Abstract
Biostimulants that improve agriculture's productivity and environmental responsibility are widely favored and used. Algae has emerged as a viable option for sustainable agriculture. In the present study, the effects of the Cladophora glomerata (L.) Kütz. aqueous extract in three concentrations (2.5, 5, and 10 mg extract per mL of tap water) was tested on the growth and productivity of garden cress (GCR) Lepidium sativum L., plants over 12 days, and the results were compared with those of the control group (irrigated only with tap water). The effect of the algae extract was studied by assessing the changes in phenolic compounds using HPLC–DAD and determining the chlorophyll content as well as the total antioxidant capacity of the GCR using DPPH and ABTS tests. Total phenolics, as well as total flavonoids, were measured. The potential role of algae extract in promoting cress plant growth was attained when compared to the control, mainly at a concentration of 2.5 mg/mL, which exhibited the highest yield growth after 12 days and presented the highest antioxidant capacity at 13.53 ± 1.16 mg Trolox equivalent (TE)/g dry weight (DW), compared to 10.44 ± 0.33 mgTE/g DW for the control. The total phenolic content significantly increased (p < 0.05) from 14.34 ± 0.84 mg gallic acid equivalent (GAE)/ g dry weight (DW) to 23.14 ± 0.55 mg GAE/g DW. Eleven phenolic compounds were identified in different tested samples of GCR, whether treated or not treated with algae extracts. Chrysin was only identified in the treated plants. Therefore, the use of algae (C. glomerata) presents promising potential as a biostimulant in agriculture, contributing to increased plant growth and improved resistance to environmental stress.
Similar content being viewed by others
Introduction
Nowadays, there is a growing demand for sustainable agricultural development to compensate for the drought factor and the deficit in irrigation1. The current reliance on chemical fertilizers and pesticides, though intended to enhance agricultural productivity, is contributing to environmental hazards. As a result, there is a growing awareness of the detrimental impact of excessive agrochemical usage on the overall sustainability of agriculture2. These chemicals have caused significant harm to global ecological security, such as decreasing beneficial soil microorganisms and degrading the local ecosystem due to an increase in pollutants in soil, water, and air. Moreover, they are harmful to human health as they enter the food chain1. Therefore, successful agricultural practices involve managing plant diseases and growth, as well as having a favorable growing environment (healthy soils, availability of nutrients, and protection from other stresses, etc.), considering important requirements for sustainable agricultural development, and prompting the scientific community to overcome this problem and find feasible solutions3. Under the current scenario, the application of biofertilizers has been suggested as a viable solution for large-scale agricultural practices and could be used as one of the environmentally friendly alternatives to synthetic fertilizers4. Amongst various available biofertilizers, microorganisms like bacteria, fungi, algae, and different metabolites are extracted from their biomass. These organic fertilizers are one of the alternatives, which are eco-friendly, cost-effective, and enhance the soil quality without degrading the ecosystem4.
The organic fertilizers from algae, for example, are considered a potential alternative to mainstream synthetic fertilizers due to their enrichment with macronutrients, micronutrients, and some growth regulators, etc. This directly aids in enhancing the growth and yield of crop plants5. Algae can be classified into different types, including microalgae, macroalgae, and unicellular to multicellular forms. Among other applications, they are commonly used as food, feed, and the source of different phytochemicals. Furthermore, both macroalgae and microalgae have long been viewed as potential commercial prospects in the field of agronomy and agro-industries due to their high biostimulant activity6. For decades, microalgae have been utilized as biofertilizers in rice fields. Now, the use of macroalgae commonly known as seaweeds, is being viewed as a potential resource of organic fertilizer or soil conditioner in many countries7. Kuwada et al.8 observed that seaweed liquid extract from Undaria pinnatifida (Harvey) Suringar and Laminaria japonica Arschoug (syn. Saccharina japonica) could be used as an arbuscular mycorrhiza fungus growth promoter. Several studies also demonstrated that seaweed liquid extract accelerates proper root development by increasing lateral root formation9 and enhancing the total volume of the root system10. Likewise, there are reports of the direct