Abstract
This prospective study aimed to investigate microvascular changes in diabetic patients undergoing cataract surgery. Foveal avascular zone (FAZ) area, as well as vessel density (VD) in the macula and radial peripapillary capillary plexus (RPC), were compared before and after surgery. Sixty eyes (72.3%) had no diabetic retinopathy (no DR group) and 23 eyes (27.7%) had non-proliferative diabetic retinopathy (DR group). In the no DR group, the FAZ area in the superficial capillary plexus (SCP) decreased from 0.41 ± 0.13 to 0.38 ± 0.11 mm2 (P = 0.036), while no significant change was observed in the DR group (0.33 ± 0.12 to 0.30 ± 0.12 mm2, P = 0.091) at 6 months postoperatively. VD in the RPC increased from 34.4 ± 2.3% to 35.6 ± 2.3% in the no DR group (P = 0.009), but there was no significant change in the DR group (33.0 ± 3.5% vs. 34.0 ± 2.3%, P = 0.051) after 6 months. VD in the macula did not change in either group. Phacoemulsification reduced the FAZ area in the SCP and increased the VD in the RPC in diabetic patients without diabetic retinopathy at six months postoperatively.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) and cataracts are prominent global causes of vision impairment1. The frequent co-occurrence of DR and cataracts is notable, given diabetes as a risk factor for cataract development2,3. While cataract surgery is intended to enhance vision, its potential impact on DR progression remains controversial. Although one report suggested no progression of DR after cataract surgery4, another indicates that phacoemulsification may have the potential to initiate or exacerbate DR5, additionally elevating the risk of macular edema6. Various factors, including the blood-aqueous barrier, vascular endothelial growth factor, and inflammatory cytokines, have been implicated in the pathological processes of macular edema, though their precise roles remain elusive7,8.
Optical coherence tomography angiography (OCTA), an innovative non-invasive three-dimensional imaging technique, has recently emerged as a tool capable of identifying early microvascular changes even before clinical signs of DR manifest. It can quantify retinal microvasculature by measuring parameters such as the foveal avascular zone (FAZ) area and vessel density (VD)9,10. Moreover, peripapillary VD has been reported to decrease in diabetic patients without clinical DR11. While the progression of DR after cataract surgery has traditionally been assessed based on fundus examination4,5, this approach could not sufficiently verify changes in microvasculature occurring after the surgery. Previous studies utilizing OCTA have reported alterations not only in the macula but also in peripapillary microvasculature following cataract surgery in both normal and diabetic patients12,13,14,15.
However, OCTA has limitations in accurately evaluating macular perfusion, particularly in cases of macular edema due to artifacts16. Additionally, variations in macular edema presentation on OCTA might arise from device measurement discrepancies17. Despite challenges in accurately assessing macular perfusion using OCTA in cases of pseudophakic macular edema, reported to occur four times more frequently in diabetic patients compared to nondiabetic patients at approximately 4%,6 there is still a lack of research documenting changes in macular perfusion following phacoemulsification in diabetic patients, excluding cases of pseudophakic macular edema. Furthermore, existing evidence on alterations in peripapillary blood flow after cataract surgery in diabetic patients remains inconclusive. Therefore, this study aimed to evaluate macular and peripapillary perfusion using OCTA after uncomplicated phacoemulsification in diabetic patients without macular edema.
Results
We included 83 eyes from 83 patients. The demographic and clinical characteristics of the patients are presented in Table 1. Of the 83 eyes, 60 eyes (72.3%) did not have DR (No DR group), and 23 eyes (27.8%) had non-proliferative diabetic retinopathy (DR group). In the DR group, there were 9 eyes (39.1%) with mild non-proliferative diabetic retinopathy (NPDR), 11 eyes (47.8%) with moderate NPDR, and 3 eyes (13.0%) with severe NPDR.
