Abstract
This study delves into the correlation between the triglyceride glucose-body mass index (TyG-BMI) index upon hospital admission and clinical outcomes among this patient population. We investigated the association between TyG-BMI at hospital admission and clinical outcomes in this patient group, and analyzed data from the Medical Information Mart for Intensive Care IV database, identifying acute pancreatitis (AP) patients admitted to ICUs and stratifying them by TyG-BMI quartiles. We assessed the relationship between TyG-BMI and mortality (both in-hospital and ICU) using Cox proportional hazards regression and restricted cubic splines. The cohort included 419 patients, average age 56.34 ± 16.62 years, with a majority being male (61.58%). Hospital and ICU mortality rates were 11.93% and 7.16%, respectively. Higher TyG-BMI was positively correlated with increased all-cause mortality. Patients in the highest TyG-BMI quartile had significantly greater risks of in-hospital and ICU mortality. An S-shaped curve in the spline analysis indicated a threshold effect at a TyG-BMI of 243 for increased in-hospital mortality risk. TyG-BMI is a reliable predictor of both in-hospital and ICU mortality in severely ill AP patients, suggesting its utility in enhancing risk assessment and guiding clinical interventions for this vulnerable population.
Similar content being viewed by others
Introduction
Pancreatitis represents a primary cause of hospitalization among patients with gastrointestinal disorders, entailing substantial morbidity, mortality, and socioeconomic burdens1,2. It also assumes a significant role in escalating hospital mortality3. Roughly one in five patients develop moderate to severe acute pancreatitis (AP), triggering severe complications such as necrosis of pancreatic or surrounding tissues and failure of multiple organs. Reported mortality rates fluctuate between 20% and 40%4,5, underscoring the urgency of promptly evaluating AP severity and implementing interventions. There are several scoring systems available to evaluate AP severity, such as Ranson criteria6, Acute Physiology and Chronic Health Evaluation II (APACHE-II)6, Balthazar grade7, and Bedside Index for Severity in Acute Pancreatitis (BISAP)8. These scoring systems typically involve a more complex assessment process that requires gathering multiple indicators. This time-consuming assessment process may lead to potential delays in identifying the optimal treatment window, thereby increasing the risk of mortality in certain patients. Thus, it is urgent to identify a simplistic, cost-efficient, and acutely sensitive predictive marker for the severity of AP.
Insulin resistance (IR) is a condition in which insulin becomes less effective in facilitating the uptake and utilization of glucose9. This condition is closely associated with the development of AP and is a significant risk factor for its progression10. The triglyceride-glucose (TyG) index, which is derived from fasting triglyceride (TG) and blood glucose (FBG) levels, has emerged as a simple surrogate marker for IR11.
Obesity, as measured by body mass index (BMI), plays a pivotal role in the development of IR, and stands as a critical determinant in its etiology12. The TyG-BMI composite index, a fusion of TyG and BMI, has shown commendable agreement with the homeostatic model assessment of IR in measuring IR in Korean9 and Chinese cohorts13. The predictive role of TyG-BMI index has been demonstrated in various clinical conditions, such as ischemic stroke14, heart failure15, and the necessity of percutaneous coronary intervention16. We hypothesize that this index is associated with prognostic outcomes in patients afflicted with AP and could potentially be utilized for early prognostic assessment. While the prognostic implications of the TyG-BMI index have been studied across a spectrum of diseases, its impact on clinical endpoints within the AP patient population has not yet been fully investigated. Therefore, the aim of our study is to ascertain the relationship between TyG-BMI and mortality in AP patients.
Methods
Database
We harnessed clinical data from the Medical Information Mart for Intensive Care (MIMIC)-IV database, an international online intensive care repository. The database contains patient-related information from the Intensive Care Units (ICUs) of Beth Israel Deaconess Medical Center from 2008 to 2019. It comprises detailed records of 299,712 hospitalized patients and 73,181 critical care patients. In our research team, the author Y.L. has completed the Collaborative Institutional Training Initiative program, (certification number 53244021).
