Abstract
To explore the influence of surfactant concentration on the pore structure and permeability of coal samples during the chemical enhancement of coalbed methane production, different kinds and different concentrations of surfactants were added to the chemical solution, and the coal samples were soaked. Methods such as low-field nuclear magnetic resonance testing (NMR), fractal theory, permeability testing, surface tension testing, and contact angle testing were employed to analyze the variation patterns of coal sample pore structure, fractal characteristics, and permeability, and to explore the correlation between surface tension, contact angle, and the degree of pore structure development. The results show that the increase in total porosity of coal samples, the increase in the seepage pore porosity, the decrease in Dt, and the growth rate of permeability increase with the increase in surfactant concentration, and are negatively correlated with the surface tension of the solution and the contact angle of the coal-solution interface, while the decrease in Ds is not significantly correlated with surfactant concentration, surface tension, or contact angle. In terms of the erosion effect of a chemical solution on coal samples, the influence of contact angle is greater than that of surface tension, while surface tension has the greatest impact on the development of adsorption pores. By adding different surfactants, the surface tension of the chemical solution and the contact angle of the coal-solution interface can be controlled, further promoting the erosion of coal samples, which is of positive significance for the chemical enhancement of coalbed methane production.
Similar content being viewed by others
Introduction
Coalbed methane (CBM) has immense development potential due to its abundant reserves, and it can help mitigate the global shortage of oil and gas resources1,2. As a clean energy source, the development of CBM helps reduce greenhouse gas emissions, contributing to the goal of achieving carbon neutrality3,4. Additionally, the complex occurrence conditions of CBM can easily lead to gas disasters, but its efficient extraction contributes to the safe production of coal mines5,6. Therefore, technologies for the safe and efficient extraction of CBM have garnered widespread attention7,8.
CBM primarily exists in an adsorbed state within the pore structures of coal. However, coal seams generally exhibit dense structures, poorly developed pore systems, and low permeability, which limit the efficiency of CBM extraction9,10. Enhancing the development of the coal’s pore structure and increasing its permeability have long been considered effective methods for improving CBM recovery11,12. In recent years, chemical methods aimed at dissolving soluble substances in coal to improve its pore structure have become a research focus13.
Organic solvents have shown excellent effectiveness in promoting the development of pores and fractures14. The erosion caused by acetone can significantly increase the porosity of coal samples, with an increase in the number of mesopores and macropores and a decrease in micropores15. Ethanol and glycol ether can enlarge the pore size of coal samples, increase the number of transitional and mesopores, and make the pore structure more open. Additionally, glycol ether can continuously reduce the bound water saturation of coal samples16. The combination of organic solvents and acids can further promote the development of pores in coal samples. Studies have shown17,18 that after acid treatment and extraction with tetrahydrofuran (THF), the pore volume of coal samples in all pore size ranges significantly increases, which is more conducive to improving CBM production.
Acid solutions have long been considered effective in improving the pore structure and increasing the permeability of coal by dissolving its mineral components19,20. Acidification of coal samples can increase their oxygen-containing functional groups, reduce aliphatic content, decrease mineral content, and these microscopic changes will affect the pore structure of the coal21,22. Researches indicate23,24 that acidification does not significantly change the pore shape, but it markedly affects the distribution of mesopores and macropores, increasing the pore volume. Acidification can also increase the surface roughness of coal pores, simplify the pore structure, and bituminous coal has higher acid sensitivity compared to anthracite. Different acids have varying effects on coal erosion: HF primarily promotes the transformation of macropores, while HNO3mainly enhances mesopore development25. Acetic acid effectively dissolves granular minerals on the coal surface, making it smoother, creating dissolution pores and microcracks, thus improving coal permeability26. Moreover, mixed acid solutions show better results in improving the pore structure of coal, expanding pores and fractures, enhancing pore connectivity, and reducing the tortuosity of the pore structure27. Xing et al28. pointed out that multicomponent acids can reduce the moisture and ash content of lignite, increase the volatile matter and fixed carbon content, reduce the proportion of micropores, increase the proportion of macropores, and improve pore connectivity. They also found that both the fractal dimension of pore surface and pore structure decreased. Additionally, citric acid can serve as an auxiliary acid to enhance coal erosion. Because coal contains various soluble substances, the effects of organic solvents and acids on coal erosion may differ. Zheng et al29. found that after THF treatment, the pore types of coal samples did not change significantly, but the number of pores increased. After HCl treatment, the number of open pores increased, and pore connectivity improved. Compared to THF, HCl effectively reduced the ash content of the coal samples. The pore surface fractal dimension decreased after HCl treatment, while it increased after THF treatment.
Researchers have also adjusted the physical properties of solutions by adding surfactants to enhance the erosion effect of chemical solutions on coal samples30. The amphiphilic structure of surfactant molecules can significantly reduce the surface tension of the solution. The interaction between surfactants and the functional groups on the coal surface can decrease the contact angle between the solution and the coal31,32,33. These changes in the solution enhance its permeability and wetting ability, which may positively influence the chemical erosion of coal samples. Xie et al34. found that the synergistic effect of surfactants reduced the ash content in coal, increased the volatile matter, fixed carbon content, and lower calorific value. The addition of surfactants also increased the hydrogen and hydroxyl group content in coal, reduced the length and branching of aliphatic chains, and significantly increased the average diameter and stacking height of aromatic layers. The synergistic acidification with surfactants can reduce both the total pore fractal dimension and the percolation pore fractal dimension of coal samples, further enhancing the connectivity of coal pores35. Compared to acidification alone, the addition of surfactants reduced the negative impact of acidification on the wettability of the coal surface, resulting in a greater increase in porosity, a larger decrease in the T2cutoff value, and more free fluid space in the coal samples36.
