Abstract
This study investigates the impact of long-term water immersion on the mechanical and hydrochemical properties of cemented calcareous soil, emphasizing the critical role of carbonate content in mechanical performance. Utilizing hydrochemical analysis and triaxial testing, the research revealed that prolonged immersion disrupts the acid-base balance of the solution, resulting in an increased concentration of ions and chemicals. Significant dissolution of carbonates and soluble minerals occurs, which reacts with carbon dioxide to generate bicarbonate ions, thereby elevating the alkalinity of the soaking solution. Additionally, the gradual dissolution of clay minerals compromises the cementitious structure, leading to particle reorientation and interlocking. The study quantitatively assesses the changes in soil properties, demonstrating a substantial reduction in soil cohesion by up to 86.1% and an increase in the internal friction angle by 37.5%. Furthermore, the gradual dissolution of clay minerals compromises the cementitious structure, resulting in particle reorientation and interlocking that contribute to the observed mechanical changes. The findings underscore the importance of understanding the effects of extended immersion on the stability and engineering applicability of cemented calcareous soils.
Similar content being viewed by others
Introduction
Cemented calcareous soils are prevalent in arid and semi-arid regions, constituting over one-third of the terrestrial area1. These soils are characterized by carbonates that serve as principal cementitious agents, facilitating the bonding of soil particles through deposition and crystallization, thereby enhancing their strength and stability2,3. However, in proximity to rivers or reservoirs, long-term soaking can induce the dissolution and segregation of calcium salts, which may compromise the integrity of the cementitious structure, as shown in Fig. 1(a)~(c). This alteration can modify the porosity and ionic composition of the soil, leading to significant geotechnical disasters such as ground subsidence, soil erosion, and landslides4,5,6,7. Moreover, the release of calcium ions can adversely affect the hydrochemical properties of surrounding aquatic environments, posing risks to local ecosystems8. Therefore, it is of paramount importance to undertake systematic investigations into the mechanical and hydrochemical properties of cemented calcareous soils under water immersion.
Despite the importance of these soils, research on their properties under long-term immersion conditions remains sparse. Existing studies have demonstrated that the degree of cementation is a crucial determinant of mechanical properties, with higher cementation levels correlating with increased load-bearing capacity. However, extended immersion typically results in substantial deterioration of both physical and chemical properties, significantly reducing soil stability and impacting foundational integrity9,10,11. Numerous scholars have conducted experimental studies on the mechanical properties of cemented calcareous soils, summarizing their formation and characteristics. For cemented calcareous soils, the degree of cementation serves as a crucial determinant of their mechanical properties; soils with higher cementation levels can withstand greater load levels12,13,14. Aire and Fahey15 conducted multiple triaxial tests on undisturbed calcareous soils and observed that soils with higher cementation typically exhibit three states: an elastic response without pore pressure accumulation, a stable state after some accumulation of pore pressure, and a failure state following significant pore pressure build-up. Ismail et al.16 treated calcareous soils with various cementitious agents and found significant differences in the effective stress paths and post-yield responses through triaxial loading tests, markedly affecting the mechanical response of the soils. Airey et al.17 investigated the increase in shear modulus and yield point size in cemented soil samples through conventional and stress path triaxial tests. Performing cyclic triaxial tests on different types of cemented calcareous soils, it was demonstrated that enhancing the degree of cementation significantly increases the initial stiffness of the soil, although the shear modulus decreases with the number of cycles18,19. The stress-strain behavior of cemented calcareous soils follows an elasto-plastic deformation model, leading to the loss of cohesion under load and often exhibiting high compressibility. This phenomenon typically occurs because the calcareous cementation bonds are prone to failure under high stress conditions20,21.
In general, cemented calcareous soils are rich in hydrophilic mineral components, thereby rendering water as a significant influencer of their properties22. Zhu et al.23 examined natural cemented calcareous soils in both naturally air-dried and saturated conditions, demonstrating that the degree of cementation and density are pivotal parameters determining the strength of soil samples. Through diverse experimental procedures, Wang et al.24 explored the mechanical performance of the soil samples over different immersion durations, revealing a pronounced decrease in soil cohesion as the immersion time increased. Han et al.25 conducted a series of physicochemical and uniaxial compression tests on sandstone after long-term immersion, and found that the dissolution of calcite and dolomite within the soil released HCO3− which consequently elevated the pH level of the immersion solution.