Study population and definitions
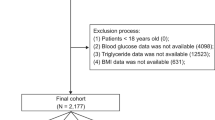
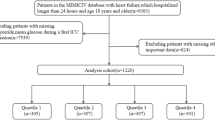
Data extraction was conducted utilizing Structured Query Language within a PostgreSQL environment (version 14.6). Based on our analysis of the MIMIC-IV database17, ICU admission is generally determined by the actual care received by patients, typically including those with hemodynamic instability, respiratory failure requiring mechanical ventilation, or other organ dysfunctions necessitating close monitoring and support. AP was diagnosed by the International Classification of Disease, 9th Revision (ICD-9) code 577.0 or 10th Revision (ICD-10) code K85. in patients aged over 18 years. Patients lacking recorded admission-day levels of FBG, TG, or BMI were excluded. The methodological flowchart is depicted in Fig. 1.
Data collection
The TyG-BMI index was designated as the primary variable of this investigation. Baseline characteristics of patients were extracted from the database, encompassing demographics such as age, sex, ethnicity, marital status, and BMI. We also collected the Simplified Acute Physiology Score II (SAPS II) at admission and the administration details of Vasopressin and Octreotide. The SAPS II, used for severity assessment, consists of 17 variables including physiological measurements, age, and admission type. Higher scores indicate greater severity and higher predicted mortality18. SAPS II is widely used for assessing severity in critically ill patients, including those with AP19. Comorbidities at baseline, including chronic lung disease, hypertension, acute kidney injury (AKI), heart failure, diabetes, and malignancy, were collected. Pertinent laboratory parameters at ICU admission were also gathered, encompassing red blood cell distribution width, red blood cell (RBC) count, white blood cell (WBC) count, platelet count, alanine aminotransferase (ALT) levels, blood glucose, hemoglobin concentration, creatinine, lactate, international normalized ratio, blood urea nitrogen, anion gap, serum chloride levels, potassium levels, sodium levels, and total bilirubin levels. To reduce potential biases, variables with over 15% missing values were excluded from the analysis. The “mice” package in the R software was used for processing missing data.
Definition and clinical outcomes
The TyG-BMI index was calculated using the following equation: In [TG (mg/dL) × FBG (mg/dL)/2] × BMI16. The primary endpoint was all-cause mortality during hospitalization, while the secondary endpoint was ICU mortality.
Statistical analysis
Continuous variables are presented as mean ± SD or median (interquartile range), depending on their distribution. Meanwhile, categorical variables were displayed as proportions. The Kolmogorov-Smirnov test was used for testing the normality of continuous data. Normally distributed variables were analyzed utilizing t-test or ANOVA, while the Mann-Whitney U-test and Kruskal-Wallis test were reserved for non-normally distributed variables.
To investigate the effect of the TyG-BMI index on short-term survival, Kaplan-Meier survival analysis was executed to ascertain 28-day and 90-day all-cause mortality in patients with different TyG-BMI levels, and log-rank testing was used for comparisons between groups. The relation between the TyG-BMI index and mortality was further explored through univariate and multivariate Cox regression analyses. Model 1 served as the unadjusted reference; Model 2 was adjusted for demographic variables including age, sex, and ethnicity. Model 3 was additionally adjusted for a comprehensive range of clinical covariates such as comorbidities (heart failure, AKI, hypertension, diabetes, sepsis, chronic obstructive pulmonary disease, and malignancies), hematologic and biochemical parameters (WBC, RBC, platelet count, ALT levels), metabolic and physiologic data (serum lactate, creatinine, total bilirubin, anion gap), SAPS II, and specific treatments (Vasopressin and Octreotide administration). The Cox regression analysis and a restricted cubic spline model with four knots were leveraged to ascertain the link between TyG-BMI index and the risk of in-hospital and ICU mortality in critically ill patients with AP. All statistical analyses were carried out using the R software, version 4.0.2.
Results
This investigation incorporated 419 patients admitted for severe AP. The median age of these patients was 56.34 years, and male patients constituted 61.58%. The median value of the TyG-BMI of the participants was 297.88. The in-hospital and ICU mortality rates were 11.93% and 7.16% respectively, as detailed in Table 1.