Current research has confirmed that organic solvents and acids can promote the development of pores in coal samples, and multicomponent solutions have a better erosion effect on coal samples compared to single-component solutions. The addition of surfactants can enhance this erosion effect. Additionally, the combination of anionic and nonionic surfactants can further reduce the surface tension of the solution and the contact angle between the coal and solution37,38 However, it has not yet been proven whether the enhancement of pore development in coal samples by adding surfactants is achieved by altering the surface tension and contact angle of the solution. The correlation between surface tension, contact angle, and the degree of pore structure development under chemical erosion remains unclear. This study selects organic solvents, organic acids, anionic surfactants, and nonionic surfactants to prepare multicomponent composite solutions. Surface tension tests and contact angle experiments are used to determine the optimal blending ratio of the two surfactants. Multicomponent composite solutions with different surfactant concentrations are prepared using the optimal blending ratio, and modification experiments are conducted on coal samples. Low-field nuclear magnetic resonance (NMR), permeability tests, and fractal theory are employed to characterize the pore structure and permeability of the coal samples. The correlation between surface tension, contact angle, and pore structure parameters is analyzed to clarify the influence of surfactant concentration on chemical erosion. The research results can further refine the theoretical basis for the role of surfactants in promoting chemical erosion in coal and provide guidance for the chemical enhancement of coalbed methane production.
Materials and methods
Materials
The coal samples used in this study were collected from Ping’an Mine in the Fuxin Basin, China, which is one of the main producing areas of low metamorphic degree bituminous coal in China. Current research results indicate that low metamorphic degree coal has a high content of small molecular organic compounds. Some small molecular organic compounds are soluble in organic solvents, and low metamorphic degree coal exhibits higher acid sensitivity24,39. Therefore, this study chose low metamorphic degree bituminous coal as the research object. Large coal blocks were collected and transported to the laboratory, where they were processed into two standard coal samples with diameters of 25 mm and heights of 50 mm, and diameters of 50 mm and heights of 100 mm using drilling and cutting methods. Coal samples with obvious damage were removed, and the remaining coal samples were subjected to ultrasonic wave velocity testing, with coal samples showing significant velocity dispersion being removed. All samples were sealed and stored and were respectively used for nuclear magnetic resonance (NMR) testing and permeability testing. The proximate analysis and ultimate analysis of the coal samples are listed in Table 1. The organic solvents used in this study were 99% diethylene glycol butyl ether (C8H18O3), organic acids were 99.5% citric acid (C6H8O7·H2O), anionic surfactants were 90% sodium dodecylbenzene sulfonate (SDBS), and non-ionic surfactants were 99% fatty alcohol polyoxyethylene ether (AEO-9).
Chemical modification process
The preparation of the solution consists of two steps: First, fixed components of 5% diethylene glycol butyl ether and 5% citric acid by mass fraction are used, with a total mass fraction of 0.2% for the surfactants (SDBS + AEO-9). Different proportions of surfactant components (SDBS/AEO-9: 0/5, 1/4, 2/3, 3/2, 4/1, 5/0) were prepared with deionized water as the balance, and the optimal surfactant combination ratio was determined through surface tension and contact angle experiments. Then, according to the optimal combination ratio, multi-component composite solutions with different total mass fractions of surfactants were prepared, with diethylene glycol butyl ether (5 wt%) and citric acid (5 wt%) as fixed components. The surface tension and contact angle of each solution were tested again. The solution numbers and the mass fractions of each component are listed in Table 2.
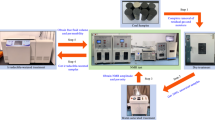
The coal samples with a diameter of 25 mm and a height of 50 mm were saturated with water for NMR testing to remove samples with large differences in porosity. Coal samples with a diameter of 50 mm and a height of 100 mm were dried to constant weight at 60 °C in a drying oven for permeability testing. After testing, the coal samples were placed in six sets of experimental solutions and soaked at 40 °C in a water bath for 72 h. NMR testing and permeability testing were conducted again following the above steps. The experimental process is illustrated in Fig. 1.
Contact angle and surface tension test
Additionally, a quantity of coal powder with a particle size of 200 mesh to 300 mesh was prepared. Circular specimens with a diameter of 20 mm and a thickness of approximately 4 mm were obtained using a tablet press under a pressure of 30 MPa. The contact angle between the solution and the coal sample surface was measured using the OCA Pro15 contact angle measurement instrument produced by DataPhysics Instruments GmbH, Germany. The surface tension of the solution was measured using the DCAT 21 surface tension meter produced by DataPhysics Instruments GmbH, Germany, employing the Wilhelmy plate method.
Low-field nuclear magnetic resonance (NMR) test
The pore structure parameters of the coal samples before and after solution erosion were determined using low-field nuclear magnetic resonance (NMR) technology. First, the T2 relaxation time of the coal samples in the saturated state was measured. Then, the coal samples were subjected to solution immersion treatment, and the T2 relaxation time of the coal samples after immersion treatment was measured to obtain the T2 curves, cumulative porosity curves, and NMR porosity of the coal samples before and after solution erosion, quantitatively analyzing the changes in the pore structure of the coal samples. The equipment used was the MesoMR23-060 H-I nuclear magnetic resonance imaging analyzer produced by Niumag Analytical Instrument Corporation, Suzhou, China.
