Abstract
A Right Ear Advantage (REA) is well-established in perceptual tasks but it has been found also during imagery. It is ascribed to the left temporoparietal activity for language, and it can be absent/reversed in some clinical conditions including auditory hallucinations. We applied 1-Hz repetitive TMS over TP3/TP4 (left/right language areas) identified through neuronavigation in 18 healthy participants, before administering a modified white noise (WN) speech illusion paradigm: a voice was presented at one ear, at the same or lower intensities with respect to binaural WN. In some trials the voice was not presented, but participants were anyway instructed to report in which ear they believed perceiving it in all trials. Results confirmed the REA both when the voice was present (perceptual REA) and when it was absent (imaginative REA). Interestingly, results suggested that the correct localization of the voice when the stimulus was ambiguous (presented at low intensity and “masked” by WN) was better when TMS was applied over the right/left hemisphere, in male participants with a low/high proneness to unusual experiences (e.g., auditory hallucinations), respectively. This interaction must be further explored to shed light on the relationship between hemispheric asymmetries and auditory hallucinations, in healthy and clinical samples.
Similar content being viewed by others
Introduction
Since the pioneering discoveries by Broca1, the left-hemispheric superiority for language is a pillar of human cerebral lateralization and it is attributed to both anatomical and functional asymmetries of the two sides of the brain2. This asymmetry is so robust that it is now considered a marker of typical brain development, so that its absence has been related to both cognitive and psychiatric disorders, including autism3 and schizophrenia4. The most known behavioural task used to investigate this asymmetry is the dichotic listening paradigm (DL), in which two different auditory inputs are delivered simultaneously in the two ears by means of headphones5. Exploiting the predominantly crossed organization of the auditory perceptual system in humans, the DL revealed the so-called Right Ear Advantage (REA)6: when asked to report which of the two stimuli is heard better, listeners report more frequently the one presented in the right than in the left ear.
The REA is explained by the left-hemispheric superiority for language, and this model received support also by neuroimaging7 and electrophysiological studies8, showing that the unbalanced activity between the left and the right auditory cortex is responsible for such a bias. Indeed bilateral but not unilateral electrical stimulation of the temporal areas can alter the REA9,10. Importantly, a reduced or even a reversed ear advantage is often described by means of the DL in patients suffering from auditory hallucinations (AHs), further confirming the temporoparietal involvement in this perceptual asymmetry11 (which has been found to be reversed also in dyslexia12). AHs are considered a symptom of psychotic disorders13, but it is less known that they are relatively frequent also in the non-clinical population14, with 4–15% of the healthy individuals experiencing non-clinical AHs15,16. In this view it is important to stress that clinical and non-clinical AHs are described as different in terms of content and emotional impact on the perceiver, with non-clinical AHs reported as less stressful and less negative compared with clinical AHs17. Anyway, it has been proposed that clinical and non-clinical AHs share the same cerebral bases, with an altered hemispheric activity of the linguistic areas18,19. As suggested by Hugdahl20, AHs would be linked to a deficit in prefrontal and cingulate outputs towards the temporal cortex: language areas would thus be disinhibited, and their unexpected activity would constitute the cerebral correlate of perceiving voices in the absence of physical inputs. This model received support also by fMRI studies with healthy participants, showing a left temporal activity (and a subsequent right homologue activity) during silence, and this activity has been described as a kind of default mode network of auditory processing21,22. To summarize, AHs should be due to a failure in the top-down inhibition of the frontal areas on the temporal cortex20.