Baseline characteristics
The patients were classified into quartiles by the level of the TyG-BMI index [quartile 1 (134.91-241.68), quartile 2 (241.68-281.39), quartile 3 (281.39-349.13), quartile 4 (349.13-683.47)], as shown in Table 1. Individuals with the uppermost quartile of the TyG-BMI index tended to be older and exhibited elevated SAPS II scores upon admission, increased prevalence of diabetes and AKI, as well as higher levels of RBC, hemoglobin, glucose, TG, creatinine, and potassium compared to individuals in lower quartiles. Despite these differences, the in-hospital and ICU mortality rates did not significantly diverge across quartiles (P > 0.05).
Kaplan–Meier analysis
Figure 2 illustrates Kaplan-Meier survival analysis curves assessing key outcomes among different TyG-BMI groups. Noteworthy, no significant disparities in mortality at 28 and 90 days were observed (log-rank P > 0.05).
Cox regression analysis
Cox proportional hazards regression was leveraged to scrutinize the correlation between the TyG-BMI and in-hospital and ICU mortality outcomes. The analytical findings showed that TyG-BMI is significantly associated with an increased risk of in-hospital mortality. This association was observed in all models: unadjusted [HR, 1.03; 95% CI: 1.01–1.06; P = 0.020], partially adjusted [HR, 1.04; 95% CI: 1.01–1.07; P = 0.014], and fully adjusted [HR, 1.05; 95% CI: 1.01–1.10; P = 0.010], when TyG-BMI was treated as a continuous variable. In addition, the index was identified as a significant risk factor for ICU mortality in AP patients in the fully adjusted model (HR: 1.10; 95% CI: 1.02–1.18; P = 0.017). The uppermost quartile of the TyG-BMI was correlated with a significant increase in the risk of in-hospital mortality [HR, 4.08; 95% CI: 1.34–12.41; P = 0.007] compared to the nadir quartile, as shown in the fully adjusted Cox regression (Table 2). similar results for ICU mortality were found in multivariate Cox regression analysis (Table 2).
Restricted cubic splines
The restricted cubic splines were employed to better characterize and graphically represent the relationship between the TyG-BMI index and in-hospital mortality. As illustrated in Fig. 3, a non-linear correlation was noted between TyG-BMI index and in-hospital mortality. Specifically, the mortality risk initially decreased within the lower spectrum of TyG-BMI, to a nadir at a TyG-BMI index value of approximately 243, and then elevated steeply (P for overall < 0.001, P for non-linearity = 0.003).
Discussion
The objective of this investigation was to scrutinize the correlation of TyG-BMI index with clinical prognoses amongst a demographic of acutely ill patients afflicted with AP. Following adjustment for confounders, our findings indicated that escalated TyG-BMI values were closely linked with an upsurge in all-cause mortality both within ICU settings and overall hospitalization among these individuals. As such, the TyG-BMI index might be instrumental as a prognostic indicator for healthcare providers, potentially serving as an independent marker of mortality in the context of AP. Our findings align with studies demonstrating the prognostic value of TyG-BMI in various clinical contexts. TyG-BMI has shown associations with cardiovascular outcomes20,21,22, stroke risk23,24, and mortality in critical care settings25,26. It has also been linked to coronary artery disease severity16,20 and long-term outcomes in heart failure21. Our study extends the utility of TyG-BMI to AP.
The pathogenic mechanisms underlying AP significantly involve inflammation within the peripancreatic adipose tissue27. IR, as a protracted, low-grade inflammatory state, is typified by heightened systemic concentrations of pro-inflammatory cytokines28,29. This increase in pro-inflammatory cytokines further intensifies inflammation following the initial trigger and magnifies damage to the pancreas and other organs, resulting in systemic inflammatory response syndrome, organ failure, and local complications. Obesity and diabetes, which serve as representative markers of metabolic disorders, have been strongly linked to the occurrence and progress of AP30,31. The association between TyG-BMI and mortality in our study could be attributed to its capture of both lipid metabolism and obesity32, which are crucial factors in the pathogenesis of AP33. Additionally, insulin resistance, reflected by TyG-BMI13,34, may exacerbate the inflammatory response in AP34.