So far, research on cemented calcareous soils remains limited, particularly concerning the properties of these soils under long-term immersion conditions. An increase in immersion duration tends to precipitate profound deterioration in both the physical and chemical properties of cemented calcareous soils, markedly reducing soil stability and, consequently, affecting the stability of foundations26,27. This study aims to systematically investigate the effects of long-term immersion on the mechanical characteristics and hydrochemical properties of cemented calcareous soils, utilizing triaxial testing and hydrochemical analysis of samples collected from the downstream banks of the Jinsha River. Section 2 delineates the preparation of samples and the experimental apparatus. Section 3 expounds on the methods and principles of experimentation. Section 4 presents the results of material composition analysis, hydrochemical analysis, and mechanical testing. Section 5 discusses the distinctions between long-term and short-term immersion effects on cemented calcareous soils and the occurrences of long-term immersion in natural settings. Section 6 summarizes the study.
Specimen preparation and experimental equipment
A broad range of particle size distribution alongside significant heterogeneity cemented calcareous soils can be easily observed28,29. All the Specimens utilized in this investigation were obtained from the downstream banks of the Jinsha River, where the area is characterized by the substantial evaporation and predominantly seasonal precipitation, as illustrated in Fig. 2a, b30. Twenty-four specimens were molded into cylindrical rock specimens with dimensions of 50 mm in diameter and 100 mm in height. These specimens were assorted into three distinct groups based on the different duration of immersion, as demonstrated in Fig. 2c. Each specimen can be represented via T/C/Y-N, where T/C/Y stand for the the duration of immersion and N denotes the ranked number. T, C and Y stand for the the duration of immersion with zero, 120 and 150 days, respectively. For instance, C-1 shows the specimen ranked number one under the water immersion with 120 days. In addition, natural Jinsha River water was transported for the immersion experiments and solution hydrochemical analyses.
Figure 3a–e show the basic physical parameters of cemented calcareous soil, involving mass, height, diameter, density and wave speed. Specimens exhibit diameters within the range of 47.77 to 50.45 mm, with a mean of 49.04 mm. Heights of these specimens span 98.19 to 103.95 mm, with an average of 100.86 mm. The masses recorded vary from 393.03 to 492.99 g, resulting in an average mass of 455.02 g. Density measurements for these specimens range from 2224.23 to 2523.67 kg/m³, with a mean density of 2384.48 kg/m³. Wave speed values are observed between 2747.25 and 3623.19 m/s, with an average speed of 3219.61 m/s. These measurements demonstrate that the specimens exhibit minimal deviation across the evaluated parameters. Specimens were grouped and placed in the three glass soaking containers. The waters from the Jinsha River were then slowly poured along the walls into two of these containers until the specimens were fully submerged, avoiding direct impact of the water on the specimen surfaces to prevent potential interference. The containers were subsequently sealed with cling film until the designated soaking duration had been reached. During the soaking period, solutions from the first and second groups of cemented calcareous soil specimens were extracted. The first group was immersed using the original Jinsha River water, and the soaking solution from the second group was collected after 90 days of immersion. Subsequent hydrochemical analyses were performed on these solutions to determine the relationship between the solution pH, ion concentrations, total hardness, and mineralization.
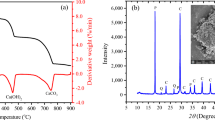
Figure 4 shows the entire process of the soaking process, ion measurement and triaxial experiments. First, the chemical composition and concentration of ions in the topsoil of the sampling area were analyzed, followed by the collection and aqueous chemical analysis of water from the adjacent Jinsha River, focusing on ion concentrations and pH values. Then, basic parameter tests were conducted on the samples, along with X-Ray Diffraction (XRD) to determine mineralogical compositions and X-Ray Fluorescence (XRF) to assess the content of chemical oxides. Transmission Electron Microscopy (TEM) was utilized to image and observe the microstructure of the samples. Following immersion of sample groups, aqueous chemical analysis of the immersion solutions was performed to study changes in ion concentrations and pH values. Finally, the immersed samples were subjected to triaxial tests to evaluate the mechanical strength after varying duration of immersion. In this investigation, mineralogical compositions of the specimens were quantitatively determined employing an X-ray diffractometer (DX-2700), as depicted in Fig. 5a. Mineral crystalline diffraction spectra were analyzed using Bragg’s Law to assess diffraction angles and intensities, thereby quantifying the mineral content31. X-ray fluorescence spectroscopy (XRF1800) was utilized to assess the chemical oxide compositions of the specimens, shown in Fig. 5b. This non-destructive method involves the excitation of atoms within the specimen, causing the emission of characteristic X-rays. The energies and intensities of these rays facilitate the determination of the chemical oxide compositions32. Furthermore, the internal structure and composition of the specimens were examined using a transmission electron microscope (TEM), as detailed in Fig. 5c. High-energy electron beams penetrate the specimen and the resultant image formed post-transmission33. Additionally, the concentrations of Cl− and SO42− in aqueous solutions were determined using ion chromatography, as illustrated in Fig. 5d. This technique involves ion exchange resin interacting with the specimen ions, facilitating their separation based on differing migration velocities between the stationary and mobile phases, thus allowing for the separation and quantitative analysis of ionic mixtures34. The concentrations of cations (K+, Na+, Mg2+ and Ca2+) in the aqueous solutions were quantified utilizing an inductively coupled plasma atomic emission spectrometer (ICP-AES), as illustrated in Fig. 5e. The specimen atoms were excited to emit characteristic spectra via importing the specimen into a high-temperature plasma, where the intensities of these spectra facilitated the analysis of the types and quantities of elements within the specimen35. Triaxial experiments were conducted using the MTS-815 servo-controlled machine, as depicted in Fig. 5f. This apparatus is capable of exerting a maximum axial load of 2700 kN with a confining pressure up to 140 MPa36. Moreover, the detailed soaking process of the cemented calcareous soil can be shown in Fig. 5g.
