Abstract
The study was to evaluate the clinical outcomes of azvudine versus nirmatrelvir-ritonavir against omicron strains of coronavirus disease 2019 infections and determine their comparative effectiveness. This retrospective study included 716 patients who received nirmatrelvir-ritonavir (NR group) or azvudine (FNC group) at Peking Union Medical College Hospital between 1 November 2022 and 27 February 2023. Patients in the FNC group (n = 304) were younger, exhibited less severe symptoms, started antiviral therapy later, received corticosteroids more frequently, and used tocilizumab less frequently than patients in the NR group (n = 412). Within 28 d of therapy, 40 (9.7%) and 20 (6.6%) deaths were in the NR and FNC groups, respectively. No differences were observed between drugs and mortality rates (odds ratio [OR] 0.78, 95% CI 0.40–1.5, P = 0.45), clinical improvement (OR 0.79, 95% CI 0.79–1.3, P = 0.38), and clinical progression (OR 1.0, 95% CI 0.58–1.8, P = 0.96). More patients in the NR group experienced platelet decline than those in the FNC group (17.6% vs. 8.9%, P = 0.034). This study indicated that the effectiveness and safety of azvudine were comparable to those of nirmatrelvir-ritonavir.
Similar content being viewed by others
Introduction
Since the emergence of the coronavirus disease 2019 (COVID-19) pandemic in early 2020, millions of lives have been claimed by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. In the initial stages of the pandemic, treatment decisions relied on armamentarium therapies, in vitro data, case reports, and case series; subsequently, higher-quality evidence became available, which led to the identification of more effective antiviral agents.
Antiviral agents are recommended for nonhospitalized COVID-19 patients with mild-to-moderate symptoms at high risk of clinical progression and hospitalized patients who do not require oxygen supplementation or conventional oxygen1. Nirmatrelvir-ritonavir, a combination of a protease inhibitor and potent enhancer, has effectively reduced hospitalization and death rates when administered to high-risk unvaccinated nonhospitalized patients within 5 days of symptom onset2. Meanwhile, azvudine, a broad-spectrum antiviral nucleoside, has received emergency use authorization to treat COVID-19 in adults3. Several single-arm trials have indicated the efficacy of nirmatrelvir-ritonavir and azvudine in treating patients with COVID-197,10. However, direct comparisons between these two antiviral drugs are lacking and controversial.
In this retrospective cohort study, we aimed to compare the effectiveness and safety of azvudine versus nirmatrelvir-ritonavir to enhance the existing knowledge and provide valuable insights into clinical decision-making regarding COVID-19 treatment.
Results
Baseline characteristics of treatment groups
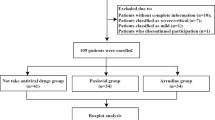
In total, 716 patients were included in the study. They were classified into the nirmatrelvir-ritonavir (NR; n = 412, 57.5%) and azvudine (FNC; n = 304, 42.5%) groups. The demographic information, comorbidities, baseline respiratory support modes, disease severity, and treatment details were listed in Table 1.
Patients in the FNC group were younger than those in the NR group (72 vs. 68 years, P = 0.043). No significant differences were observed between groups regarding sex ratio, comorbidities, or immunocompromised status. However, the NR group had a higher number of patients who were graded ≥ 3 on the seven-category ordinal scale (52.7% vs. 39.5%, P < 0.001) and who received advanced respiratory support (11.9% vs. 7.2%, P = 0.039).
The interval between disease onset and drug administration was significantly shorter in the NR group than in the FNC group (4 vs. 9 days, P < 0.001). The percentage of included patients receiving corticosteroids, tocilizumab, and baricitinib was 47.8%, 13.3%, and 5.6%, respectively. More patients in the FNC group were administered corticosteroids (47.7% vs. 58.2%, P = 0.006), whereas more patients in the NR group were administered baricitinib (7.3% vs. 3.3%, P = 0.022). The percentage of tocilizumab administration was not significantly different between the groups.
After propensity score matching, no significant differences were observed between the treatment groups regarding the baseline characteristics and concomitant therapies other than antiviral drugs (Table 1, Fig. 1). The kernel densities of the propensity scores before and after matching are presented in Supplementary Fig. S1.