Fractal dimension theory based on NMR
According to the fractal theory and nuclear magnetic resonance testing theory, when the pore structure of coal samples exhibits fractal characteristics, there exists the following relationship between the ratio of accumulated pore volume with radii smaller than r to the total pore volume (SV), the transverse relaxation time (T2) measured by nuclear magnetic resonance, and the fractal dimension (D) of the pore structure of coal samples35,40:
Where T2,max is the transverse relaxation time corresponding to the maximum pore size.
Taking the logarithm of both sides of Eq. (1), we have:
Equation (2) is the fractal geometric equation for the coal sample’s NMR T2 curve. At this point, the fractal dimension of the coal sample based on NMR can be represented as:
From Eqs. (2) and (3), it can be observed that by linearly fitting the double logarithmic curve of SV and T2 obtained from NMR, the fractal dimension of the coal sample based on the NMR T2 spectrum can be obtained through the slope of the fitted line.
Permeability test
The permeability of coal samples before and after solution erosion was tested using a coal-rock triaxial permeability testing system with nitrogen as the testing gas. To avoid damaging the coal samples during the application and release of confining pressure and to minimize the influence of non-chemical erosion on permeability, a lower confining pressure (2 MPa) was selected for permeability testing, reducing the adverse effects of these factors on the experimental results. Permeability tests were conducted at room temperature under the same confining pressure (2 MPa) but with varying pore pressures (0.2 ~ 1.0 MPa).
Results and analysis
Surface tension and contact angle
The surface tension and contact angle of multi-component composite solutions containing different proportions of SDBS and AEO-9 were measured. As shown in Fig. 2, under the condition of constant total concentration of surfactants, different proportions of SDBS and AEO-9 exhibited different surface properties. When only one surfactant was present, compared to AEO-9 (0/5), SDBS (5/0) reduced the surface tension of the solution. When both surfactants were mixed, the surface tension of the solution decreased with the increasing proportion of SDBS, indicating that SDBS had a more pronounced advantage in reducing the surface tension of the solution. Interestingly, when SDBS/AEO-9 was 4/1, the surface tension of the solution reached its minimum, which was 1.13% lower than SDBS (5/0) and 6.08% lower than AEO-9. This is because the presence of mixed micelles of surfactants in the solution weakened the electrostatic repulsion between anionic surfactants, and AEO-9 contained ether bonds that could form hydrogen bonds with water38, thereby exhibiting a synergistic effect between the two. This indicates that mixing a small amount of nonionic surfactant in anionic surfactants helps to adjust the surface properties of the solution and further reduces the surface tension of the solution. This synergistic effect is still reflected in the contact angle between the solution and coal. Although the trend of the contact angle varies with the different proportions of the two surfactants, it is still evident that SDBS wets the coal more than AEO-9. When SDBS/AEO-9 was 4/1, the contact angle between the solution and coal reached its minimum, which was 0.27% lower than SDBS (5/0) and 7.43% lower than AEO-9, indicating that the combination of the two improved the wetting of the solution to coal. The combination of anionic and nonionic surfactants further reduced the surface tension and contact angle, which may have a positive effect on the chemical solution erosion of coal samples. Therefore, in the subsequent work, we chose 4/1 (SDBS/AEO-9) as the combination ratio for the follow-up experiments.
Figure 3 shows the contact angle images between multi-component composite solutions containing different concentrations (0.05 wt% ~ 0.5 wt%) of surfactants (SDBS/AEO-9: 4/1) and coal, while Fig. 4 depicts the changes in surface tension and contact angle with the concentration of surfactants in the composite solution. Both surface tension and contact angle decrease with the concentration of surfactants, reaching the critical micelle concentration (CMC) at a concentration of 0.2 wt%, where the surface tension begins to stabilize, with a value of 31.57 mN/m. Preliminary experiments showed that the surface tension of pure water was 72.80 mN/m, while the surface tension of the solution without added surfactants was 40.99 mN/m. The addition of organic solvent and citric acid had already significantly reduced the surface tension of the solution, and the addition of surfactants further enhanced this reduction. Compared to the solution without added surfactants, the surface tension of the composite solution reaching CMC decreased by 22.98%, and this value remained relatively stable as the concentration continued to increase. The change in contact angle followed a similar pattern: before reaching CMC, the contact angle decreased rapidly with concentration, and after reaching CMC, the rate of decrease slowed significantly. The minimum value of the contact angle was 28.3°, which was 43.96% lower than the solution without added surfactants according to the preliminary test results.
NMR test
Prior to solution treatment, each coal sample was saturated with water and tested for its T2 spectrum. To minimize the impact of differences between coal samples on the experimental results, coal samples with significantly different porosity were excluded. The selected coal samples had porosity ranging from 8.3599 to 11.3868%, with an average porosity of 9.7394%. The porosity of each coal sample is listed in Table 3. Figure 5 shows the T2 spectra of coal samples before and after treatment with various solutions. The coal samples all exhibited a typical three-peak distribution, representing micropores, mesopores, and macropores, respectively. It can be observed that micropores and mesopores accounted for the vast majority of the T2 spectrum of the coal samples, while the proportion of macropores was very small. Additionally, the correlation between the peaks representing mesopores and macropores was generally low, reflecting poor connectivity between mesopores and macropores, which are unfavorable factors for coalbed methane permeability. After treatment with the solutions, the T2 spectra of the coal samples exhibited varying degrees of expansion, and the increase in T2 spectrum area showed an increasing trend with the increase in surfactant concentration. Of particular note is the significant expansion of the second and third peaks of the coal samples after solution erosion, especially the notable enhancement of the third peak of the coal samples treated with solution 5#. This indicates that the erosion of the solution has led to the conversion of some micropores and mesopores into mesopores and macropores, which will contribute to the improvement of coal sample permeability.