application of seaweed in dried powder form in agricultural fields as fertilizers8. Additionally, seaweed liquid extracts and seaweed manure displayed an extensive range of growth responses, which suggests the presence of various plant growth regulators, such as auxins, cytokinins, and gibberellins. Their fortification with certain bioactive compounds, like phenolic compounds, has become popular for numerous key crops (vegetables, cereals, flowers, etc.)11. Marine macroalgae are distributed globally in oceans and are generally divided into three categories: green, brown, and red algae. Cladophora glomerata (L.) Kütz., a green alga belonging to the Cladophoraceae family (Chlorophyta, Ulvophyceae), is one of the most commonly found algae. It has been reported that the qualitative analysis of this algae extract revealed both phenolic acids and flavonoids, known antioxidants12,13. Korzeniowska et al. 13 noted that nine phenolic compounds (gallic acid, chlorogenic acid, syringic acid, p-coumaric acid, myricetin, 3,4-dihydroxybenzoic acid, vanillic acid, 4-hydroxybenzoic acid, and rutin) were found in microwave extracts from freshwater C. glomerata13. Yarnpakdee et al. 14 reported that C. glomerata ethanolic extract contained quercetin, isoquercetin, and hydroquinone as major components14. Regardless, this seaweed serves as a natural and potentially useful source for cosmetic ingredients, food supplements, nutraceuticals, and pharmaceutical components15. Limited research investigation has been undertaken on similar subjects for the use of this algae type in agriculture development. As a consequence, our investigation aims to study the effect of C. glomerata extract on the growth of the GCR (Lepidium sativum L.) plant. The choice of GCR plant is not random as it is a rapidly growing annual plant and it is planted all over the world as an important medicinal crop with antioxidant, anti-inflammatory, and anti-fungal activities, etc. 16.
In this context, the main objective of our research is to enhance the productivity and quality of garden cress plants by adding C. glomerata extract, representing the first instance of its application for this purpose.
Materials and methods
Chemicals
All solvents and reagents were of analytical purity. Methanol, ethanol, aluminum nitrate nonahydrate (Al(NO₃)₃ * 9 H₂O), sodium Carbonate (Na2CO3), potassium persulfate (K2S2O8) were purchased from Sigma-Aldrich (Merck, Darmstadt, Germany).
2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS); 1,1-diphenyl-2-picrylhydrazyl (DPPH), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′, Folin–Ciocalteu's phenol reagent were purchased from Sigma-Aldrich (Merck, Darmstadt, Germany).
Standards
Standards for HPLC analysis such as gallic acid, chlorogenic acid, syringic acid, vanillic acid, chrysin, 3-hydroxybenzoic acid (3-HBA), ferulic acid, rosmarinic acid, quercetin, 4-hydroxybenzoic acid (4-HBA), rutin, cinnamic acid, 3,4 –dihydroxybenzoic acid (3,4-DHBA) and caffeic acid were purchased from Sigma-Aldrich (Merck, Darmstadt, Germany). Methanol HPLC grade (Riedel de Haen brand) and LC–MS grade formic acid (Fluka brand) were from Honeywell (Seelze, Germany). HPLC water was produced using a Hydrolab SPRING water treatment station (Hydrolab, Straszyn, Poland).
Algae biomass collection and extraction
Freshwater Cladophora glomerata biomass was collected from the Wielkopolska region (Lake Oporzynskie N52˚55´31.26'', E17˚9′38.372''; Nielba River N52˚48´14.996'', E17˚12′ 40.93'') in Poland. The collected freshwater algae was cleaned, washed numerous times with distilled water to remove sand and mud particles, and dried at 40 °C. The dried raw material was then ground into a fine powder using an electric grinder. The prepared algae biomass was stored at room temperature, protecting it from moisture and light.
The ground material (40 g) was extracted using the Soxhlet system for 24 h with water/ethanol (30:70). The extraction was carried out in three replications, each time using new algae material.
The mixture was filtered after each extraction, followed by concentration using a rotary evaporator at 40° C, and 60° C at the end. The resulting extract was stored at 4° C for further experimental studies.
Plant material
The plant tested in this study was Lepidium sativum L., also known as garden cress (GCR).
0.4 g (GCR) seeds (about 200 seeds) were sown in each sprouting box. The study involved planting seeds in different substrates sprouting box: (T0) tap water only (without algae extract), (T2.5) 2.5 mg of dried C. glomerata extract per mL of tap water, (T5) 5 mg of dried C. glomerata extract per mL of tap water, and (T10) 10 mg of dried C. glomerata extract per mL of tap water. After 12 days of growth, the GCR plants grown in various germination containers were collected and stored in a refrigerator for future use.