Antiviral therapies and 28-d mortality
A total of 60 (8.4%) deaths were recorded, including 40 (9.7%) in the NR group and 20 (6.6%) in the FNC group (P = 0.14) within 28 d after antiviral initiation. The median duration from the initiation of drug administration to death was 15 (6–20) d and 14 (7–20) d in the NR and FNC groups (P = 0.31), respectively. The univariate logistic regression analysis revealed no significant association between different antiviral therapies and 28-d mortality risks (odds ratio [OR] 0.65, 95% confidence interval [CI] 0.37–1.1, P = 0.14). The association remained non-significant in the age- and sex-adjusted model (OR 0.74, 95% CI 0.41–1.3, P = 0.31) and multivariate model (OR 0.78, 95% CI 0.40–1.5, P = 0.45) adjusted for continuous variables, including age and interval between symptom onset and antiviral initiation, and categorical variables, including sex, diabetes, cardiovascular disease, chronic kidney disease, baseline respiratory support modes, and therapy with corticosteroid, tocilizumab, and baricitinib. The mortality rate of the 438 patients included after matching was 7.8% and 8.2% in the FNC and NR groups, respectively (P = 0.86). The difference in 28-day mortality risk remained non-significant between the groups (OR 1.01, 95% CI 0.44–2.3, P = 0.98) (Table 2).
Antiviral therapies and clinical outcomes
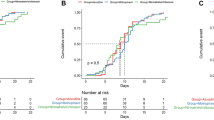
Changes in disease severity from baseline to the end of follow-up according to the seven-category ordinal scale are shown in a Sankey plot (Fig. 2). Among 337 patients with baseline categories ranging from 3 to 6, 223 (66.2%) exhibited clinical improvement at the end of follow-up, including 146/217 (67.3%) patients in the NR group and 77/120 (64.2%) patients in the FNC group (P = 0.45). Correspondingly, clinical progression was observed in 46 (11.2%) patients in the NR group and 28 (9.2%) patients in the FNC group (P = 0.40). No significant associations were observed between the antiviral therapy and clinical improvement or progression in the multivariate logistic regression model in all or the matched patients (Table 2).
Safety evaluation of antiviral drugs
Twenty patients (6.6%) discontinued azvudine early because of side effects, whereas 4 (0.97%) discontinued nirmatrelvir-ritonavir for similar complaints (P < 0.001). The most commonly reported side effect was abnormal liver function (9 cases, 37.5%), followed by diarrhea (4 cases, 16.6%), dizziness or nausea (3 cases, 12.5%), decreased white blood cell and/or platelet counts (3 cases, 12.5%), increased blood pressure (2 cases, 8.3%), gastrointestinal bleeding (1 case, 4.2%), visual hallucination (1 case, 4.2%, and allergic reactions (1 case, 4.2%). The laboratory tests were reviewed 14 days after the initiation of antiviral therapy; no significant differences were observed between the two groups regarding the percentages of patients with elevated serum alanine aminotransferase and creatinine levels or decreased white blood cell counts. However, more patients in the NR group exhibited decreased platelet levels within 14 days of drug initiation (17.1% vs. 8.9%, P = 0.047). Details of the abnormal laboratory test results are shown in Fig. 3.
Discussion
In this retrospective analysis, we examined the clinical presentation of 716 patients who received either nirmatrelvir-ritonavir or azvudine during the recent wave of SARS-CoV-2 infections caused by omicron subvariants. Both treatments showed similar 28-day mortality risks and clinical outcomes (disease improvement or progression, defined as changes in two categories on the seven-category ordinal scale). A higher proportion of patients on azvudine opted for early termination of therapy due to side effects than those on nirmatrelvir-ritonavir. However, no differences were observed in the proportions of patients exhibiting abnormal serum alanine aminotransferase, creatinine, and leukocyte levels between the two groups. Nevertheless, more patients receiving nirmatrelvir-ritonavir exhibited decreased platelet counts within 14 d after antiviral therapy initiation.
Nirmatrelvir-ritonavir comprises a protease inhibitor, nirmatrelvir, and a pharmacological enhancer, ritonavir. Nirmatrelvir, which targets the major SARS-CoV-2 proteases, has demonstrated good antiviral efficacy in vitro5. Ritonavir is a potent inhibitor of cytochrome P450 3A4 (CYP3A4), which is responsible for nirmatrelvir metabolism. The combination of ritonavir and nirmatrelvir allows for higher peak levels and longer half-lives than sole nirmatrelvir administration1. Various clinical trials have demonstrated the efficacy of nirmatrelvir-ritonavir in shortening symptom duration, promoting nucleic acid conversion, and reducing mortality rates6,10,14. However, an increased likelihood of drug interactions associated with the inhibited metabolism of other drugs by CYP3A4 is a key factor that affects its clinical use.