The FAZ area of the superficial capillary plexus (SCP) was significantly larger in the No DR group (0.41 ± 0.13 mm2) compared to the DR group (0.33 ± 0.12 mm2) preoperatively (P = 0.019) (Table 2). This difference persisted until 6 months postoperatively (0.38 ± 0.11 mm2 vs. 0.30 ± 0.12 mm2, P = 0.005). Additionally, we observed a significant difference in the FAZ area of the deep capillary plexus (DCP) between the No DR group and the DR group (0.55 ± 0.16 mm2 vs. 0.45 ± 0.14 mm2, P = 0.009) before surgery. However, this difference was not significant at 6 months postoperatively (0.53 ± 0.15 vs. 0.46 ± 0.18, P = 0.104). Preoperatively, the VD of the radial peripapillary capillary plexus (RPC) was significantly higher in the No DR group (34.4 ± 2.3%) compared to the DR group (33.0 ± 3.5%, P = 0.039). Similarly, at 6 months postoperatively, the VD of the RPC remained significantly higher in the No DR group (35.6 ± 2.3%) than in the DR group (34.0 ± 2.3%, P = 0.030).
The results of repeated measures analysis of variance and post-hoc analysis of OCTA parameters are shown in Table 3. In the overall cohort, the FAZ area of the SCP demonstrated a significant difference among preoperative, 1-month postoperative, and 6-month postoperative measurements (P = 0.017). Notably, the No DR group exhibited a significant difference in the FAZ area of the SCP (P = 0.036), while the DR group did not show a significant difference (P = 0.091) in the FAZ area of the SCP across the same time points. Furthermore, post-hoc analysis revealed a significant increase in the VD of the RPC at 6 months postoperatively compared to baseline in the No DR group (34.4 ± 2.3% at baseline vs. 35.6 ± 2.3% after 6 months, P = 0.005). On the other hand, the DR group did not show a significant difference between baseline and 6 months postoperatively (33.0 ± 3.5% vs. 34.0 ± 2.3%, P = 0.393). In the macula, the VD of the parafovea and perifovea showed no significant postoperative changes in either the SCP or DCP for all patients, as well as for the No DR group and the DR group.
Discussion
In this prospective study, we investigated alterations in macular and peripapillary microvasculature following cataract surgery in diabetic patients. Six months after uncomplicated phacoemulsification, we observed a significant reduction in the FAZ area in the SCP and an increase in VD in the RPC in the DR group, while these changes were not significant in the No DR group. No significant postoperative changes in the VD of the parafovea and perifovea were observed in either the SCP or DCP for all patients, as well as within the No DR group and the DR group.
To date, there remains a paucity of evidence addressing alterations in the FAZ following cataract surgery in diabetic patients. Remarkably, we observed a significant difference in the FAZ area of the SCP 6 months postoperatively in patients with NPDR who were free of diabetic macular edema. In contrast, Svjaščenkova et al., who included patients with diabetic macular edema, reported an increase in the FAZ area up to 3 months postoperatively in those with diabetic macular edema. They observed a decreased in the FAZ area for patients without macular edema, but it did not show statistical significance18. The absence of a significant decrease in the FAZ area could be attributed to the smaller sample size and shorter follow-up period in their study. We postulated that the reduction in the FAZ area after cataract surgery in diabetic patients might be due to the compromised blood-retinal barrier associated with DR. This hypothesis is based on our observation of different postoperative changes between eyes with and without DR. A comprehensive study highlighted an increased risk of macular edema after cataract surgery, especially in cases of severe pre-existing DR, attributing it to the impaired blood-retinal barrier function in patients with advanced vascular changes due to DR6.