Methodology
Chemical analysis for cation
The original water of Jinsha River and soaking solutions are filtered through a 0.45 μm membrane and stored in the clean polyethylene bottles to eliminate suspended particulate matter. Specimens are diluted with ultrapure water to ensure concentrations are within the operational range of the instrument and free of interfering substances. Single-element standard solutions of K+, Na+, Mg2+ and Ca2+are prepared by dissolving high-purity KCl in ultrapure water to create a stock solution of 1000 mg/L. This stock solution is sequentially diluted to produce standard solutions at the concentrations of 0.1, 1, 10, and 100 mg/L; the spectrometer is preheated for at least 30 min to stabilize the plasma. Instrument parameters of the ICP-AES are set with RF power of 1300 W, cooling gas flow of 15 L/min, auxiliary gas flow of 1.0 L/min, carrier gas flow of 0.8 L/min, and specimen introduction rate of 1.0 mL/min. Standard solutions are injected sequentially, recording the emission spectral signals of each element to construct calibration curves. The accuracy and linearity of the calibration curves are verified using a mid-range standard solution. Treated water specimens are then injected in sequence, with the spectral signals of K+, Na+, Mg2+ and Ca2+ recorded. Each specimen is tested thrice, and the mean values are calculated to enhance the reliability of the results. Concentrations of K+, Na+, Mg2+ and Ca2+ in the specimens are computed using the calibration curves, with adjustments made for the dilution factors of the specimens.
Chemical analysis for anion
Similar to the chemical analysis for cation, the natural water collected from Jinsha River and soaking solutions are filtered through a 0.45 μm membrane and stored in the clean polyethylene bottles. The specimens are diluted with ultrapure water and prepared for analysis by creating single-ion standard solutions of Cl− and SO42−. High-purity NaNO2 is dissolved in ultrapure water to prepare a stock solution of 1000 mg/L, which is subsequently diluted to generate the standard solutions at the concentrations of 0.1, 1, 10, and 100 mg/L; the analytical instrument should be preheated prior to use.
The Instrument parameters are configured with the column temperature maintained at 30°, the mobile phase flow rate at 1.0 L/min, and the injection volume set at 25 µL. The standard solutions of differing concentrations are injected, and the peak areas of Cl− and SO42− can be sequentially recorded to establish calibration curves. The accuracy and linearity of these curves are verified using a standard solution of intermediate concentration. Quantitative water from the Jinsha River and soaking solution are treated with an appropriate amount of methyl orange indicator. Titration is performed using a 0.02 mol/L HCl standard solution until the solution transitions from yellow to the red endpoint. The volume of HCl standard solution consumed is meticulously recorded; the titration reaction can be represented chemically as follows37:
the concentration of OH− can be calculated as follows38:
where c1 represents the concentration of hydroxide ions, V2 denotes the volume of the consumed HCl standard solution, c2 is the concentration of the HCl standard solution, and V3 corresponds to the volume of water from the Jinsha River or the soaking solution.
A specified volume of water from the Jinsha River or soaking solution sample is treated with a dilute solution of BaCl2 to remove SO42−. Subsequently, an adequate quantity of NaOH solution is added, and the mixture is boiled to expel CO2. Titration is then carried out using a 0.02 mol/L HCl standard solution until the color of the solution shifts from blue to colorless. The volume of the HCl standard solution expended is rigorously documented. The chemical reaction for the titration can be depicted as follows39,40:
The calculation of the concentration of carbonate ions CO32− also utilizes Eq. (2), in which c1 denotes the concentration of CO32−, V2 represents the volume of the consumed HCl standard solution, c2 specifies the concentration of the HCl standard solution, and V3 corresponds to the volume of water from the Jinsha River or the soaking solution.