IR is a pivotal contributor to the pathogenesis of type 2 diabetes mellitus, dyslipidemic disorders, adiposity, and cardiovascular pathologies35. Consequently, the TyG index was conceived as a substitute indicator for assessing IR36,37. Additionally, BMI is frequently utilized to evaluate obesity and its correlation with IR. A study utilizing data from the Korean National Health and Nutrition Examination Survey has shown that the TyG-BMI index exhibits superior accuracy in delineating IR compared to other metrics9. The TyG-BMI index has been recognized as an efficacious marker for assessing IR16, and stands as a prognostic element for ischemic cerebrovascular events, heart failure, and the necessity for percutaneous coronary intervention14,15,16. However, there is limited research on the prognostic value of TyG-BMI in determining the prognosis of patients with AP. To our knowledge, this is the inaugural analysis to probe the association of TyG-BMI index with in-hospital and ICU mortality in individuals with AP, using data from the MIMIC-IV database. Clinically, TyG-BMI could be a valuable tool for risk stratification in AP patients due to its simplicity and reliance on readily available parameters. However, further research is needed to establish optimal cut-off values and validate its use in diverse populations.
Nonetheless, our investigation has several limitations. Firstly, the study is a single-center, observational study, which hinders us from inferring causation. Secondly, due to the insufficient sample size, subgroup analysis was not conducted. We look forward to conducting subgroup analysis in a larger prospective study. Thirdly, TyG-BMI measurements were limited to the time of ICU admission in this study, and the changes during the course of hospitalization were not measured. Fourthly, there are limitations related to the MIMIC-IV database and our assessment tools. The database contains a mixture of ICD-9 and ICD-10 codes, with ICD-9 not specifying pancreatitis etiology, preventing consistent analysis of etiology for all cases. Additionally, while the SAPS II used for severity assessment is widely validated for critically ill patients, it may not capture some pancreatitis-specific factors. The database lacked variables required for pancreatitis-specific scoring systems, which might have provided more targeted prognostic information. Finally, due to the lack of longer follow-up data in the MIMIC-IV database, our study focuses solely on in-hospital and ICU mortality, which may have affected the overall prognostic assessment. Hence, additional studies are warranted to corroborate our observations.
Conclusion
Overall, our study reveals a significant association between higher TyG-BMI index and increased mortality in critically ill AP patients. This expands the index’s applicability to acute care settings and suggests its potential as a simple risk stratification tool. Future prospective, multi-center studies are needed to validate these findings, establish the index’s clinical utility, and explore its integration with established pancreatitis-specific scoring systems in AP management.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Goyal, H., Awad, H. & Hu, Z. D. Prognostic value of admission red blood cell distribution width in acute pancreatitis: a systematic review. Ann. Transl Med.5, 342. https://doi.org/10.21037/atm.2017.06.61 (2017).
Yadav, D. & Lowenfels, A. B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology144, 1252–1261. https://doi.org/10.1053/j.gastro.2013.01.068 (2013).
Scurt, F. G., Bose, K., Canbay, A., Mertens, P. R. & Chatzikyrkou, C. Acute kidney injury following acute pancreatitis (AP-AKI): definition, pathophysiology, diagnosis and therapy. Z. Gastroenterol.58, 1241–1266. https://doi.org/10.1055/a-1255-3413 (2020).
Schepers, N. J. et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut68, 1044–1051. https://doi.org/10.1136/gutjnl-2017-314657 (2019).
Bang, J. Y., Wilcox, C. M., Arnoletti, J. P. & Varadarajulu, S. Superiority of endoscopic interventions over minimally invasive surgery for infected necrotizing pancreatitis: meta-analysis of randomized trials. Dig. Endosc. 32, 298–308. https://doi.org/10.1111/den.13470 (2020).