Figure 6 shows the cumulative porosity curve of the coal samples. The porosity of the coal samples increased to varying degrees after solution erosion. The increments in porosity after erosion by solutions 1# to 6# were 2.0281%, 2.3275%, 2.5927%, 2.6366%, 3.0576%, and 3.0717%, respectively. Compared to the original coal samples, these represented increases of 24.26%, 26.40%, 27.09%, 25.97%, 30.13%, and 26.98%, respectively. The increment in porosity was positively correlated with the concentration of surfactants in the solution. According to the occurrence status of coalbed methane in pores of different scales, pores are divided into adsorption pores and seepage pores41. Adsorption pores are composed of micropores, while seepage pores are composed of mesopores and macropores. The variations in porosity at different scales of each coal sample are shown in Fig. 7. After erosion by solutions 1# to 6#, the increments in porosity of adsorption pores of coal samples were 1.1777%, 1.1970%, 1.4980%, 1.4453%, 1.5036%, and 1.3613%, respectively. The increments in porosity of seepage pores were 0.8503%, 1.1305%, 1.0946%, 1.1913%, 1.5540%, and 1.7104%, respectively. The changes in adsorption pore porosity in the coal samples did not show a clear correlation with the concentration of surfactants. However, the increment in seepage pore porosity in the coal samples showed an increasing trend with the increase in surfactant concentration. This indicates that the addition of surfactants helps in the transformation of adsorption pores into seepage pores, promoting the development of seepage pores. The development of seepage pores is conducive to the migration of gas and is beneficial for the extraction of coalbed methane.
Fractal dimension of coal samples based on NMR
Coal samples possess extremely complex pore networks and rough surface morphology, which inevitably affect the flow of gas. The complexity of coal sample pore structures is typically characterized using fractal dimensions. According to fractal theory, pore structures with fractal dimensions between 2 and 3 exhibit fractal characteristics. The closer the value is to 2, the more regular the coal sample pores, which is conducive to the flow of gas. Conversely, the closer the value is to 3, the more complex the coal sample pores, which is detrimental to the flow of gas. According to Eq. (1) to (3), the fractal dimension of coal samples is calculated using the slope of the double logarithmic curve fitting line of SV and T2 obtained through nuclear magnetic resonance, as shown in Fig. 8. The total fractal dimension (Dt) of the original coal sample pore structure ranges from 2.3346 to 2.4243, with an average of 2.3770. After solution treatment, the total fractal dimensions of coal samples all decreased (ranging from 2.3071 to 2.4108), with an average of 2.3546. The average decrease was 0.0224, with a maximum decrease of 0.0339. This indicates that under chemical erosion, not only do the pore structures of coal samples develop, but their uniformity also improves. The pore shapes become more regular, and pore connectivity increases, consistent with the results of previous studies28,35.
The double logarithmic curve is divided into adsorption pores and seepage pores. The fractal dimensions of adsorption pores (Da) and seepage pores (Ds) of coal samples are obtained by fitting straight lines. Figures 9 and 10 respectively depict Da and Ds of the original coal sample and the coal sample after solution treatment. Table 4 lists the statistical results of the fractal dimensions of coal samples. The Da values of coal samples before and after solution treatment are both less than 2, indicating that they do not exhibit fractal characteristics and will not be discussed here. The Ds values of coal samples are all between 2 and 3, and the correlation coefficients of fractal dimensions of seepage pores (0.4701 to 0.7812) are significantly higher than those of total fractal dimensions (0.4162 to 0.5183). This indicates that compared to the overall pore structure of coal samples, the seepage pore part exhibits more fractal characteristics. The original coal sample Ds ranges from 2.9329 to 2.9796, with an average of 2.9657. After solution treatment, the fractal dimensions of seepage pores of coal samples all decreased (ranging from 2.9285 to 2.9750), with an average of 2.9584. The average decrease was 0.0073, with a maximum decrease of 0.0134. The Ds values of coal samples are generally close to 3, indicating that the seepage pores of coal samples are relatively complex and less uniform. The erosion by chemical solution reduces the Ds value, making the seepage pores relatively more regular and uniform, which is conducive to gas permeation in coalbeds. After solution erosion, the correlation coefficients of the fractal dimensions of seepage pores of coal samples all significantly increased, indicating that chemical solution erosion can make the seepage pores of coal samples more fractal, making the analysis of pore fractal characteristics of coal samples after chemical erosion more meaningful. Additionally, the decrease in the total fractal dimension of coal samples is generally positively correlated with the concentration of surfactants in the solution, while the correlation in terms of seepage pores is relatively weak. This may be because surfactants enhance the permeability of the solution and are more effective at modifying micropores in coal than mesopores and macropores.