Coming back to the REA, the bias has been also found in pure imagery tasks, further confirming a shared cerebral basis among perception, imagery and possibly AHs: when asked to imagine hearing a voice lateralized in one ear, healthy participants reported more frequently to imagine it in the right than in the left ear23, at least when it contained a neutral or positive emotional valence24. Although no imaging evidence for such an “illusory/imaginative REA” exists in the literature, it is plausible to hypothesize it is based on the same left-hemispheric activity of the linguistic areas, similarly to the perceptual REA (i.e., default mode auditory network21,22). According to the same idea, patients suffering from clinical AHs do not show the imaginative REA25. Finally, interesting results have been also described in a condition experimentally created to investigate the borderline condition between imagery and perception in healthy participants, namely the white noise (WN) speech illusion paradigm, in which WN is presented either alone, or superimposed to a voice: the illusion consists in presenting the voice at different intensities (higher, same and lower with respect to the intensity of WN stream), or to not present it at all, and to ask participants to detect the voice, without informing listeners that the voice could be absent in some trials. Exploiting a WN speech illusion paradigm during transcranial Direct Current Stimulation (tDCS), a study revealed that anodal/cathodal stimulation on the left posterior superior temporal gyrus enhanced/reduced false alarms in detecting a voice in WN stream, respectively, confirming the direct involvement of the left linguistic areas also in auditory illusions26. Moreover, in a modified version of the paradigm, WN was binaurally presented either alone or superimposed to a voice presented lateralized in one ear (at different intensities)27: the expected REA was found when the voice was clearly distinguishable from WN (perceptual REA), but – importantly – it was also found when it was difficult to be perceived (low volume) or it was even absent (imaginative REA), thus confirming by means of a behavioural task the main involvement of the left hemisphere also in auditory imagery.
To investigate the causal involvement of the temporal cortex in clinical AHs, Transcranial Magnetic Stimulation (TMS) has been widely exploited28,29,30,31. In a pioneering study with three patients, it was found that 1-Hz repetitive TMS (rTMS) applied on the left temporoparietal cortex reduced the severity of AHs in all patients, and it abolished AHs until two weeks in two out of the three patients32, and this evidence was then confirmed with wider samples28,29,33.
Interestingly, an fMRI study revealed that the activity of the right homologue of Wernicke’s area after patients had experienced AHs was highly correlated with the subjective attentional salience of AHs themselves34: starting from this result, the effect of 1-Hz rTMS applied over the left and the right temporoparietal cortex was investigated by Hoffman and colleagues, who showed that the inhibitory stimulation on the left hemisphere was most efficient in affecting low salience AHs, but surprisingly rTMS applied on the right hemisphere was most efficient in reducing high salience AHs in a sample of schizophrenic patients31, even if contrasting results have been collected in different studies35,36.
There is no evidence on the possible effects of rTMS on the REA in patients, and little is known about the possible effects of the same stimulation setup also in healthy participants during WN speech illusion paradigms, auditory imagery or even dichotic listening. To the best of our knowledge, only one study explored the effect of rTMS on the REA by means of a classical DL task with 18 healthy participants37: to ensure that the noise caused by TMS did not interfere with the auditory task, 1-Hz rTMS was applied offline, immediately before the DL paradigm, for 10 min. It was applied on the planum temporale of the left hemisphere and on the homologue contralateral area; a sham-control condition was also applied by means of a placebo coil reducing magnetic field strength by 80% (either on the left or right temporal areas). The REA was not different between left-hemispheric stimulation and the sham condition, suggesting a main role of the right (instead of the left) temporoparietal areas in perceptual REA in healthy participants. The authors proposed different possible explanations for the absence of an effect of left-hemispheric stimulation on the REA, for instance they suggested that rTMS might be too focal to affect the activity of the left planum temporale, which has been shown to be strongly and widely activated during DL37. Another interesting study in this field exploited a different stimulation technique, which is the transcranial Static Magnetic Field Stimulation (tSMS)38, which induces focal changes in cortical activity by means of cylindrical permanent magnets over the scalp39. In this case, 14 healthy participants received tSMS on the primary auditory cortex (T7/T8) during the DL task, and results revealed a reduced REA during left-hemispheric than right-hemispheric stimulation. Moreover, EEG recordings on the same sample confirmed lower N1 amplitude after left tSMS compared to the other conditions (no difference emerged on N1 latency and P50 amplitude and latency). The discordance between the two studies reviewed here could be due to both the different magnetic stimulation techniques, and to the different target areas stimulated (associative vs primary auditory cortices). To conclude, however, the causal involvement of the left vs right temporoparietal areas in the REA remains debated.