Garden cress plant height and stem length measurements
The heights and stem length of plants were measured and recorded using a tape measure with 1 mm precision.
Garden cress dry and fresh weight measurements
The fresh weight of GCR was measured after 12 days using a digital scale with a precision of 0.01 g. Then, the samples were placed in an oven at 45⁰C for 6 h for drying (to constant mass) and weighed using the same scale.
Garden cress extraction
The extraction of dried GCR plants was carried out by maceration for 3 h at 45⁰C using methanol as a solvent. The extracts were centrifuged at 14,500 rpm for 5 min and stored at 4⁰C until analysis.
Total phenolic content (TPC)
The total phenolic content (TPC) assay was modified from Sánchez-Rangel et al. 17 . In brief, 100 μL of the GCR plant macerate solution with a concentration of 10 mg/mL was mixed with 2.5 mL of Folin-Ciocalteu reagent (diluted 10 times with distilled water). The mixture was stirred and allowed to stand for 2 min. Then, 0.5 mL of 20% (w/v) Na2CO3 was added. The prepared samples were mixed and kept in the dark for 2 h at room temperature. The absorbance was measured at 760 nm. The samples were performed in triplicate, and the results were presented as means ± standard deviation in units of mg gallic acid equivalent per gram of dry biomass weight (mg GAE/g DW). The gallic acid equivalents were calculated from measured absorbances using the linear equation based on the prepared standard calibration curve.
Total flavonoid content (TFC)
900 μL of MeOH was mixed with 100 μL of extract GCR solution (concentration 10 mg/mL). Then, 100 uL of aluminum nitrate nonahydrate solution in methanol was added to the mixture and allowed to stand for 5 min. The absorbance was measured at 420 nm at room temperature. A yellow color indicated the presence of flavonoids. Quercetin was used as a standard for the calibration curve, and the total flavonoid content was expressed as quercetin equivalent (QE) in mg per g of dry biomass weight (mg QE/g DW) 13,18. All determinations were made in three replications.
Determination of the content of chlorophyll and total carotenoids
The content of chlorophyll was determined by the method described by Osório et al. 19 with some modifications. All the extractions were carried out in triplicate using methanol solvent by vortexing the dry biomass for 2 min and shaking via ultrasonic cleaner for 10 min at room temperature, all done with protection from light. Then, the extracts were centrifuged at 6000 rpm for 5 min and the collected supernatant was subjected to another centrifugation (14,000 rpm for 5 min). The absorbance of the solutions was measured in a UV-7205 ultraviolet–visible spectrophotometer (Jenway), at the wavelengths corresponding to the maximum absorption of the pigments under study, according to Wellburn 20. The equations described below were used to calculate the chlorophyll’s content, and the results were expressed in µg/g of dry biomass19.
High-performance liquid chromatography with diode array detection
Qualitative analysis of phenolic compounds in different samples of extracts was analyzed according to Kebal et al. 21 using high-performance liquid chromatography (HPLC). The assay was performed using a Dionex Ultimate 3000 system (Thermo Fischer, CA, US) with a diode array detector (DAD). The chromatographic separation was carried out in the Gemini NX C18 column (150 mm × 4.6 mm × 3 µm, Phenomenex, Torrance, US) with a dedicated pre-column. The column oven was set at 25⁰C. The injection volume was 10 µL. The elution solvents were 0.1% formic acid in water (A) and methanol (B). The UV absorption spectra of the standards and samples were measured between 190 and 400 nm.
The following phenolic compounds standards were used for analyses: gallic acid, 3,4-dihydroxybenzoic acid (3,4-DHBA), 4-hydroxybenzoic acid (4-HBA), chlorogenic acid, vanillic acid, caffeic acid, 3-hydroxy-benzoic acid (3-HBA), syringic acid, ferulic acid, rutin, rosmarinic acid, cinnamic acid, quercetin, and chrysin. The identification of compounds was based on retention time, UV absorption spectrum, and the addition of standards to the analyzed samples. The different samples of the algae and GCR extracts were spiked with the stock solution of 14 phenolic compounds standards. Prepared samples were stored at 4 °C until analysis.
Antioxidant activity determination
Two different colorimetric and spectrophotometric methods were used to evaluate the antioxidant activity of different samples of GCR and algae C. glomerata extract, namely DPPH and ABTS tests. The analyses for each extract were carried out in at least three replications.
ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulpfonic acid)] assay
ABTS radical scavenging activity was determined using the method of Korzeniowska et al. 13. A standard curve was prepared using Trolox in the range of (0.03–0.15 mg/mL). The absorbance of the resulting solution was measured at 734 nm. The activity was expressed as milligrams of Trolox equivalent per g of dry biomass weight (mg TE/g DW).
DPPH (2,2-diphenyl-1-picrylhydrazyl) assay
DPPH radical scavenging activity was determined using the method of Górnaś et al. 22. The reaction was initiated by mixing the sample (0.1 mL) with the DPPH solution (2.9 mL). Absorbance was measured at a wavelength of 517 nm after 30 min storage in the dark. Gallic acid (GA) with the range of (0–0.06 mg/mL) was used as a standard for calibration curve preparation. The activity was expressed as milligrams of GA per gram of dry biomass weight (mg GA/g DW).
Statistical analysis
The results were expressed as the mean ± standard deviation (SD) of three measurements for the analytical determination. The significant differences between the values of all parameters were determined at p < 0.05 according to the one-way ANOVA: Tukey post-hoc test. This statistical analysis was performed using the SPSS Statistics (V20.0) program. The principal component analysis (PCA) was applied to the data set of all analyses performed for garden cress plants treated or not treated with algae extract. PCA plots analysis was performed using XLSTAT software for Windows (https://www.xlstat.com/en/v.2023.3.0, Addinsoft, New York, US).
Results and discussion
Algae extraction
The extraction of C. glomerata was effected in triplicate using the Soxhlet method for 24 h. The weight of the dry algae extract obtained was 5.01 ± 0.02 g, resulting in an extraction yield of 4.37%.
Growth and morphological characteristics
After harvesting the plants from the four different pots, GCR height was measured from the root tip to the apex of the plant using a measuring tape. Similarly, the fresh biomass of plants was measured in each treatment, and dry weight was measured after drying in an oven at 45 °C.
The effect of algae extract on GCR growth parameters, including fresh weight, dry weight, plant height, and stem length after 12 days of growth, is presented in Figs. 1 and 2. Under control conditions, with tap water (T0), the mass of harvested GCR was 3.67 g (Fig. 1a). A considerable increase of biomass was observed in T2.5, attaining the highest mass of 5.12 g per sprouting box, compared to 3.08 g and 2.52 g for T5 and T10, respectively. Similar results were attained for GCR (T2.5) at dry weight parameter compared to other samples (Fig. 1 b). In addition, Fig. 2a shows an increased average GCR plant height of 11.33 cm for the T2.5 compared to the control (T0) exhibiting 9.17 cm.
Al-Shareefi et al. 23 noted that the presence of such nutrients, macro, micronutrients, and phytohormones in algae extract stimulated root growth and increased stem thickness of the Freesia hybrida L. plant. This report is in line with our present results indicated in Fig. 2b. Stem growth was greater for all the GCR plants treated with algae extract: (T2.5), (T5), and (T10) with respective average stem lengths of 5.5, 4.67, and 4.33 cm, compared to the control plant, which had average stem length of 3.67 cm.
Due to the information above, the treatment with algae extract, especially with a concentration of 2.5 mg/mL, enhances the morphological characteristics of the garden cress plants. Our results are in agreement with previous studies, which observed an increase in vegetative growth of plants caused by the application of seaweed extract 24,25. The presence of growth-promoting substances in C. glomerata extract, including phytohormones, along with macro- and micronutrients may be the reason for the promoted growth25.
Total phenolic content (TPC) and total flavonoid content (TFC)
The presence of bioactive metabolites (phenols, carbohydrates, flavonoids, and vitamins) with different biological activities has been documented in various macroalgae such as C. glomerata 26. Therefore, we tested TPC and TFC in both algal and GCR extracts. The standard calibration curves were prepared for each test (Fig. S1).