Limited information is available on the clinical effectiveness of azvudine against SARS-CoV-2. As a nucleoside analogue, azvudine can be converted intracellularly to inhibit viral RNA polymerase as a prodrug, thereby preventing viral replication8. A randomized open-label controlled clinical trial (n = 20) including patients with mild or common COVID-19 revealed that azvudine could shorten the interval between disease onset and the first time of nucleic acid conversion (2.6 ± 0.97 days), compared with standard support treatment (5.6 ± 3.06 days)4. Another randomized single-arm controlled clinical trial involving patients with severe (n = 5) and common (n = 26) types of COVID-19 showed that treatment with azvudine led to a 100% negative conversion of nucleic acids within 4 days of treatment, whereas only 30% of patients in the control group achieved this outcome. Furthermore, all patients in the azvudine group were discharged after 9 ± 4.93 days9.
Direct comparisons between nirmatrelvir-ritonavir and azvudine have been restricted to retrospective studies. A retrospective analysis in Beijing YouAn Hospital reviewed the clinical information of 245 hospitalized patients. The cycle threshold (Ct) values for azvudine effectiveness against SARS-CoV-2 were lower than those for nirmatrelvir-ritonavir after 3 and 5 days of antiviral therapies. Patients who received nirmatrelvir-ritonavir tended to experience a shorter time to the negative conversion of the first nucleic acids than those who received azvudine. However, the clinical outcomes of the two antiviral drugs were not compared10. In a previous study on SARS-CoV-2, the nucleic acid positivity did not exactly coincide with that of the live virus culture. Inactive viral debris lacking pathogenicity may lead to positivity in nucleic acid11. Therefore, the correlation between Ct values and the time to negative conversion of coronavirus nucleic acids or the clinical prognosis of patients requires further exploration. A recent retrospective cohort study of hospitalized patients with COVID-19 who did not need oxygen supply at admission showed that 281 patients treated with azvudine had lower risks of composite outcomes for disease progression and all-cause mortality than the 281 patients treated with nirmatrelvir-ritonavir12.
Our study extended to patients who were not admitted and included patients with different intensities of oxygen support. Patients who received nirmatrelvir-ritonavir were older and tended to have more severe disease symptoms. Our results partially reflect clinician preferences and a lack of clinical data on azvudine use in patients with COVID-19. Nevertheless, no significant differences were observed between the two antiviral drugs regarding the 28-day mortality risks and clinical improvement or progression based on changes in the seven-category ordinal scale, even after propensity score matching. Thus, we hypothesized that azvudine was not associated with a higher risk of poor clinical outcomes than nirmatrelvir-ritonavir. Therefore, azvudine might be an effective antiviral drug against COVID-19, specifically for patients concerned with the side effects of nirmatrelvir-ritonavir in complex combined therapies for comorbidities.
In a previous single-arm study, 16.12% of patients treated with azvudine experienced transient dizziness and nausea early in the treatment9. While no adverse side effects were reported in a study conducted by Ren et al.4, 20 patients treated with azvudine in this study reported side effects. The most common adverse effects were abnormal liver function, dizziness, and nausea. However, no differences were observed between azvudine and nirmatrelvir-ritonavir in the occurrence of abnormal serum alanine aminotransferase, creatinine, and white blood cell levels within 14 days of treatment, although more patients in the nirmatrelvir-ritonavir group exhibited decreased platelet levels. In conclusion, azvudine is a comparatively safe antiviral drug for COVID-19 but may require surveillance of liver transaminase levels.
The EPIC-HR trial (n = 2,246) showed that nirmatrelvir-ritonavir was associated with a significantly lower viral load after 5 days of treatment than the placebo, and it prevented symptomatic COVID-19 patients from developing severe disease. The trial also reported a reduced risk of hospitalization or death by day 28 when nirmatrelvir-ritonavir was initiated within 5 d of symptom onset, compared with a placebo2. In this study, a higher percentage of patients treated with nirmatrelvir-ritonavir after 5 days of disease onset died by day 28 compared to those treated with nirmatrelvir-ritonavir within the first 5 days (17.3% vs. 4.2%, P < 0.001); however, a similar trend was not observed in the FNC group (P = 0.27). These findings partially support the importance of administering nirmatrelvir-ritonavir within 5 days of disease onset. However, further cohort studies and randomized controlled trials are required to determine a reasonable timeframe for azvudine administration.