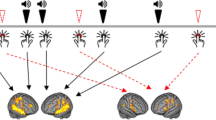
Starting from this framework, we decided to explore the possible effect of the TMS protocol most used with patients suffering from AHs (1-Hz rTMS on the linguistic areas; see Fig. 1), but in a non-clinical sample, during a lateralized WN speech illusion paradigm. The aim was to shed light on the role of the left vs right linguistic areas in both the perceptual and the imaginative REA, and to this aim we exploited the same paradigm already used with healthy participants27, adding left/right TMS before the experimental sessions. The only difference with respect to the paradigm already used27 is the exclusion of one of the intensities exploited in the original study, namely that in which the voice was presented with a higher intensity with respect to WN, because in this condition the REA was so strong that we did not expect a modulation due to the stimulation. Indeed, we expected a perceptual REA in the conditions in which the voice was clearly perceived (same intensity as bilateral WN), and also in the conditions in which the voice was hardly perceived (lower intensities than bilateral WN), or it was even absent (illusory REA), as already found in the previous behavioral study27. Importantly, considering that 1-Hz rTMS on the left linguistic cortex reduced AHs symptomatology in patients28,29,32, being associated to inhibitory effects, we hypothesized that 1-Hz rTMS applied over the left/right temporoparietal linguistic areas in healthy participants (TP3/TP4) would lead to a weaker/stronger REA, respectively. In fact, if in the clinical population the inhibitory effects of 1-Hz rTMS on the left hemisphere reduce AHs (which are related to the spontaneous hyperactivity of the left temporal cortex), we can hypothesize that in healthy participants it would inhibit the left-hemispheric activity responsible for the perceived and the imaginative REA (and thus it would weaken the REA), and that the contralateral stimulation would indeed enhance the REA (see Fig. 1). As in the study by Hirnstein and colleagues37, here we used an offline protocol to avoid the influence of the TMS noise on the auditory task, and we decided to stimulate at 90% of the individual threshold, as suggested by the safety guidelines on AHs patients29.
Finally, we also administered a questionnaire measuring participants’ susceptibility to hallucinatory experiences, to shed light on the role of each hemisphere in the proneness to non-clinical AHs. Specifically, starting from the evidence of stronger effects of a left vs right-hemispheric stimulation in affecting low vs high salience AHs in schizophrenia patients31, we expected a stronger REA or a lower difficulty in correctly localizing the voice in the WN stream during the right-hemispheric stimulation in healthy participants with higher (vs lower) proneness to experience AHs.
Results
Overall analysis: perceived and imaginative REA
The percentage of “right ear” responses for both the left TMS (l-TMS) and the right TMS (r-TMS) session in each of the three conditions (WN: binaural WN; LE: binaural WN superimposed to a voice presented in the Left Ear; RE: binaural WN superimposed to a voice presented in the Right Ear) was compared with the chance level (50%) by means of exact t-tests (significance threshold = 0.017 after Bonferroni correction for multiple comparisons in each session). Data are provided in the Supplementary information file, and as it can be seen in Fig. 2, results confirmed that participants correctly localized the voice in the RE both in the l-TMS (t(17) = 8.19, p < 0.001) and in the r-TMS session (t(17) = 13.93, p < 0.001), and they correctly localized the voice presented in the LE both during the l-TMS (t(17) = − 6.82, p < 0.001) and r-TMS (t(17) = -6.79, p < 0.001). Importantly, results showed that in the absence of vocal stimuli, namely in the WN condition, participants localized the voice more frequently in the right ear than expected by chance, both during the l-TMS (t(17) = 3.29, p = 0.004) and the r-TMS session (t(17) = 4.44, p < 0.001), confirming an imaginative REA independently of the side of stimulation.