Permeability
Figure 11 depicts the change in permeability of coal samples before and after solution treatment. The experimental confining pressure was kept constant at 2 MPa to minimize irreversible damage to the samples during loading and unloading, which could affect the test results. The selected pore pressures for the experiment ranged from 0.2 to 1.0 MPa, with a pore pressure gradient of 0.2 MPa. The permeability of the original coal samples ranged from 0.3031 to 1.6511 mD, with an average of 0.8273 mD. Due to the Klinkenberg effect42,43, the permeability of coal samples was highest at a pore pressure of 0.2 MPa. In the low pore pressure range, the permeability decreased significantly with increasing pore pressure. However, as the pore pressure continued to increase, the permeability showed a slow upward trend. This pattern remained evident after solution treatment, with the permeability of the treated coal samples ranging from 0.6269 to 2.9285 mD, with an average of 1.5396 mD. The permeability of all coal samples significantly increased after solution erosion. At a pore pressure of 0.2 MPa, the maximum increase in permeability was 1.6076 mD, with the highest growth rate reaching 121.70%. This indicates that erosion by the solution promotes the development of coal sample pore structure, increases the number of flow-through pores, enhances pore connectivity, facilitates gas flow channels, and improves coal sample permeability. Furthermore, the addition of surfactants promotes this erosive effect, suggesting that adding surfactants to chemical solutions helps to improve the efficiency of coalbed methane recovery.
Discussion
Under chemical erosion, the change in coal permeability is due to the dissolution of soluble components in the coal by the solution, leading to the development of pore-fissure networks, thereby providing more space for gas flow. In this paper, the soluble components in the multi-component composite solution consist of organic solvents and organic acids. Organic solvents can dissolve small organic molecules in coal, while organic acids dissolve carbonate minerals and iron minerals in coal. In this paper, the concentrations of organic solvents and organic acids in the 6 sets of solutions are the same, while different concentrations of surfactants are added to the solutions. Although the pore structure and permeability of the 6 sets of coal samples have developed and increased, there are differences in the extent of change, which seems to be correlated with the concentration of surfactants. The main function of surfactants is to reduce the surface tension of the solution and lower the contact angle between the solution and coal31,32,33,37,38. Current research results suggest that this effect enhances the permeability of the solution, increases the contact area between the solution and coal, and promotes the erosion of coal samples by the chemical solution34,35,36,44. Therefore, the correlation between surface tension, contact angle and pore structure and permeability of coal samples is discussed in this paper.
Figure 12 illustrates the relationship between the porosity increment of coal samples and surface tension and contact angle. The surface tension of the solution is negatively correlated with the increment of pore volume in coal samples, including adsorption pore porosity, seepage pore porosity, and total porosity (Fig. 12-a), indicating that the surface tension of the solution can affect the erosion effect of the chemical solution on coal samples. The correlation between surface tension and the increment of adsorption pore porosity is the highest, with a correlation coefficient of 0.8011, while the correlation with the increment of seepage pore porosity is relatively weak, with a correlation coefficient of 0.5094. The magnitude of the correlation between the surface tension of the solution and the increment of porosity in coal samples is in the order of adsorption pore > total pore > seepage pore. Interestingly, as shown in Fig. 12-a, when the surface tension of the solution reaches the critical micelle concentration, indicating little change in surface tension, there is a better correlation between the increment of adsorption pore porosity in coal samples and the fitted line, while the increment of seepage pore porosity shows greater dispersion from the fitted line. This may be because the reduction in surface tension facilitates the entry of the solution into smaller-scale pore spaces, thereby having a greater impact on the development of adsorption pores during chemical erosion, whereas its effect on seepage pores is relatively weaker. The contact angle of the solution on the coal surface is negatively correlated with the increment of adsorption pore porosity, seepage pore porosity, and total porosity in coal samples (Fig. 12-b). The correlation with the increment of porosity for each type of pore is relatively strong. Except for adsorption pores, the correlation between the contact angle and the increment of seepage pore porosity and total porosity is higher than that for surface tension, with the correlation coefficient in the order of total pore > seepage pore > adsorption pore. This indicates that the change in contact angle has a greater impact on the chemical erosion process, as the reduction in contact angle not only increases the permeability of the solution but also promotes the spreading of the solution on the pore surface, thereby enlarging the contact area between the solution and the soluble substances in coal. Therefore, during the chemical erosion process of coal samples by the solution, the reduction in contact angle can further promote the comprehensive development of pores, while the reduction in surface tension has a greater impact on adsorption pores.
Figure 13 shows the relationship between the fractal dimension of coal pore and surface tension and contact angle. The decrease in surface tension of the solution and the contact angle at the coal-solution interface is negatively correlated with the decrease in the Dt, while no correlation is observed with the Ds. It can be observed that the correlation between the contact angle (Fig. 13-b, R2 = 0.6810) and the decrease in Dt of coal samples is higher than that of surface tension (Fig. 13-a, R2 = 0.5099). Since the fractal dimension of adsorption pores is less than 2 and lacks fractal characteristics, no discussion is made regarding the change in the fractal dimension of adsorption pores. The erosion by chemical solution promotes the comprehensive development of coal pore structure, leading to a decrease in the fractal dimension of pores. It is evident that under the influence of surface tension and contact angle, the overall pore structure of coal tends to be more uniform and regular, which is beneficial for the flow of coalbed methane. However, the transition of seepage pores from complexity to regularity may be more advantageous for the flow of coalbed methane, but the decrease in the fractal dimension of seepage pores does not seem to be influenced by surface tension and contact angle.