The inherent limitations of retrospective studies make it difficult to acquire and extrapolate definitive conclusions. First, data on respiratory symptoms and pulmonary physical examination were not fully collected, but we used respiratory support to reflect the severity of the disease. Second, patient heterogeneity may influence the prognostic prediction. Therefore, we performed propensity score matching to reduce the imbalance between groups, and the results remained comparable. Third, 15 patients who needed invasive mechanical ventilation were included, 14 of whom received nirmatrelvir-ritonavir, which might have contributed to a bias in mortality rates. Only one patient with intubation was included in the analysis after propensity score matching. Therefore, our results do not reflect antiviral effectiveness in patients with severe COVID-19. Accordingly, the effectiveness of antiviral therapies in these patients requires further exploration.
Conclusion
In this study, similar clinical outcomes were observed for azvudine and nirmatrelvir-ritonavir treatment. Current conclusions regarding the antiviral effects of nirmatrelvir-ritonavir and azvudine remain inconclusive. Nevertheless, our study provides a reference and research foundation for future high-quality studies.
Methods
Study participants
This retrospective study reviewed clinical data from electronic medical records of adult Chinese patients diagnosed with COVID-19 at Peking Union Medical College Hospital between 1 November 2022 and 27 February 2023. We specifically included adult patients infected with SARS-CoV-2 Omicron subvariants, confirmed by nucleic acid or antigen tests, who had received either nirmatrelvir-ritonavir or azvudine. The exclusion criteria were patients who received more than one type of antiviral therapy against COVID-19, when the initiation of antiviral therapy could not be confirmed, and when follow-up clinical outcomes were missing (Fig. 1). This study was approved by the Ethics Committee of Peking Union Medical College Hospital (approval number: I-23PJ946). The requirement for informed consent was waived owing to the study’s retrospective nature. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Supplementary Table 1)13. All procedures followed the Declaration of Helsinki and its amendments.
Data collection, definitions, and outcomes
In the retrospective data collection, we gathered birthdate, sex, comorbidities, symptom onset date, and antiviral therapy details (type, dose, initiation time, and therapy course). The index date was the date each patient started taking azvudine or nirmatrelvir-ritonavir. All patients were followed-up until 28 days after antiviral initiation. Information on respiratory support modes, laboratory tests (serum alanine aminotransferases, creatinine, white blood cell, and platelet levels), therapeutic strategies other than antiviral drugs (corticosteroids, tocilizumab, and baricitinib), time of admission, discharge or death, and reported side effects were obtained at baseline (defined as the time of antiviral drug initiation) and during follow-up. Immunocompromised status was defined according to the consensus of Julio et al.14. Respiratory support modes were classified into no respiratory support, conventional oxygen therapy (patients who needed nasal cannula, Venturi masks or < 10 L/min using reservoir masks), and advanced respiratory support (patients who required respiratory support at > 10 L/min using reservoir masks, high-flow nasal cannula, noninvasive mechanical ventilation, invasive mechanical ventilation or extracorporeal membrane oxygenation). Disease severity was evaluated using a seven-category ordinal scale15. The standard therapy was 100 mg ritonavir and 150 mg nirmatrelvir twice daily for 5 days or 5 mg azvudine once daily for 7 days16.
The primary outcome was 28-day mortality risk, whereas the secondary outcome was a clinical improvement or progression possibility within 28 days from drug initiation. Clinical improvement or progression was defined as a decline or an increase (or death) in two categories on the seven-category ordinal scale, respectively. The two treatment groups were also compared for the reported adverse effects and abnormal laboratory results. Adverse events were evaluated using the Common Terminology Criteria for Adverse Events (version 5.0)17.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation or median (interquartile range) and compared using the t- or Wilcoxon rank-sum test. Categorical variables are presented as numbers (percentages) and compared using the chi-square test. A logistic regression model assessed the association between antiviral drug administration and primary or secondary outcomes. In addition to age and sex, factors significantly related to outcomes in the univariate regression model were included for multivariate adjustment. Considering the imbalance between groups, propensity score matching was applied regarding age, sex, comorbidities, baseline disease severity (on the seven-category ordinal scale), the interval between symptom onset and antiviral initiation, and corticosteroid, tocilizumab, and baricitinib therapy for 1:1 matching of patients who were administered different antiviral therapies (Fig. 1)18. Associations between antiviral therapies and primary or secondary outcomes in patients were re-validated after matching. For safety evaluation, we compared the number of patients in the two groups reporting side effects and percentages of patients who exhibited elevation of serum alanine aminotransferase and creatinine and decline of white blood cell or platelet counts within 14 days of antiviral therapy. All statistical analyses were performed using Stata software (version 16.0; StataCorp, College Station, TX, USA).