Regarding alterations in RPC VD in non-diabetic patients, variations exist among previous studies. Two studies indicated no change in VD in the peripapillary area, while showing an increase in VD in the optic disc 3 months postoperatively12,19. Contrary to these findings, Karabulut et al. reported an increase in VD in the peripapillary area from 49.5% preoperatively to 51.8% at one month postoperatively, speculating that this observation might be attributed to the peripapillary blood supply and retinal vascular autoregulation15. In diabetic patients, the available evidence regarding changes in RPC VD is limited. We observed that the increase of RPC VD up to 1 month after surgery was maintained until 6 months. In contrast to our study, a prior study following diabetic and non-diabetic patients for 1 month after phacoemulsification reported no significant changes in RPC VD for either diabetic or non-diabetic patients postoperatively14. However, this discrepancy might be ascribed to their exclusion of patients with DR, coupled with a restricted follow-up period. While the pathophysiologic mechanism remains unclear, we hypothesized that the alterations in peripapillary blood supply could potentially result from retinal vascular autoregulation due to a decrease in intraocular pressure following cataract surgery or from postoperative inflammation13,15.
Contrast to other previous reports suggesting a significant increase in SCP and DCP VD following phacoemulsification13,14,20,21, our study observed an elevation in both SCP and DCP VD at the parafovea and perifovea after six months postoperatively, though it did not attain statistical significance. The discrepancies in VD observed between our study and others could be explained by variations in OCTA equipment. Due to the diversity in methodologies used for segmenting the retinal plexus layers and identifying vascular flow on OCTA devices9, comparing results obtained from different equipment can pose challenges22. Moreover, previous reports indicated that measurements varied with axial length, astigmatism, and media opacity23,24,25. Another possible reason could be the variation in phacoemulsification equipment and surgeon-related factors, because the energy level of phacoemulsification employed during surgery could influence microvascular parameters21.
Since macular blood flow may not fully represent the overall retinal circulation26, it remains unclear which OCTA metrics of the macula are most useful for monitoring DR progression after cataract surgery in diabetic patients. Many studies have reported an increase in FAZ area and a decrease in macular VD with DR progression9,26,27. While VD can be assessed purely by its numerical value, FAZ allows for the evaluation of both its area and morphological changes. Therefore, we suggest that FAZ in both the SCP and DCP may serve as a sensitive OCTA metric of the macula for monitoring DR progression in clinical practice. However, it is important to note that a smaller FAZ has been reported even in advanced DR stages26. Thus, incorporating fundus examination findings with OCTA metrics is crucial for a thorough assessment during follow-up. Additionally, considering that pseudophakic macular edema can occur in diabetic patients after cataract surgery and affect the measurement of the FAZ or VD in the macula6,28, tracking vascular changes using RPC VD might be more useful. However, further research is needed to determine whether changes in RPC VD after cataract surgery can reflect overall retinal vascular changes.
Our study has several limitations, such as a small sample size and the lack of investigation into factors associated with phacoemulsification. However, the study’s strength lies in its prospective design, relatively large scale, and extended follow-up period compared to previous studies. Furthermore, unlike other studies that included patients with macular edema, potentially introducing errors in OCTA measurements, our study did not involve macular edema, thereby possibly providing more accurate data. Given the limited evidence regarding post-cataract surgery changes in macular and peripapillary perfusion in diabetic patients, future long-term observational studies with larger sample sizes are warranted. In conclusion, alterations in macular and peripapillary microvasculature post-surgery are believed to be driven by postoperative inflammation and hemodynamic changes, persisting for up to 6 months after cataract surgery. Further investigation is required to ascertain whether these microvascular alterations impact DR and the optic nerve.
Methods
This prospective study received approval from the Institutional Review Board of Hallym University Sacred Heart Hospital (IRB number: HALLYM 2019-05-031) and adhered to the tenets of the Declaration of Helsinki. Written informed consent for study participation was obtained.
We enrolled 83 eyes from 83 patients with type 2 diabetes mellitus undergoing uncomplicated phacoemulsification at Hallym University Sacred Heart Hospital between May 2019 and July 2023. Inclusion criteria involved eyes with visually significant cataracts but without DR (No DR group) or with NPDR (DR group). Exclusion criteria were as follows: (1) Age under 18 years; (2) Presence of macular edema or proliferative DR; (3) Severe cataracts causing poor OCTA image quality; (4) History of previous ocular interventions (laser treatment, injection, etc.); (5) Presence of other retinal or optic nerve diseases; (6) High myopia with an axial length 26.5 mm or more; (7) Complicated cataract surgery.
