled to evaluate effects of photoperiod and time of day. ANOVA of SA with food available ad libitum indicated no main effect of photoperiod (F1,36 = 0.341, p = 0.563) or test time (F3,108 = 0.695, p = 0.557) and no interaction (F3,108 = 0.619, p = 0.604) (Fig. 3a,b). During restricted feeding, SA again showed no main effect of photoperiod (F1,34 = 0.497, p = 0.485) or test time (F3,102 = 2.53, p = 0.061), and no interaction (F3,102 = 0.588, p = 0.624) (Fig. 3c). Averaging across test times, SA did not vary with either photoperiod or food access condition (Fig. 3d).
(A) Graphical representations of Spontaneous Alternation (SA) test of spatial working memory. SA scores were calculated with food available ad-libitum (B) or restricted to a 4 h daily meal (C), at each of 4 test times, and averaged across test times (D). Stars denote SA % scores significantly greater than chance (1-sample t-tests, p < 0.05). Test times are denoted using Zeitgeber Time (ZT) and External Time (ExT) notation. Yellow and grey shading denotes lights-on or -off at each test time.
Food intake, body weight and test performance
Food intake and body weight were measured daily during the last week of ad libitum food access and throughout restricted feeding. Intake did not differ significantly by photoperiod in either condition and so the L6 and L18 groups were pooled to quantify effects of disc access and feeding condition. ANOVA of average intake during the week of ad libitum measures and week 4 of restriction revealed a significant main effect of disc access (F1,35 = 52.97, p < 0.0001) and feeding condition (F1,34 = 31.33, p < 0.0001), with no interaction (F1,34 = 0.119, p = 0.73). Mice with discs ate more than mice without discs in both feeding conditions (mean grams/day: ad-libitum = 4.4 ± 0.6 vs 3.6 ± 0.4, restricted = 4.0 ± 0.4 vs 3.2 ± 0.2; Fig. 4a). When availability was restricted, food intake was reduced equivalently in the two groups (91 ± 2.5% of ad-libitum in the disc group, and 89 ± 2.4% in the no-disc group).
(A) Food intake and (B) body weight with food available ad-libitum or restricted to a 4 h daily meal, separately for mice housed with or without a running disc. Long-day and short-day groups did not differ and were combined. (C) Association between starting body weight and weight loss in grams after 4 weeks of restricted feeding. (D,E) Association between performance on the Novel Object Preference test (Discrimination Index, DI%) and body weight, with food ad-lib or restricted. (F) Association between DI% during restricted feeding and weight loss in grams.
ANOVA of body weights taken on the last day of ad-libitum food access and after 4 weeks of restriction confirmed a main effect of feeding condition (F1,66 = 86.2, p < 0.0001) and revealed a main effect of disc access (F1,66 = 49.3, p < 0.0001) with a significant interaction (F1,66 = 4.47, p = 0.038). Despite eating more, mice with a running disc weighed less, both with food available ad-libitum (29.3 ± 1.1 g vs 34.7 ± 0.9 g) and restricted (25.9 ± 1.6 g vs 27.9 ± 1.8 g; Fig. 4b). During restricted feeding, mice without a running disc lost more weight, and the group difference decreased from 4.5 g to 2.7 g. Weight loss was proportional to starting weight, in both groups separately (disc group: Pearson r = + 0.89; no-disc: r = + 0.88, both p < 0.001) and combined (r = + 0.85; p < 0.001; Fig. 4c); heavier mice lost more weight.
To determine whether weight or weight loss might affect object memory, Pearson correlation coefficients were calculated using DI scores for each mouse, averaged across the 4 test times, separately for the ad libitum and restricted feeding conditions. DI scores showed a modest positive correlation with body weight, both during ad libitum food access (r = 0.45, p = 0.022; Fig. 4d) and restricted feeding (week 4 measure; r = 0.35, p = 0.048; Fig. 4e). DI scores during restricted feeding showed no relationship with weight loss (r = 0.02, p > 0.9; Fig. 4f).
Discussion
Bright light can improve alertness, cognition and mood in humans, both acutely (e.g., alerting by light exposure at night)28 and with repeated exposure over time (e.g., remission of seasonal depression by daily bright daytime light exposure)29. The processes and neural circuits that mediate these effects have not been fully delineated. The most widely used animal models in translational neuroscience are nocturnal rats and mice, species in which bright light acutely suppresses activity and promotes sleep. These species also do not exhibit photoperiodic regulation of reproduction and metabolism, and thus might not be expected to show cognitive or affective benefits of long days. Nonetheless, nocturnal mice do show marked effects of photoperiod on SCN output5,6,7, and mice entrained to a long-day photoperiod have been reported to perform better on several memory tests13. Having previously shown that a daytime restricted feeding schedule can block aftereffects of photoperiod on τ in DD19, we asked whether this might also block effects of photoperiod on memory. We found instead that performance on tests of long-term object recognition memory and spatial working memory were equivalent in mice entrained to long- and short-day photoperiods, with food available ad libitum or restricted to a 4 h daytime meal.