Percentage of “right ear” responses in the Left Ear (LE), the Right Ear (RE) and the White Noise (WN) conditions. All comparisons between each condition (LE, RE, WN) in both the left TMS (l-TMS) and the right TMS (r-TMS) sessions were significantly different from the chance level (50%; p < 0.005). Bars represent standard errors.
Then, an analysis of variance (ANOVA) was carried out by using Sex of participants (female, male) and propensity to unusual experience as measured by O-LIFE (low, high) as between-subjects factors, and using Stimulation (l-TMS, r-TMS) and Condition (LE, RE, WN) as within-subjects factors. The percentage of “right ear” responses was used as the dependent variable (see Supplementary information). The main effect of Condition was significant (F(2,28) = 103.01, MSE = 26,263.2, p < 0.001, ηp2 = 0.88), and all post-hoc comparisons, carried out by means of Duncan test, were significant (all ps < 0.001): the percentage of “right ear” responses was higher in the RE condition (83.22% ± 1.74) with respect to both the LE condition (28.18% ± 2.2) and the WN condition (67.22 ± 4.47), and it was significantly lower in the LE condition than in the WN condition.
The interaction between Sex of participants and O-LIFE was significant (F(1,14) = 6.24, MSE = 2471.8, p = 0.025, ηp2 = 0.31). Post-hoc comparisons revealed that in male participants, the percentage of “right ear” responses was higher in the subsample scoring high (62.72% ± 3.57) rather than low in the O-LIFE (49.15% ± 3.48; p = 0.033). No other main effects and interactions reached significance.
Analysis on vocal targets
To further explore the laterality bias during voice presentation, as in Prete and colleagues27 (same paradigm), in a second ANOVA only LE and RE conditions were considered (WN condition was excluded): Sex of participants (female, male) and O-LIFE (low, high) were used as between-subjects factors, Stimulation (l-TMS, r-TMS), Ear (left, right), Intensity (42 dB, 48 dB, 60 dB), and Sex of voice (female, male) were used as within-subject factors. Since in each trial included in this analysis, participants can give a correct or incorrect response (the voice being presented in all trials), the analysis was carried out considering the Inverse Efficiency Score (IES) as the dependent variable27 (see also40,41). IES was obtained by dividing response times comprised between 500 and 1500 ms for correct responses by the proportion of correct responses in each condition, so that lower scores correspond to a better and faster performance (see Supplementary information).
Intensity was significant (F(2,28) = 64.24, MSE = 15,846,002, p < 0.001, ηp2 = 0.82), and post-hoc comparisons showed that the performance was better for the higher intensity (60 dB: 827.24 ± 33.21) with respect to 48 dB (996.86 ± 38.82; p = 0.007) and 42 dB (1494.8 ± 81.32; p < 0.001), and it was better for voices presented at 48 dB than 42 dB (p < 0.001). Ear did not reach significance as main effect (F(1,14) = 4.33, p = 0.056), but it significantly interacted with Intensity (F(2,28) = 5.09, MSE = 2,179,351, p = 0.013, ηp2 = 0.27; Fig. 3). Post-hoc comparisons confirmed that the performance was better in the RE than in the LE condition with the lowest intensity (42 dB, p < 0.001). Moreover, the performance was worse with intensity 42 dB than both other intensities for the LE condition (ps < 0.001 for all comparisons; 48 dB vs 60 dB: p = 0.061), and for the RE condition (42 dB > 48 dB: p = 0.014, and 42 dB > 60 dB: p = 0.001; 48 dB vs 60 dB: p = 0.326).