Data availability
The data underlying this article are available within the article and its online supplementary material.
References
National Institutes of Health (USA). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines, <https://www.covid19treatmentguidelines.nih.gov/> (2021).
Hammond, J. et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 386, 1397–1408. https://doi.org/10.1056/NEJMoa2118542 (2022).
National Medical Products Administration. Domestically developed drug joins virus battle, <http://english.nmpa.gov.cn/2022-08/15/c_797867.htm> (2022).
Ren, Z. et al. A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study. Adv. Sci. (Weinh) 7, e2001435, https://doi.org/10.1002/advs.202001435 (2020).
Owen, D. R. et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 374, 1586–1593. https://doi.org/10.1126/science.abl4784 (2021).
Pesko, B. et al. Safety and Tolerability of Paxlovid (Nirmatrelvir/Ritonavir) in High-risk Patients. Clinical Infectious Diseases 75, 2049–2050. https://doi.org/10.1093/cid/ciac588 (2022).
Najjar-Debbiny, R. et al. Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients. Clin. Infect. Dis.https://doi.org/10.1093/cid/ciac443 (2022).
Yu, B. & Chang, J. Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct. Target Ther. 5, 236. https://doi.org/10.1038/s41392-020-00351-z (2020).
Zhang, J. L. et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct. Target. Ther. 6, 414. https://doi.org/10.1038/s41392-021-00835-6 (2021).
Gao, Y. et al. Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19. J. Infect. 86, e158–e160. https://doi.org/10.1016/j.jinf.2023.03.023 (2023).
Chu, V. T. et al. Comparison of Home Antigen Testing With RT-PCR and Viral Culture During the Course of SARS-CoV-2 Infection. JAMA Intern. Med. 182, 701–709. https://doi.org/10.1001/jamainternmed.2022.1827 (2022).
Deng, G. et al. Real-world effectiveness of Azvudine versus nirmatrelvir–ritonavir in hospitalized patients with COVID-19: A retrospective cohort study. J. Med. Virol. 95, https://doi.org/10.1002/jmv.28756 (2023).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–1457. https://doi.org/10.1016/s0140-6736(07)61602-x (2007).
Ramirez, J. A. et al. Treatment of Community-Acquired Pneumonia in Immunocompromised Adults: A Consensus Statement Regarding Initial Strategies. Chest 158, 1896–1911. https://doi.org/10.1016/j.chest.2020.05.598 (2020).
Wang, Y. et al. Comparative Outcomes of Adults Hospitalized With Seasonal Influenza A or B Virus Infection: Application of the 7-Category Ordinal Scale. Open Forum Infect. Dis. 6, ofz053, https://doi.org/10.1093/ofid/ofz053 (2019).
General Office of the National Health Commission. Diagnosis and treatment protocol for COVID-19 in China (trial version 10). <https://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm> (
US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 [Internet], 2017., <https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf > (
Zhang, Z., Kim, H. J., Lonjon, G. & Zhu, Y. Balance diagnostics after propensity score matching. Ann. Transl. Med. 7, 16. https://doi.org/10.21037/atm.2018.12.10 (2019).
Acknowledgements
We thank all the hospital staff members for their efforts in COVID-19 treatment and data collecting that used in this study; thank the patients who participated in this study.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2023YFC3041900) and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant number 2021-I2M-1-048). The sponsor had no role in the study design or execution, analyses or interpretation of data, or decision to submit.
Author information
Authors and Affiliations
Contributions
M.W. and X.T. engaged in conceptualization. H.X. engaged in methodology, formal analysis and writing - original draft. Y.W. engaged in methodology and formal analysis. All authors contributed to writing - review & editing. All authors read and approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Peking Union Medical College Hospital (approval number: I-23PJ946). The requirement for informed consent was waived owing to the study’s retrospective nature.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, H., Wang, Y., Xu, Y. et al. Effectiveness and safety of azvudine versus nirmatrelvir-ritonavir in adult patients infected with COVID-19 omicron strains: a retrospective study in Beijing. Sci Rep 14, 23974 (2024). https://doi.org/10.1038/s41598-024-74502-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74502-5
Keywords
This article is cited by
-
Impact of early and delayed azvudine administration on COVID-19 mortality: a retrospective study
Scientific Reports (2025)
-
Azvudine efficacy in reducing mortality in COVID-19 patients
European Journal of Medical Research (2024)