One interpretation of these results is that encoding of photoperiod in SCN output, independent of other factors, is not sufficient to modulate object and spatial memory processes. If long days significantly modify SCN output but not memory, then the retinal pathways that mediate effects on memory likely bypass the SCN and act directly elsewhere in the brain, or they act through the SCN, but independent of photoperiodic effects on SCN cycling30.
Alternatively, in the context of previous findings, the results could be interpreted as evidence for a dose–response relationship between photoperiod and memory processes. A significant benefit of a long-day photoperiod was reported in mice entrained to LD 20:4, compared to mice in LD 12:12, with lights (type not specified) providing ~ 1100 lx illumination13. By contrast, we compared mice in LD 18:6 with mice in LD 6:18, to avoid possible unstable entrainment at longer photoperiods (preliminary observations;8). We used blue-enriched white LEDs providing ~ 15 lx illumination, to be within recommended guidelines for long-term housing of nocturnal rodents20. Although the group difference in daylength was greater in our study (12 h vs 8 h), more than 18 h between ‘dawn’ and ‘dusk’ (lights-on and -off) may be necessary to impact memory. Given that the separation between dawn and dusk is the critical parameter for the photoperiodic response8, light intensity may be less important. Differences in aftereffects induced by 18 h and 20 h photoperiods are likely to be small (if detectable), but parametric studies of photoperiodic modulation of SCN output are lacking. Pending further studies, the data allow us to conclude that, 1. memory enhancement is absent under an 18 h photoperiod that does significantly alter circadian rhythm parameters in LD and subsequent DD, and 2. regardless of the role of SCN encoding of photoperiod, memory enhancement may require more light than was used in our study.
Performance of rats and mice on tests of object and spatial memory has been reported to vary with time of day31,32. Time of day effects (e.g., day versus night advantage) are variable across studies, and not always evident, e.g.,26,31,32,33,34. This suggests that true effect sizes are likely small, which would explain why in the history of animal learning and memory research, for matters of convenience, tests were often scheduled during regular working hours when the lights were on, without obvious and systematic failures of animals to learn despite training and testing in the daily rest phase. In addition to real effect size, there are a range of other factors that may contribute to replicability of findings, including sampling error and differences in methodology and statistical analysis35. In previous studies, we did not see significant day-night differences in 24 h object recognition, fear conditioning or spatial working memory in mice entrained to LD 12:12, despite efforts to match procedures used in other work that did report differences26,36. In the present study, we found only weak time of day effects on object recognition; when food was available ad libitum, discrimination scores (the metric for object memory) were above chance at 2 of 4 test times (one sample t-tests), but differences across and between test times were not significant (ANOVA with Sidak multiple comparisons tests). Notably, mice in long and short days performed above chance at the same two test times (ZT 9 and 15, i.e., 2–4 h before and after lights-off, respectively), and the long-day photoperiod did not improve performance above chance at the other two test times (ZT21 and 3). When food was restricted to a 4 h meal in the light period, all groups performed above chance, and there was again no variation across test times. In the test of spatial working memory, mice in both photoperiods performed above chance and equivalently at all 4 test times. These results are consistent with small true effect sizes, and suggest that time of day variation may be related primarily to modulatory factors (e.g., motivation, arousal, attention) and less so to memory encoding and retrieval capacity.
Mealtime controls the phase of circadian clocks in many tissues and brain regions, but has a much smaller or no effect on the timing of the SCN pacemaker in mice entrained to LD15,16. Consequently, phase relationships among oscillators across the extended circadian clock system are flexible, and can be extensively reorganized. This reorganization seems obviously adaptive, as it aligns foraging and physiology with predictable feeding opportunities. There may a temptation to view shifting of food-entrained oscillators relative to the SCN pacemaker as an experimental model of internal desynchrony, with presumed adverse consequences for physiological functioning, but if there are such consequences, it is clear that memory functions are exempt. There is one report of memory impairments in mice fed in the mid-day compared to the mid-night, but using similar procedures we found that day- and night-fed mice performed equally well26,36. In the present study, we extend this finding by showing that memory performance is actually improved when food is restricted to the daytime, compared to when it is available ad-libitum. This result is consistent with numerous findings that caloric restriction, without malnutrition, improves many functions, including cognition37,38,39,40.