The interaction between Intensity and Sex of voice was significant (F(2,28) = 4.74, MSE = 924,910, p = 0.017, ηp2 = 0.25), and post-hoc comparisons showed that only with the lowest intensity (42 dB), the performance was better with male than with female voices (p = 0.006). Moreover, with both female and male voices it was worse for stimuli presented at 42 dB than at 48 and 60 dB (all ps < 0.001), and only for male voice it was worse at 48 than at 60 dB (p = 0.008).
The interaction among Stimulation, Intensity, Sex of participants and O-LIFE was significant (F(2,28) = 4.03, MSE = 613,263, p = 0.029, ηp2 = 0.22; Fig. 4). Post-hoc comparisons confirmed that in each subgroup the performance was worse for stimuli presented in each ear when voices were presented at 42 dB than at 48 and 60 dB (only for male participants with high O-LIFE scores the comparison between voices presented in the left ear at 42 and 48 dB did not differ significantly). Importantly, in the male subsample, when the voice was presented at the lowest intensity (42 dB), the performance was better when TMS was applied over the right than the left hemisphere in participants with lower O-LIFE scores (p < 0.001), but it was worse when TMS was applied over the right than the left hemisphere for participants with higher O-LIFE scores (p = 0.003).
Interaction among Sex of participants (females: left panel; males: right panel), O-LIFE (Low scores, High scores), Intensity of the voice (42 dB, 48 dB, 60 dB), and Stimulation (left TMS: white columns; right TMS: black columns) on the Inverse Efficiency Score. Bars represent standard errors and asterisks show the most important significant comparisons (p < 0.05). Note that in each comparison 42 dB < 48 dB < 60 dB, except for the comparison between 42 and 48 dB for males with high O-LIFE scores.
Finally, a series of exact t-tests were carried out on the percentage of accuracy in the RE condition subtracted to that of the LE condition for each intensity and for female and male voices separately (see27). The resulting scores were compared against 0 (absence of right-left asymmetry) and after the Bonferroni correction for multiple comparisons the threshold of significance was set at p = 0.008 for the l-TMS and for the r-TMS condition. None of the comparisons reached significance for l-TMS, whereas in the r-TMS condition the result was significantly higher than the chance level (showing a REA) for female and male voices presented at 42 dB (female: t(17) = 3.33, p = 0.004; male: t(17) = 4.49, p = 0.003) and for female voices presented at 48 dB (t(17) = 4.19, p < 0.001).
Correlation analysis
A Laterality Index (LI) was calculated by using the formula (R – L)/(R + L) X 100, where R and L correspond to the accuracy in the RE and in the LE condition, respectively. LI, in which higher scores correspond to a stronger perceptual REA, was calculated separately for l-TMS and for r-TMS session and it was correlated with the percentage of “right ear” responses in the WN condition (in which the voice was not presented: imaginative REA). As in Prete and colleagues27, a positive correlation emerged in both the l-TMS (r = 0.68, p = 0.002) and in the r-TMS (r = 0.73, p < 0.001; see Fig. 5), confirming that a higher perceptual REA when the vocal stimulus is present corresponds to a higher imaginative REA in the absence of vocal stimuli.