A measured volume of water from the Jinsha River or soaking solution is initially treated with an appropriate amount of methyl red indicator. Titration is then performed using a 0.02 mol/L H2SO4 standard solution until the endpoint is reached, where the solution transitions from yellow to red. The volume of the H2SO4 standard solution utilized is meticulously recorded. The chemical reaction formula for the titration is provided as follows41:
the concentration of bicarbonate ions HCO3− is also calculated using Eq. (2), where c1 represents the concentration of HCO3−, V2 is the volume of the consumed H2SO4 standard solution, c2 stands for the concentration of the H2SO4 standard solution, and V3 the volume of water from the Jinsha River or the soaking solution sample.
Triaxial test
Triaxial experiments are conducted on five specimen groups after variable soaking durations, corresponding with confining pressures of 1, 2, 3, 4, and 5 MPa respectively. The application of axial load occurs at a rate of 0.2 kN/s, and the confining pressure is applied at a rate of 0.1 MPa/s. Once the designated confining pressure is achieved and stabilized, the loading methodology transitions to displacement control, imposing the load at a rate of 0.005 mm/s until sample failure. From the results of these triaxial tests, the optimal relationship between σ1 and σ3 can be determined, facilitating the calculation of the cohesion C and the angle of internal friction φ for cemented calcareous soils under various soaking durations. The solution can be derived from the following formula42:
where C represents the cohesion of the rock, φ denotes the angle of internal friction of the rock; σc refers to the intercept of the optimal relationship curve on the ordinate, in megapascals; m indicates the slope of the optimal relationship curve.
Results
Specimen and water chemical characteristics of Jinsha River
Figure 6a shows the content of mineral components in the cemented calcareous soil. The cementitious material samples predominantly consist of minerals such as montmorillonite, illite, glauconite, plagioclase, calcite, and dolomite. Among these, quartz and dolomite represent the principal mineral constituents, comprising 23% and 22% respectively, followed by illite at 16%, calcite at 15%, glauconite at 11%, montmorillonite at 8%, and plagioclase at 5%. Notably, quartz, though abundant, exhibits considerable resistance to dissolution in water at ambient temperatures. Consequently, in aqueous environments over extended periods, the minerals that predominantly dissolve are dolomite and calcite. The composition and proportions of chemical oxides in the samples are delineated in Fig. 6b, where SiO₂ accounts for 45.876%, CaO for 19.6364%, MgO for 6.9441%, Al₂O₃ for 13.0423%, Fe₂O₃ for 8.4733%, K₂O for 2.4075%, Na₂O for 0.9645%, and SO₃ for 0.4331%.
Figure 7a and b display the Scanning Electron Microscope (SEM) image of a cemented calcareous soil, revealing its microstructural characteristics. It can be observed that the surfaces of gravel and crushed stone within the cemented calcareous soil exhibits as rough granular textures with random cementing degree. Moreover, the clay minerals tightly encircle the non-clay particles, forming the dense and unevenly distributed agglomerates. The pores within these agglomerates show heterogeneous distribution and significant variations in size, resulting in the complex spatial arrangements of the filling of the clay mineral. Clay minerals emerge as large particles formed via the fine clay material, complexly stacking and intertwining with other counterparts. In addition, pores with various sizes distribute around these particles accompanied with numerous cracks, which might be induced via external influences or the decomposition of the cementing substance.
Figure 8a and b show the chemical ions and pH value of topsoil in sampling site. The pH value of topsoil ranges from 7.15 to 7.82, indicative of a weakly alkaline condition. Ca²⁺ and Mg²⁺ play the dominate role in the cation distribution, with average concentrations of 139.175 mg/kg and 33.4625 mg/kg, respectively. The principal anions, SO42− and HCO3− have average concentrations of 162.9525 mg/kg and 336.075 mg/kg, respectively. Hydrochemical characteristics of Jinsha River water can be shown in Fig. 9. Within the cation spectrum, calcium ions manifest the highest concentration at 46.79 mg/L, followed by potassium and sodium ions at a maximum of 26.7 mg/L, and magnesium ions at 22.33 mg/L. Among the anions, bicarbonate ions reach a peak concentration of 221.0 mg/L, while the sulfate and chloride ions are recorded at 45.33 mg/L and 26.83 mg/L, respectively. The waters of the Jinsha River display a weakly alkaline pH of 7.77, with a total hardness of 208.7 mg/L, a permanent hardness of 27.5 mg/L, and a mineralization level of 278.2 mg/L.