Due to the significant differences in the original coal sample permeability, here we analyze the relationship between the growth rate of permeability after solution treatment and surface tension, and contact angle. Taking the pore pressure of 1 MPa as an example, as shown in Fig. 14. Both surface tension and contact angle are negatively correlated with the growth rate of permeability, indicating that the decrease in surface tension and contact angle can further promote the development of pores in coal samples under chemical solution erosion, thereby further increasing the permeability of coal samples. It is worth noting that the correlation between the contact angle (Fig. 14-b, R2 = 0.3865) and the growth rate of permeability is higher than that of surface tension (Fig. 14-a, R2 = 0.1853). As mentioned earlier, the decrease in surface tension has a greater effect on adsorption pores, while the contribution of adsorption pores to coal sample permeability is relatively weak. Therefore, the effect of surface tension on the increase in permeability of coal samples is not as significant as that of contact angle.
The experimental results indicate that in the process of using chemical methods to improve the permeability of coal, the surface tension of the solution and the coal-solution interface contact angle can be controlled by adding different surfactants. The reduction of surface tension and contact angle can promote the erosion of coal samples by chemical solutions, improve the seepage space of coal samples, and further increase the permeability of coal samples. Therefore, in the process of implementing chemical methods to improve the efficiency of coalbed methane extraction, surface tension, and contact angle are factors worthy of consideration, and adding different surfactants is one of the effective means to control these factors. The experiments found that the effect of contact angle on chemical erosion is greater than that of surface tension. Specifically, when the concentration of surfactants in the solution reaches the critical micelle concentration, the surface tension no longer changes significantly. However, when the concentration is further increased, there is still an enhanced trend of the solution’s erosion on the coal samples, resulting in a weakening correlation between surface tension and chemical erosion.
The coal-solution interface contact angle not only depends on the surface tension of the solution but also is affected by the adsorption between the hydrophobic groups of surfactants and the functional groups on the coal surface. As the solution expands inside the coal sample, a large number of surfactant molecules adsorb on the adsorption sites of the functional groups on the coal surface, which not only leads to the rapid spreading of the solution inside the coal sample but also may result in the loss of surfactant concentration. Surfactants with concentrations slightly higher than the critical micelle concentration can alleviate the loss of solution permeability and wettability during the chemical erosion process. Therefore, the decrease in surfactant concentration during the chemical erosion process leads to a decrease in the solution’s permeability and wettability, which further affects the erosion of coal samples. How to compensate for the loss of surfactant concentration during the chemical erosion process is an issue that needs to be studied in the future.
Conclusion
In this study, multicomponent composite solutions were prepared using organic solvents, organic acids, anionic surfactants, and nonionic surfactants, and modification experiments were conducted on coal samples. NMR tests, permeability tests, and fractal theory were applied to investigate the correlation between surface tension, contact angle, and pore development in coal samples during chemical erosion. The main conclusions are as follows:
-
(1)
The increment of total porosity and seepage pore porosity of coal samples increases with the increase of surfactant concentration, while the correlation between the increment of adsorption pore porosity and the concentration of surfactant is not significant. The increments of adsorption pore porosity, seepage pore porosity, and total porosity of coal samples are negatively correlated with the surface tension of the solution and the coal-solution interface contact angle. The correlation between surface tension and the increment of adsorption pore porosity is the highest, while the correlation with the increment of seepage pore porosity is relatively weaker. There is an obvious correlation between the contact angle and the increments of both adsorption and seepage pore porosity.
-
(2)
After chemical treatment, both Dt and Ds of coal sample pores decrease, resulting in a more uniform and regular overall pore structure and seepage pore structure. The correlation coefficient of fractal dimensions of seepage pores increases significantly, indicating a more obvious fractal characteristic. The decrease in Dt of coal samples is generally positively correlated with surfactant concentration, while there is no significant correlation between the decrease in Ds and surfactant concentration. Surface tension and contact angle are negatively correlated with the decrease in Dt but show no significant correlation with the decrease in Ds. The reduction in surface tension of the solution and coal-solution interface contact angle can make the overall pore structure of coal samples more uniform and regular, while the decrease in fractal dimensions of seepage pores is not affected by this.
-
(3)
The maximum increase in coal sample permeability is 133.85%, and the rate of permeability increase is positively correlated with the concentration of surfactant in the solution. At a pore pressure of 1 MPa, both surface tension and contact angle are negatively correlated with the rate of permeability increase. Due to the greater influence of surface tension reduction on micropores, the correlation between surface tension and the rate of permeability increase is less significant than that of the contact angle.
Data availability
All data generated or analysed during this study are included in this published article.
References
Hein, F. J., Ambrose, W. A., Hackley, P. & Mead, J. S. Amer Assoc Petr Geologists, E. Unconventional Energy Resources: 2017 review. Nat. Resour. Res. 28, 1661–1751. https://doi.org/10.1007/s11053-018-9432-1 (2019).
Li, S. et al. A comprehensive review of deep coalbed methane and recent developments in China. Int. J. Coal Geol. 279. https://doi.org/10.1016/j.coal.2023.104369 (2023).
Xu, F. Y. et al. The status and development strategy of coalbed methane industry in China. Petrol. Explor. Dev+. 50, 765–783. https://doi.org/10.1016/s1876-3804(23)60427-6 (2023).
Xu, F. Y. et al. Development Strategy and countermeasures of China’s CBM Industry under the goal of Carbon Peak and Neutrality. J. Earth Sci-China. 34, 975–984. https://doi.org/10.1007/s12583-022-1647-8 (2023).
Wang, K. & Du, F. Coal-gas compound dynamic disasters in China: a review. Process. Saf. Environ. 133, 1–17. https://doi.org/10.1016/j.psep.2019.10.006 (2020).