The results obtained and presented in Table 1 demonstrate higher values of TPC and TFC in the algae extract, with 19.35 mg of GAE/ g DW and 12.80 mg of QE/g DW, respectively. This observation is in line with previous results13. Regarding the garden cress extracts, a significant increase (p < 0.05) in TPC and TFC was observed in GCR (T2.5), when compared to control and other treatments, accounting for 23.14 mg of GAE/g DW and 15.07 mg of QE/g dry extract, respectively. The values for control plants were at the level of 14.34 mg of GAE/ g DW for TPC and 11.17 mg of QE/g DW for TFC. GCR plants treated with 10 mg/mL algae extract demonstrated also, an considerable content of phenolic and flavonoid compounds, namely 18.68 mg of GAE/g DW and 11.27 mg of QE/g dry extract, respectively. Whereas, the content for GCR (T5), was observed with values of 12.08 mg of GAE/g DW and 11.27 mg of QE/g DW, respectively. As a consequence, we can notice that the application of C. glomerata extract improves the biochemical composition of GCR plants, particularly the treatment T2.5, which provides the best results compared to other treatments. These findings are in agreement with Baroud et al.27, who reported that the brown algae extract could be regarded as an excellent bio-stimulant to improve the tomato biochemical composition 27.
Chlorophyll and total carotenoid contents
The algae C. glomerata has been reported as having a high supply of chlorophylls 15 which is per our results. The amount of chlorophyll a and b were determined in C. glomerata extract at levels of 559.55 and 2009.49 µg/g of dry weight, respectively. While the content of total carotenoids reached 385.30 µg/g of DW (Table 2).
In Fig. 3, we presented the result of the determination of chlorophyll a and b, as well as the content of total carotenoids, in differently-treated GCR plant samples. Compared to the control, the positive effect of algal extract on chlorophyll content was mainly observed through their increment in all GCR samples. The amount of chlorophyll a increased significantly (p < 0.05) from 620.70 μg/g of DW for GCR (T0) to 747.63, 782.99, and 702.52 μg/g of DW for GCR (T 2.5), (T5) and (T10), respectively. Similarly, considering chlorophyll b content, the highest value was obtained in GCR (T2.5) with 2087.11 µg/g of DW, compared to 2039.71 and 1736.20 μg/g of DW for GCR (T5) and (T10), respectively. Whereas, the lowest content was observed in GCR (T0) as 1614.59 μg/g of DW. A considerable increase was additionally obtained in the total carotenoid content with 675.66 and 774.56 μg/g of DW for GCR (T2.5) and (T5), respectively compared to GCR (T0) with 577.46 and 572.22 μg/g of DW for GCR(T10). The present findings are in line with a previous work of Dziergowska et al.28 that mentioned that C. glomerata extract increased the content of chlorophyll in soybean seedlings compared to the control group treated with distilled water 28.
Antioxidant activities: ABTS and DPPH
In general, algal extracts that are rich in natural polyphenolics can function as antioxidants. Yarnpakdee et al. 14, reported that quercetin, isoquercetin, and hydroquinone were found as major compounds in C. glomerata extracts, which were noted to be a high-extent antioxidant.
Therefore, to evaluate the effect antioxidant of this algae extract on GCR plants, ABTS and DPPH activities were determined. As per results presented in Table 3, a significant increase (p < 0.05) was observed and the highest ABTS and DPPH radical scavenging activities were found in GCR (T2.5) with 13.53 mg TE/g DW and 3.55 mg GAE/g DW, compared to control GCR (T0) with 10.44 mg TE/g DW and 2.28 mg GAE/g DW, respectively.
While the GCR plants (T5) and (T10) showed similar antioxidant capacity with values of 10.30 and 10.03 mg TE/g DW, they differed significantly in the content of phenolics (1.67 and 2.83 mg GAE/g DW, respectively).
The above-presented results are in agreement with our presented findings on the total phenolic and flavonoid compounds contents. We can observe the influence of C. glomerata extract in improving the biochemical composition and antioxidant effect of garden cress plants, particularly at a concentration of 2.5 mg/mL.
Qualitative HPLC–DAD analysis of phenolic compounds
The comprehensive profiling of phenolic compounds in the biomass of C. glomerata and GCR plant extracts was accomplished using the HPLC–DAD technique. The initial step was to adopt an HPLC method using 14 commercial standards of chosen phenolic compounds. The results are presented in Table 4. In the applied conditions, all studied analytes, except caffeic acid and 3-HBA (peak 6 + 7), were resolved (Fig. 4).