Characteristics of hydrochemical changes of soaking solution
Ion concentrations and pH values within solutions subjected to varying durations of immersion are delineated in Table 1. It can be observed that the pH value escalates from 7.64 to 8.18 as the immersion time extends. A negative correlation with time emerges solely for K+ & Na+, whereas other ions exhibit positive correlations. The mineralization increases from 253.3 mg/L to 278.3 mg/L, indicative of an augmentation in the summation of ions, molecules, and chemical substances, reflecting the material leaching of cemented calcareous soil within aquatic environments. The increment magnitude of anions in the solution can be ordered as HCO3−> SO42−> Cl−. Among cations, the increase is most pronounced for Mg2+ followed by Ca2+. In alkaline conditions, HCO3− reacts with OH− to produce H2O and CO32–43:
However, the absence of CO32− was observed based on the analytical findings, with the solution demonstrating weak alkalinity. This phonomenon suggests a relatively low degree of alkalinity impeding the forward reaction where HCO3− transforms into CO32−. The hydrolysis of HCO3− proceeds at a rate surpassing that of its dissociation, implying that HCO3− along with other weak acid anions contribute to the elevation of the pH value. The profusion of HCO3− in the solution arises from multifaceted sources. On the one hand, a portion of HCO3− originates from the dissolution of mineral salts. On the other hand, an additional amount of HCO3− results from the reaction of carbonates with free CO2 within water44:
Due to the greater solubility of HCO3− compared to CO32− in water, the reaction persists in the presence of free CO2. Overall, the aforementioned reaction facilitates the dissolution of carbonate minerals such as CaCO3. Under conditions of alkaline solution and the presence of CO2, the increasing concentration of HCO3− continuously promotes the dissolution of carbonate minerals and the liberation of ions.
The cemented calcareous soil inherently comprises readily soluble minerals, which can be characterized by well-developed porosity, resulting in a leaching effect under the interaction with the waters of the Jinsha River. The outcome of this interaction dissolves the calcite, dolomite, and surface clay minerals; the hydrolysis reactions can be represented as follows45:
In addition, clay minerals such as illite and chlorite also undergo hydrolysis46:
where Y primarily represents Mg2+, Fe2+, Al3+ and Fe3+, with the equation being balanced for divalent ions.
The cemented calcareous soil contains clay minerals with structural defects commonly observed in their crystal lattice, notably the absence of cations, leading a permanently negative charge on the mineral surfaces. These negative charges generally attract cations towards the mineral surface for electrical neutrality, forming a layer of exchangeable cations. These cations may exchange with the other cations in the water, transferring initially adsorbed cations into the solution. The capacity for cation adsorption decreases in the following order: H+> Fe2+> Al+> Ca2+> Mg2+> K+> Na+. The adsorption affinity is strongest for K+ and Na+, consequently reducing the concentrations of these cations in the solution.
Characteristics of changes in mechanical properties of specimens
Triaxial experiments were conducted on the cemented calcareous soil under natural conditions (no immersion), as well as after 120 and 150 days of immersion. Upon exclusion of anomalous data points, a relationship between the axial stress σ1 and the confining stress σ3 at failure was established, as depicted in Fig. 10. Figure 11 illustrates the fitted curve of the relationship between σ1 and σ3 for the cemented calcareous soil. The cohesion C and the angle of internal friction φ for different immersion durations are presented in Table 2. A significant alteration in the mechanical properties of the cemented calcareous soil was observed after long-term immersion. The cohesion C decreases to 0.9 MPa with a reduction of 78.6% 150 days of submersion, demonstrating a negative correlation with immersion duration. This result might be interpreted as follows. The cementitious materials within the cemented calcareous soil can be dissolved due to long-term aqueous environments, leading to a breakdown of the cementation structure. This weakening of the cementation bond among particles resulted in a reduction in shear strength and a decrease in soil strength. Conversely, the angle of internal friction φ shows an increase to 44.2° after 150 days of immersion with an increase of 37.5%, featuring a positive correlation with immersion time. This increase is attributed to changes in the pore structure of the cemented calcareous soil and a weakening of cementation within the cemented calcareous soil, shifting the soil structure from a cemented to a more granular state. This transformation facilitates the greater particle rearrangement and interlocking during the shearing process. The dissolution of cementitious substances may also render particle surfaces rougher, thus enhancing the frictional resistance between grains. Additionally, the leaching of fine particles results in a coarser soil texture, typically exhibiting a higher friction angle.
Discussion
The high content of carbonates play an important role for the mechanical performance of cemented calcareous soil with the presence of water, highlighting the significance of studying the mechanical and hydrochemical properties of this soil when subjected water immersion. In general, cemented calcareous soil is characterized by the formation of a hard structural layer resulting from the enrichment of calcium salts., significant alterations in the strength, stability, and structure of cemented calcareous soil are observed under water immersion, with the extent of these changes closely associated with the duration of immersion. This study investigated the mechanical properties and hydrolysis characteristics of cemented calcareous soil under long-term immersion. Compared to short-term immersion, the degradation of performance in cemented calcareous soil is more profound25.