Another factor that can affect cognitive performance is regular physical activity41. In the present study, we did not see a difference in discrimination or alternation scores between mice housed with free access to a running disc and mice housed without a disc. Horizontally rotating discs mounted on igloo-shaped nest houses are a relatively recent innovation in lab animal housing, and as an ‘exercise’ intervention, comparability to traditional running wheels or treadmills has not been established. Our recording method (a motion sensor mounted above the disc) was not calibrated to permit estimates of running distance or intensity.
One additional observation that merits highlighting is that when food was restricted, weight loss in grams and as a percentage of starting weight was proportional to starting weight. When food was available ad libitum, mice housed without running discs (what could be called sedentary housing) were significantly heavier than mice that had access to a running disc. When food was limited to the daytime, body weights in mice without discs stabilized at 19% below starting weight, compared to 13% in mice with discs, and the difference in weights between groups was reduced from 4.5 g to 2.7 g (a 67% change). Despite the differential weight loss, mice in the two groups remained healthy by visual inspection and performed equivalently on the memory tests. Guidelines may specify 10–15% weight loss as a threshold for terminating a food restriction procedure. It is clear, however, that such thresholds need to be adjusted for housing condition, starting body weight and likely other factors. Mice and rats housed under sedentary conditions may carry excess body weight, which they shed more rapidly when access to food is restricted.
The results reported here contribute to the limited empirical data base on how the performance of mice on tests of learning and memory responds to variations in environmental lighting schedules, food access, test times and availability of a running apparatus. Evidence that either daylength or total daily light exposure can affect cognitive processes in a nocturnal rodent in a manner similar to effects observed in humans merits further study to establish necessary and sufficient stimulus parameters.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Schibler, U. et al. Clock-talk: Interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb. Symp. Quant. Biol. 80, 223–232 (2015).
Brown, A. J., Pendergast, J. S. & Yamazaki, S. Peripheral circadian oscillators. Yale J. Biol. Med. 92(2), 327–335 (2019).
Patton, A. P. & Hastings, M. H. The mammalian circadian time-keeping system. J. Huntingt. Dis. 12(2), 91–104 (2023).
Welsh, D. K., Takahashi, J. S. & Kay, S. A. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 72, 551–577 (2010).
Porcu, A., Riddle, M., Dulcis, D. & Welsh, D. K. Photoperiod-induced neuroplasticity in the circadian system. Neural Plast. 13, 5147585 (2018).
Michel, S. & Meijer, J. H. From clock to functional pacemaker. Eur. J. Neurosci. 51(1), 482–493 (2020).
Evans, J. A. & Schwartz, W. J. On the origin and evolution of the dual oscillator model underlying the photoperiodic clockwork in the suprachiasmatic nucleus. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. https://doi.org/10.1007/s00359-023-01659-1.10.1007/s00359-023-01659-1 (2023).
Pittendrigh, C. S. & Daan, S. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J. Comp. Physiol. 106, 223–252 (1976).
Tackenberg, M. C., Hughey, J. J. & McMahon, D. G. Distinct components of photoperiodic light are differentially encoded by the mammalian circadian clock. J. Biol. Rhythm 35(4), 353–367 (2020).
Goldman, B. D. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythm 16(4), 283–301 (2001).
Tackenberg, M. C. & McMahon, D. G. Photoperiodic programming of the SCN and its role in photoperiodic output. Neural Plast. 9, 8217345 (2018).
Nelson, R. J. Photoperiodic responsiveness in house mice. Physiol. Behav. 48(3), 403–408 (1990).
Dellapolla, A. et al. Long days enhance recognition memory and increase insulin-like growth factor 2 in the hippocampus. Sci. Rep. 7(1), 3925 (2017).
Boulos, Z. & Terman, M. Food availability and daily biological rhythms. Neurosci. Biobehav. Rev. 4, 119–133 (1980).
Stokkan, K. A., Yamazaki, S., Tei, H., Sakaki, Y. & Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 (2001).
Damiola, F. et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14(23), 2950–2961 (2000).
Mistlberger, R. E. A multifactor model of food anticipatory circadian rhythms in mammals. In Biological Clocks (eds Homna, K.-I. & Honma, S.) (Hokkaido Press, Sapporo, 2023).
Stephan, F. K. The “other” circadian system: Food as a Zeitgeber. J. Biol. Rhythm 17, 284–292 (2002).