A chromatogram (λ = 270 nm) for phenolic compounds standards: 1: gallic acid; 2: 3,4-dihydroxybenzoic acid (3,4-DHBA); 3: 4-hydroxybenzoic acid (4-HBA); 4: chlorogenic acid; 5: vanillic acid; 6: caffeic acid; 7: 3-hydroxy-benzoic acid (3-HBA); 8: syringic acid; 9: ferulic acid; 10: rutin; 11: rosmarinic acid; 12: cinnamic acid; 13: quercetin; 14: chrysin.
For each phenolic profile, the identification of the compounds was pursued by merging the information of the DAD detector, and retention times together with the standard addition method 13,21. Quantitative analysis was impossible within the scope of the applied HPLC methodology due to the high interference from the matrix. The phenolic compounds were identified as presented in Table 5. The qualitative analysis revealed the presence of eleven phenolic compounds, either in algae extract or GCR extracts: gallic acid, 4-HBA (Fig. 5 a), 3,4-DHBA (Fig. 5 b), vanillic acid, caffeic acid (Fig. 5 c), 3-HBA, syringic acid (Fig. 5 d), ferulic acid, rutin, rosmarinic acid, and chrysin.
Examples of HPLC chromatograms. a: 4-HBA peaks in a chromatograms of algae extract  and algae extract spiked
and algae extract spiked  . b: 3,4-DHBA peaks in a chromatograms of GCR T2.5
. b: 3,4-DHBA peaks in a chromatograms of GCR T2.5  and GCR T0
and GCR T0  . c Caffeic acid peaks in a chromatograms of GCR T 2.5
. c Caffeic acid peaks in a chromatograms of GCR T 2.5  and GCR T0.
and GCR T0.  d Syringic acid peaks in a chromatograms of GCRT2.5
d Syringic acid peaks in a chromatograms of GCRT2.5  and GCRT2.5 spiked
and GCRT2.5 spiked  .
.
While chlorogenic acid (Fig. 6), cinnamic acid, and quercetin were not detected in all the tested samples (Table 5). Chrysin was exclusively absent in GCR (T0), confirming the subsequent validation of the beneficial impact of C. glomerata extracts on enhancing the phenolic composition of GCR-treated samples. Chrysin, a natural bioactive flavone compound, occurs in many plants, honey, and propolis with numerous biological potentials and pharmaceutical effects including anti-inflammatory, antiviral, and antioxidant. Due to their therapeutic properties, research has been undertaken on its production in the hairy roots of plants, obtained by genetic modification with the help of Agrobacterium rhizogenes29.
Previous studies of phenolic compounds in garden cress plants and C. glomerata extracts 13,30,31 described the detection of the same compounds as indicated by us in Table 5.
Chemometric analysis
PCA was applied to reduce the data's redundant information and group the correlated responses into principal components. The PCA plot (1st principal component vs 2nd principal component) as presented in Fig. 7, explains 95.23% of the total system variance. Each of the first and second principal components explains a variance of 65.35% (PC1) and 29.87% (PC2), respectively. In the present study, PCA was applied to the dataset of all analyses performed for GCR treated or not treated with algae extract during growth.
Eleven variables (TPC, TFC, chlorophyll a content, chlorophyll b content, total carotenoids content, ABTS activity, DPPH activity, fresh weight, dry weight, total height of GCR plant, and stem length) were selected and presented in the loading plot (Fig. 7a) and 4 observations (related to a control group and three different treatments with algal extracts) were shown in the score plot (Fig. 7b).
Notably, PC1 separates the GCR plant (T2.5) with the greatest growth and best biochemical characteristics from the other groups of tested plants, while PC2 distinguishes each treatment session and separates the lowest growth results of GCR plants (T5) with positive contribution (Fig. 7b).
Regarding the control (T0), it is positioned on the left side of the score plot, reflecting a negative relation to PC1, which is mainly correlated with the significant reduction in the TFC, and the lowest growth of the plant, as indicated by both the total height and the stem length. Furthermore, the GCR (T2.5) is highly and positively related to PC1. As depicted in Fig. 7a, it is characterized by the highest values of TPC and TFC, the best growth expressed via the fresh weight as well it contains the greatest antioxidant activities with ABTS and DPPH tests.
Conclusion
Due to their potential health benefits, C.glomerata is used in a wide range of applications, including food pigments, cosmetic components, and pharmaceuticals. However, despite its environmentally friendly nature and its potential use as a biofertilizer and biostimulant in sustainable agriculture practices, such use of these algae has not been pursued to date.