Staubli, S. M., Oertli, D. & Nebiker, C. A. Assessing the severity of acute pancreatitis (ASAP) in Switzerland: a nationwide survey on severity assessment in daily clinical practice. Pancreatology17, 356–363. https://doi.org/10.1016/j.pan.2017.02.006 (2017).
Xiao, B., Xu, H. B., Jiang, Z. Q., Zhang, J. & Zhang, X. M. Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging. Quant. Imaging Med. Surg.9, 1973–1985. https://doi.org/10.21037/qims.2019.11.10 (2019).
Arif, A., Jaleel, F. & Rashid, K. Accuracy of BISAP score in prediction of severe acute pancreatitis. Pak J. Med. Sci.35, 1008–1012. https://doi.org/10.12669/pjms.35.4.1286 (2019).
Lim, J., Kim, J., Koo, S. H. & Kwon, G. C. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS One14, e0212963. https://doi.org/10.1371/journal.pone.0212963 (2019).
Cho, S. K., Huh, J. H., Yoo, J. S., Kim, J. W. & Lee, K. J. HOMA-estimated insulin resistance as an independent prognostic factor in patients with acute pancreatitis. Sci. Rep.9, 14894. https://doi.org/10.1038/s41598-019-51466-5 (2019).
Su, J. et al. Triglyceride glucose index for the detection of the severity of coronary artery disease in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc. Diabetol.21, 96. https://doi.org/10.1186/s12933-022-01523-7 (2022).
Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes.18, 139–143. https://doi.org/10.1097/MED.0b013e3283444b09 (2011).
Er, L. K. et al. Triglyceride glucose-body Mass Index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One11, e0149731. https://doi.org/10.1371/journal.pone.0149731 (2016).
Du, Z., Xing, L., Lin, M. & Sun, Y. Estimate of prevalent ischemic stroke from triglyceride glucose-body mass index in the general population. BMC Cardiovasc. Disord.20, 483. https://doi.org/10.1186/s12872-020-01768-8 (2020).
Dou, J. et al. Association between triglyceride glucose-body mass and one-year all-cause mortality of patients with heart failure: a retrospective study utilizing the MIMIC-IV database. Cardiovasc. Diabetol.22, 309. https://doi.org/10.1186/s12933-023-02047-4 (2023).
Cheng, Y. et al. Association between triglyceride glucose-body mass index and cardiovascular outcomes in patients undergoing percutaneous coronary intervention: a retrospective study. Cardiovasc. Diabetol.22, 75. https://doi.org/10.1186/s12933-023-01794-8 (2023).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data10, 1. https://doi.org/10.1038/s41597-022-01899-x (2023).
Le Gall, J. R., Lemeshow, S. & Saulnier, F. A new simplified Acute Physiology score (SAPS II) based on a European/North American multicenter study. JAMA270, 2957–2963. https://doi.org/10.1001/jama.270.24.2957 (1993).
Juneja, D., Gopal, P. B. & Ravula, M. Scoring systems in acute pancreatitis: which one to use in intensive care units? J. Crit. Care25 358.e359–358.e315 (2010).
Yang, X. et al. Association of the triglyceride glucose-body mass index with the extent of coronary artery disease in patients with acute coronary syndromes. Cardiovasc. Diabetol.23, 24. https://doi.org/10.1186/s12933-024-02124-2 (2024).
Lyu, L. et al. Association between triglyceride glucose-body mass index and long-term adverse outcomes of heart failure patients with coronary heart disease. Cardiovasc. Diabetol.23, 162. https://doi.org/10.1186/s12933-024-02213-2 (2024).
Li, F. et al. Association between the cumulative average triglyceride glucose-body mass index and cardiovascular disease incidence among the middle-aged and older population: a prospective nationwide cohort study in China. Cardiovasc. Diabetol.23, 16. https://doi.org/10.1186/s12933-023-02114-w (2024).
Huo, R. R., Zhai, L., Liao, Q. & You, X. M. Changes in the triglyceride glucose-body mass index estimate the risk of stroke in middle-aged and older Chinese adults: a nationwide prospective cohort study. Cardiovasc. Diabetol.22, 254. https://doi.org/10.1186/s12933-023-01983-5 (2023).