Power, S. C. & Mistlberger, R. E. Food anticipatory circadian rhythms in mice entrained to long or short day photoperiods. Physiol. Behav. 222, 112939 (2020).
Lucas, R. J. et al. Recommendations for measuring and standardizing light for laboratory mammals to improve welfare and reproducibility in animal research. PLoS Biol. 22(3), e3002535 (2024).
Warburton, E. C. & Brown, M. W. Neural circuitry for rat recognition memory. Behav. Brain Res. 285, 131–139 (2015).
Chao, O. Y., Nikolaus, S., Yang, Y. M. & Huston, J. P. Neuronal circuitry for recognition memory of object and place in rodent models. Neurosci. Biobehav. Rev. 141, 104855 (2022).
Basile, B. M., Waters, S. J. & Murray, E. A. What does preferential viewing tell us about the neurobiology of recognition memory?. Trends Neurosci. S0166–2236(24), 00040–00047 (2024).
d’Isa, R., Comi, G. & Leocani, L. Apparatus design and behavioural testing protocol for the evaluation of spatial working memory in mice through the spontaneous alternation T-maze. Sci. Rep. 11, 21177 (2021).
Daan, S., Marrow, M. & Roennenberg, T. External time–internal time. J. Biol. Rhythm 17(2), 107–109 (2002).
Power, S. C., Michalik, M. J., Couture-Nowak, S., Kent, B. A. & Mistlberger, R. E. Midday meals do not impair mouse memory. Sci. Rep. 8(1), 17013 (2018).
Mistlberger, R. E., Bossert, J. M., Holmes, M. M. & Marchant, E. G. Serotonin and feedback effects of behavioral activity on circadian rhythms in mice. Behav. Brain Res. 96, 93–99 (1998).
Mahoney, H. L. & Schmidt, T. M. The cognitive impact of light: Illuminating ipRGC circuit mechanisms. Nat. Rev. Neurosci. 25, 159–175 (2024).
Wirz-Justice, A., Benedetti, F. & Terman, M. Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light and Wake Therapy 2nd edn. (Karger Medical, Basel, 2013).
Fernandez, D. C. et al. Light affects mood and learning through distinct retina-brain pathways. Cell 175(1), 71–84 (2018).
Smarr, B. L., Jennings, K. J., Driscoll, J. R. & Kriegsfeld, L. J. A time to remember: The role of circadian clocks in learning and memory. Behav. Neurosci. 128(3), 283–303 (2014).
Hartsock, M. J. & Spencer, R. L. Memory and the circadian system: Identifying candidate mechanisms by which local clocks in the brain may regulate synaptic plasticity. Neurosci. Biobehav. Rev. 118, 134–162 (2020).
Takahashi, Y., Sawa, K. & Okada, T. The diurnal variation of performance of the novel location recognition task in male rats. Behav. Brain Res. 256, 488–493 (2013).
Beeler, J. A., Prendergast, B. & Zhuang, X. Low amplitude entrainment of mice and the impact of circadian phase on behavior tests. Physiol. Behav. 87(5), 870–880 (2006).
Gulinello, M. et al. Rigor and reproducibility in rodent behavioral research. Neurobiol. Learn Mem. 165, 106780 (2019).
Loh, D. H. et al. Misaligned feeding impairs memories. Elife 4, e09460 (2015).
Ingram, D. K., Weindruch, R., Spangler, E. L., Freeman, J. R. & Walford, R. L. Dietary restriction benefits learning and motor performance of aged mice. J. Gerontol. 42, 78–81 (1987).
Orsini, C., Buchini, F., Conversi, D. & Cabib, S. Selective improvement of strain-dependent performances of cognitive tasks by food restriction. Neurobiol. Learn. Mem. 81(1), 96–99 (2004).
Fontan-Lozano, A. et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J. Neurosci. 27, 10185–10195 (2007).
Talhati, F. et al. Food restriction increases long-term memory persistence in adult or aged mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 50, 125–136 (2014).
Cooper, C., Moon, H. Y. & van Praag, H. On the run for hippocampal plasticity. Cold Spring Harb. Perspect. Med. 8(4), a029736 (2018).
Acknowledgements
This research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (04200) to REM, the Canada Research Chairs program (BAK), and graduate student fellowships from NSERC (SCP and MJM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.C.P., B.A.K., and R.E.M. designed the experiments. S.C.P. and M.J.M. collected data. S.C.P., B.A.K., and R.E.M. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Power, S.C., Michalik, M.J., Kent, B.A. et al. Photoperiod, food restriction and memory for objects and places in mice. Sci Rep 14, 21566 (2024). https://doi.org/10.1038/s41598-024-72548-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72548-z