Preoperative ophthalmologic examinations included measurements of best-corrected visual acuity, intraocular pressure, and axial length, along with slit-lamp examination, dilated fundus examination, and OCTA. Phacoemulsification and posterior chamber intraocular lens implantation (Tecnis® ZCB00, Abbott Medical Optics, Santa Ana, California, USA) were performed by a skilled surgeon (SK) using the Whitestar Signature® system (Abbott Medical Optics, Santa Ana, California, USA). Follow-up assessments were conducted at one month and six months post-surgery.
Images of 4.5 × 4.5 mm centered on the fovea and 3 × 3 mm centered on the optic disc were obtained using a swept-source OCTA device (DRI OCT Triton, Topcon, Tokyo, Japan) operating at a wavelength of 1050 nm. The device had an acquisition speed of 100,000 A-scans per second, and axial and transverse resolutions of 7 and 20 μm in tissue, respectively. Only images with a signal strength intensity above 50 were included, while those with motion artifacts or segmentation errors were excluded.
The SCP and DCP slabs were segmented using built-in software (IMAGEnet6, version 1.34). SCP was defined from the internal limiting membrane to 15.6 μm below the junction between the inner plexiform and inner nuclear layers. DCP was delineated from 15.6 to 70.2 μm below the boundary of the inner plexiform and inner nuclear layers. The parafovea was defined between circles of 1.5 mm and 3 mm diameter, and the perifovea between the 3 mm diameter circle and a 4.5 × 4.5 mm square. For the RPC, delineation extended from the internal limiting membrane to the boundary between the retinal nerve fiber layer and the ganglion cell layers, excluding a 1.5 mm diameter circle located at the center of the optic disc.
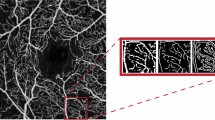
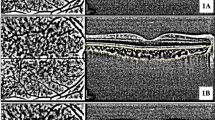
The FAZ area of both the SCP and DCP was independently measured by two researchers using built-in software, and the average value was utilized. To calculate VD, en face images of the macula and disc were exported and analyzed using ImageJ (software version 2.0.0, National Institutes of Health, Bethesda, Maryland; available at https://rsb.info.nih.gov/ij/index.html) based on the method outlined by Borrelli et al.29 Blood vessels were identified as pixels with decorrelation values above the threshold level. VD measurements were obtained for SCP and DCP in the parafovea and perifovea, as well as the RPC (Fig. 1).
Optical coherence tomography angiography of the superficial capillary plexus (A), deep capillary plexus (C), and radial peripapillary capillary plexus (E). Binarized images for measuring vessel density of the superficial capillary plexus (B), deep capillary plexus (D), and radial peripapillary capillary plexus (F).
To compare the continuous variables between the two groups, an independent t-test was utilized. Repeated measures analysis of variance was used to compare OCTA parameters at three different time points: pre-surgery, one month post-surgery, and 6 months post- surgery. The Bonferroni method was applied for post-hoc analysis of pairwise comparisons between any two time points. Statistical significance was set at P < 0.05, and all analyses were performed using IBM SPSS version 26 (IBM Corporation, Armonk, New York, USA).
Data availability
The clinical datasets used in current study are not publicly available due to privacy constraints. The data can be requested for sharing for peer-review or research purposes by contacting Soonil Kwon (magicham@daum.net).
References
Flaxman, S. R. et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob Health. 5, e1221–e1234. https://doi.org/10.1016/s2214-109x(17)30393-5 (2017).
Klein, B. E., Klein, R. & Lee, K. E. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5-year incidence of age-related cataract and progression of lens opacities: The Beaver Dam Eye Study. Am. J. Ophthalmol. 126, 782–790. https://doi.org/10.1016/s0002-9394(98)00280-3 (1998).