Short-term immersion predominantly affects the surface structure of cemented calcareous soils, leading to the dissolution of superficial calcium salts. This dissolution initiates a loosening of the previously dense cementation, manifesting as honeycombed or fissured areas, while the internal structure substantially retains its integrity. The loosening of the surface layer marginally increases porosity, while the principal pore structure unchanged, and the enhancement in permeability is confined to the surface. In addition, the degradation of the surface structure decrease the compressive and shear strengths in a limited magnitude. Short-term immersion results in the leaching of internal calcium salts, causing localized changes in ionic composition, though the overall alterations remain minimal. In contrast, the effects of long-term immersion on cemented calcareous soils prove more severe, thoroughly disrupting both internal and external cementation structures, resulting in a complete loss of the original structure. A multitude of tubular and honeycombed pores collapse with new fissures and voids emerging extensively. In this case, porosity and permeability of cemented calcareous soil show a substantial enhancement. A considerable leaching of calcium ions occurs, accompanied by the expulsion of soil mass and the infiltration of other ions from external aqueous sources, thereby altering the overall ionic composition of the soil. The destruction of the cementation structure precipitates a severe reduction in compressive and shear strengths, potentially leading to the loss of load-bearing capacity. Under the influence of gravitational forces or other external stresses, the cemented calcareous soil is highly susceptible to serious deformations such as collapses and subsidences.
The long-term immersion of cemented calcareous soils frequently occurs in natural environments, closely linked to factors such as geographic environments, climatic conditions, and water changes. In regions with significant rainfall and long rainy seasons, the disruption of both internal and external structures of cemented calcareous soils can be observed, leading to the reductions in strength and stability, potentially triggering natural disasters like landslides and collapses. Overall, the effects of immersion on cemented calcareous soils manifest progressively. Short-term immersion primarily impacts the superficial structure and localized properties. However, the extended immersion irreversibly damages the internal and external structures, alters chemical compositions, and significantly degrades the mechanical performance. Therefore, it should be paid sufficient attention on the long-term immersion of cemented calcareous soils, which might compromise the stability and engineering applicability.
Conclusion
Extended periods of immersion can precipitate the dissolution and segregation of calcium salts, thereby potentially undermining the cohesive integrity of the cementitious matrix. This study elucidates the effects of extended water immersion on the hydrochemical and mechanical properties of cemented calcareous soils, highlighting the critical implications for soil stability and engineering applications. The primary findings indicate that prolonged immersion leads to the dissolution and segregation of calcium salts, which significantly undermines the cohesive integrity of the cementitious matrix. Key observations reveal that quartz and dolomite are the predominant soluble minerals within the cemented calcareous soil, with notable concentrations of SiO2 and CaO reflecting its silicate and carbonate nature. The microstructural analysis indicates that calcium and iron are the primary agents of cementation, while the soil exhibits weakly alkaline conditions characterized by elevated levels of Ca2+, Mg2+, SO42− and HCO3 − ions. For the Jinsha River, calcium ions and bicarbonate ions prevail in concentration, presenting a weakly alkaline pH, while the degree of mineralization and hardness remain moderate.
As immersion duration increases, the solution undergoes alkalinity intensifying, evidenced by a rise in pH from 7.64 to 8.18. An enhancement in mineralization from 253.3 mg/L to 278.3 mg/L demonstrates an augmentation in the aggregate ion and molecule content, reflecting substantive material efflux from cemented calcareous soils in aqueous environments. This demonstrates a significant release of ions and molecules from the cemented calcareous soils into the aqueous environment, underscoring the dynamic chemical interactions occurring during immersion. With long-term immersion, a significant diminution in the cohesion of cemented calcareous soil occurs, with a decrease amounting to 86.1%, while the angle of internal friction experiences an increase, escalating by 37.5%. This paradoxical enhancement in friction angle is attributed to the dissolution of calcite and dolomite, which disrupts the cementation structure and alters the pore configuration, facilitating particle reorientation and interlocking. Additionally, the increased roughness of particle surfaces due to the dissolution of cementitious materials contributes to the observed rise in frictional resistance.
In conclusion, the findings underscore the necessity for careful consideration of the long-term effects of water immersion on cemented calcareous soils, as these changes can significantly compromise soil stability and engineering viability. In addition, the specific context of the Jinsha River may limit the generalizability of our results. Therefore, we propose that subsequent studies should consider a wider range of geographic locations and hydrological conditions to validate and extend our findings.
This research was supported by the Natural Science Foundation of Sichuan Province, China (Grant: No. 2024NSFSC0983), the National Natural Science Foundation of China (No. 42477179), and Open Fund of Sichuan Engineering Research Center for Mechanical Properties and Engineering Technology of Unsaturated Soil (No. SC-FBHT2024-06).
Data availability
The data supporting the findings of this study are available within the article. The models or code generated or used during the study are available from the corresponding author by request.
References
Taalab, A. S., Ageeb, G., Siam, W., Mahmoud, S. A. & H. S. & Some characteristics of calcareous soils. A review AS Taalab1, GW Ageeb2, Hanan S. Siam1 and Safaa A. Middle East. J. Agric. Res. 8, 96–105 (2019).