Zhao, W., Dong, H. Z., Wang, K., Liu, S. M. & Yan, Z. D. Evolution from gas outburst to coal outburst: an analysis from the perspective of asynchronous transfer difference of gas mass and coal deformation. Int. J. Heat. Mass. Transf. 217, 12. https://doi.org/10.1016/j.ijheatmasstransfer.2023.124736 (2023).
Wang, A. K., Shao, P., Lan, F. J. & Jin, H. Organic chemicals in coal available to microbes to produce biogenic coalbed methane: a review of current knowledge. J. Nat. Gas Sci. Eng. 60, 40–48. https://doi.org/10.1016/j.jngse.2018.09.025 (2018).
Karimpouli, S., Tahmasebi, P. & Ramandi, H. L. A review of experimental and numerical modeling of digital coalbed methane: imaging, segmentation, fracture modeling and permeability prediction. Int. J. Coal Geol. 228, 21. https://doi.org/10.1016/j.coal.2020.103552 (2020).
Li, Y. B. et al. Full-scale pore characteristics in coal and their influence on the adsorption capacity of coalbed methane. Environ. Sci. Pollut. 30, 72187–72206. https://doi.org/10.1007/s11356-023-27298-2 (2023).
Li, H. Y., Lau, H. C. & Huang, S. China’s coalbed methane development: a review of the challenges and opportunities in subsurface and surface engineering. J. Petrol. Sci. Eng. 166, 621–635. https://doi.org/10.1016/j.petrol.2018.03.047 (2018).
Pan, J. N. et al. Micro-nano-scale pore stimulation of coalbed methane reservoirs caused by hydraulic fracturing experiments. J. Petrol. Sci. Eng. 214, 10. https://doi.org/10.1016/j.petrol.2022.110512 (2022).
Dang, Z., Su, L. A., Wang, X. M. & Hou, S. H. Experimental study of the effect of ClO2 on coal: implication for coalbed methane recovery with oxidant stimulation. Energy. 271, 15. https://doi.org/10.1016/j.energy.2023.127028 (2023).
Li, H. et al. A review of laboratory study on enhancing coal seam permeability via chemical stimulation. Fuel. 330, 16. https://doi.org/10.1016/j.fuel.2022.125561 (2022).
Wang, Z., Lin, B. Q., Yang, W., Li, H. & Lin, M. H. Fracture and pore development law of coal under organic solvent erosion. Fuel. 307, 15. https://doi.org/10.1016/j.fuel.2021.121815 (2022).
Wang, Z. et al. Acetone erosion and its effect mechanism on pores and fractures in coal. Fuel. 253, 1282–1291. https://doi.org/10.1016/j.fuel.2019.05.034 (2019).
Mao, G. T., Li, Z. P., Lai, F. P. & Wei, H. X. Experimental investigation on the effect of organic solvents on gas development of coalbed methane reservoir. Fuel. 287, 8. https://doi.org/10.1016/j.fuel.2020.119497 (2021).
Zheng, C. S., Li, J. T., Xue, S., Jiang, B. Y. & Liu, B. J. Experimental study on changes in components and pore characteristics of acidified coal treated by organic solvents. Fuel. 353, 13. https://doi.org/10.1016/j.fuel.2023.129215 (2023).
Wang, F. F., Zhang, X. D., Zhang, S. & Wang, K. Mechanism of solvent extraction-induced changes to nanoscale pores of coal before and after acidification. Fuel. 310, 9. https://doi.org/10.1016/j.fuel.2021.122467 (2022).
Jing, Z. H., Balucan, R. D., Underschultz, J. R., Pan, S. Q. & Steel, K. M. Chemical stimulation for enhancing coal seam permeability: Laboratory study into permeability variation and coal structure examination. Int. J. Coal Geol. 219, 11. https://doi.org/10.1016/j.coal.2019.103375 (2020).
Chen, S. Y. et al. Chelating agent-introduced unconventional compound acid for enhancing coal permeability. J. Petrol. Sci. Eng. 199, 13. https://doi.org/10.1016/j.petrol.2020.108270 (2021).
Wei, Z., Lin, B. Q., Tong, L., Ting, L. & Wei, Y. Effect of acidification on microscopic properties and pore structure of coal. Fuel. 343, 12. https://doi.org/10.1016/j.fuel.2023.127834 (2023).
Zhang, B. X., Deng, Z., Fu, X. H., Yu, K. & Zeng, F. H. An experimental study on the effects of acidization on coal permeability: implications for the enhancement of coalbed methane production. Energy. 280, 15. https://doi.org/10.1016/j.energy.2023.128145 (2023).
Wang, L. et al. Changes in mineral fraction and pore morphology of coal with acidification treatment: contribution of clay minerals to methane adsorption. Environ. Sci. Pollut. 30, 114886–114900. https://doi.org/10.1007/s11356-023-30414-x (2023).
He, J. W. et al. Experimental study on erosion mechanism and pore structure evolution of bituminous and anthracite coal under matrix acidification and its significance to coalbed methane recovery. Energy. 283, 16. https://doi.org/10.1016/j.energy.2023.128485 (2023).
Dou, H. R. et al. Study on the mechanism of the influence of HNO3 and HF acid treatment on the CO2 adsorption and desorption characteristics of coal. Fuel. 309, 12. https://doi.org/10.1016/j.fuel.2021.122187 (2022).