Tham, Y. C. et al. Association of cataract surgery with risk of diabetic retinopathy among Asian participants in the Singapore Epidemiology of Eye diseases Study. JAMA Netw. Open. 3, e208035–e208035. https://doi.org/10.1001/jamanetworkopen.2020.8035 (2020).
Squirrell, D., Bhola, R., Bush, J., Winder, S. & Talbot, J. F. A prospective, case controlled study of the natural history of diabetic retinopathy and maculopathy after uncomplicated phacoemulsification cataract surgery in patients with type 2 diabetes. Br. J. Ophthalmol. 86, 565–571. https://doi.org/10.1136/bjo.86.5.565 (2002).
Hong, T. et al. Development and progression of diabetic retinopathy 12 months after phacoemulsification cataract surgery. Ophthalmology. 116, 1510–1514. https://doi.org/10.1016/j.ophtha.2009.03.003 (2009).
Chu, C. J. et al. Risk factors and incidence of macular edema after cataract surgery: A database study of 81984 eyes. Ophthalmology.123, 316–323. https://doi.org/10.1016/j.ophtha.2015.10.001 (2016).
Liu, Y., Luo, L., He, M. & Liu, X. Disorders of the blood-aqueous barrier after phacoemulsification in diabetic patients. Eye. 18, 900–904. https://doi.org/10.1038/sj.eye.6701349 (2004).
Patel, J. I., Hykin, P. G. & Cree, I. A. Diabetic cataract removal: Postoperative progression of maculopathy–growth factor and clinical analysis. Br. J. Ophthalmol. 90, 697–701. https://doi.org/10.1136/bjo.2005.087403 (2006).
Waheed, N. K. et al. Optical coherence tomography angiography in diabetic retinopathy. Prog .Retin Eye Res. 97, 101206. https://doi.org/10.1016/j.preteyeres.2023.101206 (2023).
Vujosevic, S. et al. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina. 39, 435–445. https://doi.org/10.1097/iae.0000000000001990 (2019).
Lee, M. W. et al. Peripapillary retinal nerve Fiber layer and microvasculature in prolonged type 2 diabetes patients without clinical diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 62, 9. https://doi.org/10.1167/iovs.62.2.9 (2021).
Zhu, Z. H. et al. Evaluation of optic nerve head vessels density changes after phacoemulsification cataract surgery using optical coherence tomography angiography. Int. J. Ophthalmol. 16, 884–890. https://doi.org/10.18240/ijo.2023.06.08 (2023).
Feng, L., Azhati, G., Li, T. & Liu, F. Macular vascular density changes following cataract surgery in diabetic patients: An optical coherence tomography angiography study. J Ophthalmol. 6641944. https://doi.org/10.1155/2021/6641944 (2021).
Tarek, N., Khalil, N. M., ElSheikh, H. F. & Shousha, S. M. Evaluation of macular and peri-papillary blood vessel density following uncomplicated phacoemulsification in diabetics using optical coherence tomography angiography. Indian J. Ophthalmol. 69, 1173–1177. https://doi.org/10.4103/ijo.IJO_2187_20 (2021).
Karabulut, M., Karabulut, S., Sül, S. & Karalezli, A. Optic nerve head microvascular changes after phacoemulsification surgery. Graefes Arch. Clin. Exp. Ophthalmol. 257, 2729–2733. https://doi.org/10.1007/s00417-019-04473-1 (2019).
Podkowinski, D. et al. A swept source optical coherence tomography angiography study: Imaging artifacts and comparison of non-perfusion areas with fluorescein angiography in diabetic macular edema. PLoS ONE. 16, e0249918. https://doi.org/10.1371/journal.pone.0249918 (2021).
Parravano, M. et al. Appearance of cysts and capillary non perfusion areas in diabetic macular edema using two different OCTA devices. Sci. Rep. 10, 800. https://doi.org/10.1038/s41598-020-57680-w (2020).