Gao, G. Microstructure of loess soil in China relative to geographic and geologic environment. Acta Geol. Sinica. 3, 265–274 (1984). (In Chinese).
Acharya, S. S. S. Characterisation of cyclic behaviour of calcite cemented calcareous soils. Ph.D. thesis, Univ. Western Australia. Australia (2004).
Huang, R. & Li, W. Formation, distribution and risk control of landslides in China. J. Rock. Mech. Geotech. 3, 97–116 (2011).
Li, P., Vanapalli, S. & Li, T. Review of collapse triggering mechanism of collapsible soils due to wetting. J. Rock. Mech. Geotech. 8, 256–274 (2016).
Xia, J. W. & Guo, H. Z. Distribution characteristics and main controlling factors of landslides in the upper reaches of Yangtze River. Hydrogeol. Eng. Geol. 23, 19–22 (1997).
McClelland, B. Calcareous sediments: An engineering enigma. Proc. Int. Cong Calcareous Sediments pp, 777–784 (1988).
Bolan, N. et al. Distribution, characteristics and management of calcareous soils. Adv. Agron. 182, 81–130 (2023).
Allman, M. A. & Poulos, H. G. Stress-strain behaviour of an artificially cemented calcareous soil. Proc. Int. Conf. Calcareous Sediments Volume. 1, 51–60 (2021).
Fookes, P. G. The geology of carbonate soils and rocks and their engineering characterisation and description. Proc. Int. Conf. Calcareous Sediments pp, 787–806 (1988).
Afifipour, M. & Moarefvand, P. Failure patterns of geomaterials with block-in-matrix texture: Experimental and numerical evaluation. Arab. J. Geosci. 7, 2781–2792 (2014).
Carter, J. P., Airey, D. W. & Fahey, M. A. A review of laboratory testing of calcareous soils. University of Sydney. Department of Civil Engineering, Centre for Geotechnical Research, (1999).
Coop, M. R. & Atkinson, J. H. The mechanics of cemented carbonate sands. Geotechnique. 43, 53–67 (1993).
Zhu, C. Q., Bin, Z. & Liu, H. F. State-of-the-art review of developments of laboratory tests on cemented calcareous soils. Rock. Soil. Mech. 36, 311–319 (2015).
Airey, D. W. & Fahey, M. Cyclic response of calcareous soil from the North-West Shelf of Australia. Geotechnique. 41, 101–121 (1991).
Ismail, M. A., Joer, H. A., Sim, W. H. & Randolph, M. F. Effect of cement type on shear behavior of cemented calcareous soil. J. Geotech. Geoenviron. 128, 520–529 (2002).
Airey, D. W. Triaxial testing of naturally cemented carbonate soil. J. Geotech. Eng. 119, 1379–1398 (1993).
Sharma, S. S. & Fahey, M. Deformation characteristics of two cemented calcareous soils. Can. Geotech. J. 41, 1139–1151 (2004).
Sharma, S. S. & Fahey, M. Evaluation of cyclic shear strength of two cemented calcareous soils. J. Geotech. Geoenviron. 129, 608–618 (2003).
Frydman, S. Calcareous sands of the Israeli coastal plain. Geotechnical Properties, Behavior, and Performance of Calcareous Soils. ASTM International, (1982).
Chen, X., Xu, D., Shen, J., Wei, H. & Wang, R. Effect of particle size and particle distribution pattern on dynamic behavior of cemented calcareous sand. Mar. Georesour Geotec. 41, 412–424 (2023).
Abdallah, K., Abdelkader, B., Ahmed, A., Djamel Eddine, B. & Marwan, S. A laboratory study of shear strength of partially saturated sandy soil. Geomech. Geoengin. 17, 741–750 (2022).
Zhu, C. Q., Zhou, B. & Liu, H. F. Investigation on strength and microstructure of naturally cemented calcareous soil. Rock. soil. mech. 35, 1655–1663 (2014).
Wang, X. M., Chen, S. X. & Cheng, C. B. Experimental study on physico-mechanical characteristics of undisturbed loess soaked in acid solution. Chin. J. Geotech. Eng. 35, 1619–1626 (2013). (In Chinese).
Han, P., Zhang, C., Wang, X. & Wang, L. Study of mechanical characteristics and damage mechanism of sandstone under long-term immersion. Eng. Geol. 315, 107020 (2023).
Razouki, S. S. & El-Janabi, O. A. Decrease in the CBR of a gypsiferous soil due to long-term soaking. Q. J. Eng. Geol. Hydroge. 32, 87–89 (1999).
Carter, J. P. et al. Triaxial testing of North Rankin calcarenite. Proc, Int. Conf On calcareous sediments. pp, 515–530 (1988).