Shao, Y. et al. Link between triglyceride-glucose-body mass index and future stroke risk in middle-aged and elderly Chinese: a nationwide prospective cohort study. Cardiovasc. Diabetol.23, 81. https://doi.org/10.1186/s12933-024-02165-7 (2024).
Hu, Y., Zhao, Y., Zhang, J. & Li, C. The association between triglyceride glucose-body mass index and all-cause mortality in critically ill patients with atrial fibrillation: a retrospective study from MIMIC-IV database. Cardiovasc. Diabetol.23, 64. https://doi.org/10.1186/s12933-024-02153-x (2024).
Huang, Y., Li, Z. & Yin, X. Long-term survival in stroke patients: insights into triglyceride-glucose body mass index from ICU data. Cardiovasc. Diabetol.23, 137. https://doi.org/10.1186/s12933-024-02231-0 (2024).
Mikolasevic, I. et al. Metabolic syndrome and acute pancreatitis. Eur. J. Intern. Med.32, 79–83. https://doi.org/10.1016/j.ejim.2016.04.004 (2016).
Lee, Y. H. & Pratley, R. E. The evolving role of inflammation in obesity and the metabolic syndrome. Curr. Diab Rep.5, 70–75. https://doi.org/10.1007/s11892-005-0071-7 (2005).
Shen, Z., Wang, X., Zhen, Z., Wang, Y. & Sun, P. Metabolic syndrome components and acute pancreatitis: a case-control study in China. BMC Gastroenterol.21, 17. https://doi.org/10.1186/s12876-020-01579-3 (2021).
Huh, J. H. et al. Diabetes mellitus is associated with mortality in acute pancreatitis. J. Clin. Gastroenterol.52, 178–183. https://doi.org/10.1097/mcg.0000000000000783 (2018).
Krishna, S. G. et al. Morbid obesity is associated with adverse clinical outcomes in acute pancreatitis: a propensity-matched study. Am. J. Gastroenterol.110, 1608–1619. https://doi.org/10.1038/ajg.2015.343 (2015).
Zhang, M. et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the rural Chinese cohort study. Cardiovasc. Diabetol.16, 30. https://doi.org/10.1186/s12933-017-0514-x (2017).
Navina, S. et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci. Transl. Med.3, 107ra110. https://doi.org/10.1126/scitranslmed.3002573 (2011).
Patel, K. et al. Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation. Am. J. Pathol.185, 808–819. https://doi.org/10.1016/j.ajpath.2014.11.019 (2015).
Tello-Flores, V. A. et al. Role of long non-coding RNAs and the Molecular mechanisms involved in insulin resistance. Int. J. Mol. Sci.22. https://doi.org/10.3390/ijms22147256 (2021).
Tao, L. C., Xu, J. N., Wang, T. T., Hua, F. & Li, J. J. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc. Diabetol.21, 68. https://doi.org/10.1186/s12933-022-01511-x (2022).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab.95, 3347–3351. https://doi.org/10.1210/jc.2010-0288 (2010).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yang Zhu was responsible for the initial drafting of the manuscript. Yang Zhu, Ye Li, Xuan Li, Sheng Huang, and Yihui Li participated in the material preparation, data collection, and analysis. All authors critically reviewed and provided feedback on previous versions of the manuscript. Yang Zhu made significant contributions to revising and finalizing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Y., Li, Y., Li, X. et al. Association between triglyceride glucose-body mass index and all-cause mortality in critically ill patients with acute pancreatitis. Sci Rep 14, 21605 (2024). https://doi.org/10.1038/s41598-024-72969-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72969-w
Keywords
This article is cited by
-
Association of red blood cell distribution width with short- and long-term all-cause mortality in patients with acute pancreatitis and sepsis
BMC Gastroenterology (2025)
-
The association between triglyceride glucose-body mass index and mortality in intensive care unit patients: a propensity score matching analysis
European Journal of Medical Research (2025)
-
Construction of a nomogram for hypertriglyceridemic severe acute pancreatitis that includes metabolic indexes
Lipids in Health and Disease (2025)