Svjaščenkova, L., Laganovska, G. & Tzivian, L. Microstructural changes in the Macula following cataract surgery in patients with type 2 diabetes mellitus detected using optical coherence tomography angiography. Diagnostics. 13, 605. https://doi.org/10.3390/diagnostics13040605 (2023).
Özkan, B. & Çiloğlu, E. Evaluation of the effect of uncomplicated cataract surgery on retina and optic disc: Optical coherence tomography angiography study. Korean J. Ophthalmol. 36, 287–295. https://doi.org/10.3341/kjo.2021.0172 (2022).
Yao, H., Yang, Z., Cheng, Y. & Shen, X. Macular changes following cataract surgery in eyes with early diabetic retinopathy: An OCT and OCT angiography study. Front. Med. 10, 1290599. https://doi.org/10.3389/fmed.2023.1290599 (2023).
Wang, Z., Wang, E. & Chen, Y. Transient reduction in macular deep capillary density on optical coherence tomography angiography after phacoemulsification surgery in diabetic patients. BMC Ophthalmol. 20, 1–9. https://doi.org/10.1186/s12886-020-01605-8 (2020).
Corvi, F. et al. Reproducibility of vessel density, fractal dimension, and foveal avascular zone using 7 different optical coherence tomography angiography devices. Am. J. Ophthalmol. 186, 25–31. https://doi.org/10.1016/j.ajo.2017.11.011 (2018).
Zhang, J., Tang, F. Y., Cheung, C., Chen, X. & Chen, H. Different effect of media opacity on automated and manual measurement of foveal avascular zone of optical coherence tomography angiographies. Br. J. Ophthalmol. 105, 812–818. https://doi.org/10.1136/bjophthalmol-2019-315780 (2021).
Youssef, M. M., Sadek, S. H. & Hatata, R. M. Macular and Optic nerve microvascular alteration in relation to axial length, by optical coherence tomography angiography (OCTA). Clin. Ophthalmol. 16, 885–892. https://doi.org/10.2147/opth.S354235 (2022).
Vidal-Oliver, L., Gallego-Pinazo, R. & Dolz-Marco, R. Astigmatism influences quantitative and qualitative analysis in optical coherence tomography angiography imaging. Transl Vis. Sci. Technol. 13, 10. https://doi.org/10.1167/tvst.13.1.10 (2024).
Or, C. et al. Combined multimodal analysis of peripheral retinal and macular circulation in diabetic retinopathy (COPRA Study). Ophthalmol. Retina. 3, 580–588. https://doi.org/10.1016/j.oret.2019.03.001 (2019).
Sun, Z. et al. OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: A prospective study. Ophthalmology. 126, 1675–1684. https://doi.org/10.1016/j.ophtha.2019.06.016 (2019).
Ghasemi Falavarjani, K. et al. Effect of segmentation error correction on optical coherence tomography angiography measurements in healthy subjects and diabetic macular oedema. Br. J. Ophthalmol. 104, 162–166. https://doi.org/10.1136/bjophthalmol-2019-314018 (2020).
Borrelli, E. et al. A comparison study among different algorithms. Retina. 41, 1799–1808. https://doi.org/10.1097/iae.0000000000003145 (2021).
Author information
Authors and Affiliations
Contributions
J.K.: Investigation, Methodology, Formal analysis, Visualization, Writing - Original Draft, Writing - Review & Editing. S.K.: Investigation, Formal analysis. E.B.: Methodology, Visualization. M.S.P.: Investigation. B.-J.C: Investigation. S.K.: Conceptualization, Methodology, Writing - Original Draft, Writing - Review & Editing, Supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, J., Kim, S., Borrelli, E. et al. Alterations in optical coherence tomography angiography parameters after cataract surgery in patients with diabetes. Sci Rep 14, 23814 (2024). https://doi.org/10.1038/s41598-024-73830-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73830-w