Yang, H. T., Yu, Y. B., Cheng, W. M., Rui, J. & Xu, Q. F. Influence of acetic acid dissolution time on evolution of coal phase and surface morphology. Fuel. 286, 11. https://doi.org/10.1016/j.fuel.2020.119464 (2021).
Xie, H. G. et al. Acidification-Induced Micronano Mechanical properties and microscopic permeability enhancement mechanism of coal. Langmuir. 40, 4496–4513. https://doi.org/10.1021/acs.langmuir.3c04022 (2024).
Xing, M. Y., Xu, C. C., Zhou, G., Sun, L. L. & Du, W. Z. Experimental investigation for effect of multicomponent inorganic-organic acid solution on pore structure of lignite. Powder Technol. 392, 503–513. https://doi.org/10.1016/j.powtec.2021.07.014 (2021).
Zheng, C. S., Liu, S. L., Xue, S., Jiang, B. Y. & Chen, Z. W. Effects of chemical solvents on coal pore structural and fractal characteristics: an experimental investigation. Fuel. 327, 11. https://doi.org/10.1016/j.fuel.2022.125246 (2022).
Zhou, G. et al. Experimental investigation on physicochemical and wetting characteristics of Modified Gas coal: effects of Multicomponent acids and surfactant. J. Energ. Resour-Asme. 145, 9. https://doi.org/10.1115/1.4062320 (2023).
Jin, H. et al. Molecular dynamics simulations and experimental study of the effects of an ionic surfactant on the wettability of low-rank coal. Fuel. 320, 9. https://doi.org/10.1016/j.fuel.2022.123951 (2022).
Gan, J. et al. Experimental and molecular dynamics investigations of the effects of ionic surfactants on the wettability of low-rank coal. Energy. 271, 18. https://doi.org/10.1016/j.energy.2023.127012 (2023).
Meng, J. Q. et al. Molecular simulation of the regulation mechanism of the hydrophilic structure of surfactant on the wettability of bituminous coal surface. J. Mol. Liq. 383, 13. https://doi.org/10.1016/j.molliq.2023.122185 (2023).
Xie, J. N. et al. The effect of adding surfactant to the treating acid on the chemical properties of an acid-treated coal. Powder Technol. 356, 263–272. https://doi.org/10.1016/j.powtec.2019.08.039 (2019).
Xie, H. C. et al. The influence of surfactant on pore fractal characteristics of composite acidized coal. Fuel. 253, 741–753. https://doi.org/10.1016/j.fuel.2019.05.073 (2019).
He, J. W. et al. Variations in the pore structure and fluid mobility under anionic surfactant assisted matrix acidification of coal based on nuclear magnetic resonance T1-T2 spectra. Fuel. 355, 16. https://doi.org/10.1016/j.fuel.2023.129488 (2024).
Zhou, Q., Qin, B. T., Zhou, B. H. & Huang, H. X. Effects of surfactant adsorption on the surface functional group contents and polymerization properties of coal dust. Process. Saf. Environ. 173, 693–701. https://doi.org/10.1016/j.psep.2023.03.049 (2023).
Nie, W. et al. Synergistic effect of binary mixture of anionic nonionic surfactant on inhibiting coal dust pollution: experiment and simulation. J. Environ. 11, 13. https://doi.org/10.1016/j.jece.2023.110099 (2023).
Xie, K. C. Structure and Reactivity of Coal: A Survey of Selected Chinese Coals 1 edn (Springer Berlin, 2015).
Zhou, S. D., Liu, D. M., Cai, Y. D. & Yao, Y. B. Fractal characterization of pore-fracture in low-rank coals using a low-field NMR relaxation method. Fuel. 181, 218–226. https://doi.org/10.1016/j.fuel.2016.04.119 (2016).
Zhang, J. J. et al. Stress sensitivity characterization and heterogeneous variation of the pore-fracture system in middle-high rank coals reservoir based on NMR experiments. Fuel. 238, 331–344. https://doi.org/10.1016/j.fuel.2018.10.127 (2019).
Liu, C. et al. Effective stress effect and slippage effect of gas migration in deep coal reservoirs. Int. J. Rock. Mech. Min. 155. https://doi.org/10.1016/j.ijrmms.2022.105142 (2022).
Meng, Y. & Li, Z. P. Laboratory investigation on gas slippage phenomenon in coal sample and its research significance. Phys. Fluids. 35, 11. https://doi.org/10.1063/5.0167526 (2023).
Cao, Z. Z., Yang, X. Q., Li, Z. H. & Du, F. Evolution mechanism of waterconducting fractures in overburden under the infuence of waterrich fault in underground coal mining. Sci. Rep. 14, 5081. https://doi.org/10.1038/s41598-024-54803-5 (2024).
Acknowledgements
This work was supported by the National Key R&D Projects (2017YFC1503102), the University-local Government Scientific and Technical Cooperation Cultivation Project of Ordos Institute-LNTU (YJY-XD-2024-A-006), the Basic Scientific Research Project of Liaoning Provincial Department of Education (JYTMS20230791).
Author information
Authors and Affiliations
Contributions
H.C. wrote the main manuscript text, H.C. and W.A. responsible for organizing information, H.C. prepared all the experiments, L.W. reviewed and corrected the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, H., Wang, L. & An, W. Effects of surface tension and contact angle on pore structure development of coal samples under chemical solution erosion. Sci Rep 14, 23161 (2024). https://doi.org/10.1038/s41598-024-74971-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74971-8