In this study, we present for the first time the effect of C.glomerata extract on the growth and biochemical composition of garden cress, chosen as a model plant. Several groups of bioactive compounds, such as phenolic compounds, flavonoids, and pigments, along with antioxidant properties were investigated.
The addition of algae extract had a considerable effect on the growth of the studied plants when compared to the control treatment. The results of this study indicated that when treated with the algae extract at a concentration of 2.5 mg/mL, the garden cress plant exhibited superior productivity and biochemical characteristics compared to all other treatments. Certainly, the utilization of macroalgae, specifically C. glomerata, has the potential to become a cost-efficient substitute for traditional farming in the future. This approach could offer plants a secure and resilient environment, thus fostering growth and productivity.
Data availability
Data supporting the findings of this study are available within the article and its supplementary material. Raw data of this study is available from the corresponding author, upon request.
Abbreviations
- ABTS:
-
2,2′-Azino-bis (3-ethyl benzo-thiazoline-6-sulfonic acid)
- DAD:
-
Diode array detector
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- DW:
-
Dry weight
- GCR:
-
Garden cress
- HPLC:
-
High-performance liquid chromatography
- QE:
-
Quercetin equivalent
- TE:
-
Trolox equivalent
References
Kumar, R., Kumar, R., & Prakash, O. Chapter 5: The impact of chemical fertilizers on our environment and ecosystem. Chief Ed, 35(69), 1173–1189 (2019).
Meena, R. S. et al. Impact of agrochemicals on soil microbiota and management: A review. Land 9. https://doi.org/10.3390/land9020034 (2020).
Sangha, J. S., Kelloway, S., Critchley, A. T. & Prithiviraj, B. Seaweeds (Macroalgae) and their extracts as contributors of plant productivity and quality. the current status of our understanding. in Advances in Botanical Research 71, 189–219 (Academic Press Inc., 2014).
Win, T. T., Barone, G. D., Secundo, F. & Fu, P. Algal biofertilizers and plant Growth stimulants for sustainable agriculture. Ind. Biotechnol. 14, 203–211. https://doi.org/10.1089/ind.2018.0010 (2018).
Sharma, H. S. S., Fleming, C., Selby, C., Rao, J. R. & Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 26, 465–490. https://doi.org/10.1007/s10811-013-0101-9 (2014).
Kapoore, R. V., Wood, E. E. & Llewellyn, C. A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 49. https://doi.org/10.1016/j.biotechadv.2021.107754 (2021).
Kumar, G. & Sahoo, D. Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. J. Appl. Phycol. 23, 251–255. https://doi.org/10.1007/s10811-011-9660-9 (2011).
Kuwada, K., Wamocho, L. S., Utamura, M., Matsushita, I. & Ishii, T. Effect of red and green algal extracts on hyphal growth of arbuscular mycorrhizal fungi, and on mycorrhizal development and growth of papaya and passionfruit. Agron. J. 98, 1340–1344 (2006).
Vernieri, P., Borghesi, E. & Ferrante, A. Application of biostimulants in floating system for improving rocket quality. www.world-food.net (2005).
Mancuso, S., Azzarello, E., Mugnai, S. & Briand, X. Marine bioactive substances (IPA Extract) improve foliar ion uptake and water stress tolerance in potted vitis vinifera plants. Adv. Hort. Sci 20 (2006).
Mohin, T. J. Environmental sustainability. in Changing Business from the Inside Out 85–96 (2018). https://doi.org/10.4324/9781351277884-6.
Borowitzka, M. A. Developments in Applied Phycology 8. http://www.springer.com/series/7591.
Korzeniowska, K., Łęska, B. & Wieczorek, P. P. Isolation and determination of phenolic compounds from freshwater Cladophora glomerata. Algal Res. 48 (2020).
Yarnpakdee, S., Benjakul, S. & Senphan, T. Antioxidant activity of the extracts from freshwater Macroalgae (Cladophora glomerata) grown in Northern Thailand and its preventive effect against lipid oxidation of refrigerated eastern little tuna slice. Turkish J. Fish. Aquat. Sci. 19 (2019).
Petchsomrit, A. et al. Extraction, antioxidant activity, and hydrogel formulation of marine Cladophora glomerata. Algal Res. 71, 103011. https://doi.org/10.1016/j.algal.2023.103011 (2023).