Wu, Y. & Lan, H. Landslide Analyst—a landslide propagation model considering block size heterogeneity. Landslides. 16, 1107–1120 (2019).
Lan, H., Bao, H., Sun, W. & Liu, S. Multi-scale heterogeneity of rock mass and its mechanical behavior. J. Eng. Geol. 30, 37–52 (2022).
Li, D., Lu, X. X., Yang, X., Chen, L. & Lin, L. Sediment load responses to climate variation and cascade reservoirs in the Yangtze River: A case study of the Jinsha River. Geomorphology. 322, 41–52 (2018).
Bunaciu, A. A., UdriŞTioiu, E. G. & Aboul-Enein, H. Y. X-ray diffraction: Instrumentation and applications. Crit. Rev. Anal. Chem. 45, 289–299 (2015).
Karathanasis, A. D. & Hajek, B. F. Elemental analysis by X-ray fluorescence spectroscopy. Methods Soil. Analysis: Part. 3 Chem. Methods. 5, 161–223 (1996).
Inkson, B. J. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization. Materials characterization using nondestructive evaluation (NDE) methods. Woodhead publishing, pp, 17–43 (2016).
Michalski, R. Principles and applications of ion chromatography. Application of IC-MS and IC-ICP-MS in Environmental Research. pp, 1–46 (2016).
Charles, B. & Fredeen, K. J. Concepts, instrumentation and techniques in inductively coupled plasma optical emission spectrometry. Perkin Elmer Corp. 3, 115 (1997).
Chen, L., Wu, S. & Guo, L. Experimental Study on Initial Damage Point of Deep Granite under Step Cyclic Loading Method. Adv. Civ. Eng. 2021(16), 8846539 (2021).
Ding, Y., Cai, P. & Wen, Z. Electrochemical neutralization energy: From concept to devices. Chem. Soc. Rev. 50, 1495–1511 (2021).
Gran, G., Dahlenborg, H., Laurell, S. & Rottenberg, M. Determination of the equivalent point in potentiometric titrations. Acta chem. Scand. 4, 559–577 (1950).
Gilbert, K., Bennett, P. C., Wolfe, W., Zhang, T. & Romanak, K. D. CO2 solubility in aqueous solutions containing Na+, Ca2+, Cl–, SO42- and HCO3-: The effects of electrostricted water and ion hydration thermodynamics. Appl. Geochem. 67, 59–67 (2016).
Self, D. E. & Plane, J. M. Absolute photolysis cross-sections for NaHCO3, NaOH, NaO, NaO2 and NaO3: Implications for sodium chemistry in the upper mesosphere. Phys. Chem. Chem. Phys. 4, 16–23 (2002).
Oickle, A. M., Goertzen, S. L., Hopper, K. R., Abdalla, Y. O. & Andreas, H. A. Standardization of the Boehm titration: Part II. Method of agitation, effect of filtering and dilute titrant. Carbon. 48, 3313–3322 (2010).
Tang, M. M., Wang, Z. Y., Sun, Y. L. & Ba, J. Experimental study of mechanical properties of granite under low temperature. Chin. J. Rock. Mech. Eng. 29, 787–794 (2010).
Vega, J. A. & Mustain, W. E. Effect of CO2, HCO3- and CO32- on oxygen reduction in anion exchange membrane fuel cells. Electrochim. Acta. 55, 1638–1644 (2010).
Aresta, M. The fixation of carbon dioxide in inorganic and organic chemicals. Energ. Convers. Manage. 34, 745–752 (1993).
Morse, J. W. & Arvidson, R. S. The dissolution kinetics of major sedimentary carbonate minerals. Earth-Sci. Rev. 58, 51–84 (2002).
Wang, S. Study on the feature of argillitization and the evolution lay of formation about weak interlayer in red strata area. Master’s thesis, Chengdu University of Technology. (In Chinese) (2017).
Author information
Authors and Affiliations
Contributions
P.F.: Conceptualization; Formal analysis; Investigation; Methodology; Resources; Supervision; Visualization; Roles/Writing—original draft. P.C.: Supervision; Investigation; Validation; Visualization, Roles/Writing—original draft. S.R.: Resources; Investigation, Validation; Visualization. J.R.: Investigation, Supervision, Roles/Writing—review & editing. Y.D.: Investigation, Roles/Writing—review & editing. G.W.: Investigation, Roles/Writing—review & editing. R.T.: Investigation, Supervision, Roles/Writing—review & editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Feng, P., Cao, P., Ren, S. et al. The mechanical and hydrochemical properties of cemented calcareous soil under long-term soaking. Sci Rep 14, 24532 (2024). https://doi.org/10.1038/s41598-024-75540-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75540-9
Keywords
This article is cited by
-
Mechanical properties and mechanisms of soda residue and fly ash stabilized soil
Scientific Reports (2025)