Prajapati, V. D., Maheriya, P. M., Jani, G. K., Patil, P. D. & Patel, B. N. Lepidium sativum Linn.: A current addition to the family of mucilage and its applications. Int. J. Biol. Macromol. 65, 72–80. https://doi.org/10.1016/j.ijbiomac.2014.01.008 (2014).
Sánchez-Rangel, J. C., Benavides, J., Heredia, J. B., Cisneros-Zevallos, L. & Jacobo-Velázquez, D. A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 5, 5990–5999 (2013).
Messyasz, B. et al. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 30, 591–603 (2018).
Osório, C. et al. Pigments content (Chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 7, 1–14 (2020).
Wellburn, A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144, 307–313 (1994).
Kebal, L. et al. HPLC-DAD profile of phenolic compounds and In vitro antioxidant activity of Ficus carica L. fruits from two Algerian varieties. Biomed. Pharmacother. 155, (2022).
Górnaś, P., Šne, E., Siger, A. & Segliņa, D. Sea buckthorn (Hippophae rhamnoides L.) leaves as valuable source of lipophilic antioxidants: The effect of harvest time, sex, drying and extraction methods. Ind. Crops Prod. 60, 1–7 (2014).
Al-Shareefi, M. J., Kadhim, Z. K. & Hakim, R. A. M. Effect of Algae extract and bio-fertilizer on vegetative growth and flowering of freesia hybrida L. J. Kerbala Agric. Sci. (2019).
Sivasankari, S., Venkatesalu, V., Anantharaj, M. & Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 97, 1745–1751 (2006).
Lewandowska, S. et al. Impact of freshwater Macroalga (Cladophora glomerata) extract on the yield and morphological responses of glycine max (L.) Merr. Agric. 12 (2022).
Shah, Z. et al. Investigation of important biochemical compounds from selected freshwater macroalgae and their role in agriculture. Chem. Biol. Technol. Agric. 9, (2022).
Baroud, S. et al. Effect of brown algae on germination, growth and biochemical composition of tomato leaves (Solanum lycopersicum). J. Saudi Soc. Agric. Sci. 20, 337–343 (2021).
Dziergowska, K. et al. Soybean germination response to algae extract and a static magnetic field treatment. Appl. Sci. 11. https://doi.org/10.3390/app11188597 (2021).
Gharari, Z., Bagheri, K., Danafar, H. & Sharafi, A. Enhanced flavonoid production in hairy root cultures of Scutellaria bornmuelleri by elicitor induced over-expression of MYB7 and FNSП2 genes. Plant Physiol. Biochem. 148, 35–44. https://doi.org/10.1016/j.plaphy.2020.01.002 (2020).
Rababah, T. M. et al. Antioxidant activities, total phenolics and HPLC analyses of the phenolic compounds of extracts from common Mediterranean plants. Nat. Prod. Res. 25, 596–605. https://doi.org/10.1080/14786419.2010.488232 (2011).
Oszmiański, J., Kolniak-Ostek, J. & Wojdyło, A. Application of ultra performance liquid chromatography-photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS) method for the characterization of phenolic compounds of Lepidium sativum L. sprouts. Eur. Food Res. Technol. 236, 699–706 (2013).
Acknowledgements
This project is financed in the framework of grant UMO-2021/41/B/NZ9/02584 entitled “Epiphytic diatoms growing on freshwater macroalgae as a source of plant available silicon” attributed by the National Science Centre.
Author information
Authors and Affiliations
Contributions
Conceptualization: IBH, BM, RP, BL, PPW; investigation: IBH, KP, LT; methodology: IBH, KP, LT; formal analysis: IBH, resources: BL, PPW, BM, RP; writing—original draft: IBH; writing—review and editing: IBH, KP, BL, BM, PPW, RP; visualization: IBH; project administration: BM, PPW; supervision: PPW, BL, BM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ben Hammouda, I., Pokajewicz, K., Pankiewicz, R. et al. Biostimulant effect of Cladophora glomerata extract on garden cress plant growth. Sci Rep 14, 26614 (2024). https://doi.org/10.1038/s41598-024-74180-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74180-3

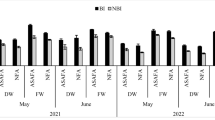









 ; algae extract
; algae extract  ;GCRT2.5
;GCRT2.5  and
and  GCRT0.
GCRT0.