Discussion
The aim of the present study was to assess the causal involvement of the left vs right temporoparietal linguistic areas in perceived and imaginative REA. To this aim, a lateralized White Noise speech illusion paradigm was used: although the voice was not always present, participants were forced to report its presentation side within a binaural WN stream. Besides confirming the REA both when the vocal stimulus was clearly perceived and when it was attenuated (presented at low intensity), the results also confirmed the imaginative REA in healthy participants, with a higher frequency of “right ear” responses during the presentation of the sole WN stream, as already shown in a previous behavioural study27, in pure imagery tasks23,24, and as statistically confirmed here by means of exact t-tests. Moreover, the first ANOVA carried out here showed that participants correctly localized the vocal stimuli in the WN speech illusion paradigm, with a higher percentage of right ear responses when the voice was really present in the right ear than when it was not present at all (WN condition), and with a lower percentage of right ear responses when the voice was present in the left ear than when it was not presented. Importantly, however, no direct effect of inhibitory low frequency rTMS applied on the left/right temporoparietal cortex emerged: our results did not reveal a main effect of stimulation on the performance of the healthy participants tested. This evidence contrasts with the results found in a DL task with the same rTMS protocol applied on the planum temporale37, in which it was found that right-hemispheric stimulation significantly reduced the REA. It must be underlined that a study exploiting transcranial Static Magnetic Field Stimulation (tSMS)38 on the primary auditory cortex revealed a reduced REA during left- instead of right-hemispheric stimulation during DL. In the present study we did not target the primary auditory cortex, but we decided to focally apply an inhibitory stimulation on TP3 and TP4, namely the temporoparietal cortex of each hemisphere. This choice was due to the literature revealing positive effects of such a stimulation in patients suffering from clinical AHs28,29,32, but no direct evidence with healthy samples was collected so far, definitely not by means of WN speech illusion paradigms. Our main conclusion, indeed, is that neither the left nor the right temporoparietal inhibition is sufficient to modulate the perceptual and the imaginative REA in healthy participants, and this conclusion is in line with findings deriving from electrical stimulation studies9,10. Interestingly, when tRNS was applied simultaneously on the left and on the right auditory cortex (bilateral stimulation), the REA was enhanced, suggesting the concurrent involvement of both hemispheres at the basis of this asymmetry in healthy participants9. Anyway, beyond the classical DL task, when tDCS was applied on the auditory cortex during a WN speech illusion paradigm, more false alarms were recorded during the anodal stimulation of the left hemisphere, thus indicating that the hyperactivity of the left-linguistic areas can be related to vocal illusory perceptions26, indirectly confirming the evidence collected with patients hearing voices34. It must be considered that electrical stimulation exploits large electrodes, so that the spatial resolution of this stimulation technique is very low, whereas TMS allows us to target a specific area with a higher spatial resolution, and we can conclude that this kind of stimulation could be too focal to alter the well-established REA in healthy participants, as already suggested by Hirnstein and colleagues37.
The results of the analysis carried out considering the intensity of the vocal stimuli (excluding the WN condition) confirmed that in our sample the performance was higher for vocal stimuli presented in the right than in the left ear, mainly when the voice was presented at lower intensity and thus it was covered by binaural WN stream. This means that when the vocal input is attenuated by noise, healthy participants localize it more frequently in the right ear (showing a kind of illusory REA), again suggesting that the activity of the left-hemispheric linguistic areas is crucial in the proposed default mode network which has been suggested to be activated also during silence21,22. Nevertheless, exact t-tests carried out to assess the possible behavioural asymmetry during the lateralized stimulation showed that the inhibition of the left temporoparietal cortex did not lead to a modulation of the REA, whereas when rTMS was applied on the right linguistic areas, the REA increased, mainly for voices presented at intensities lower than the WN stream (42 and 48 dB). This interesting evidence suggests that the inhibition of the right linguistic areas is most effective in altering the REA with respect to the inhibition of the left temporoparietal cortex, further confirming that this lateral bias is due to the unbalanced activity of the two hemispheres, more than to the hyperactivity of the left one, at least in healthy participants. We could speculate that the hypoactivity of the right hemisphere due to 1-Hz rTMS leads to a weaker inhibitory control of this region on the contralateral homologue area, and that this resulting stronger activity of the left linguistic area is responsible for the strongest REA found here. This idea agrees with the structural model5 according to which the REA is due to the specialization of the left-hemispheric temporal cortex for language (see Fig. 1). Finally, correlation analysis confirmed the previous evidence of a positive relationship between perceptual and illusory REA, with higher preference for the right ear during the real presentation of the voice being correlated to a right ear preference even in the absence of physical stimuli27. Also this finding is in line with the idea of a default mode auditory network21,22: in participants in whom this network, leftward lateralized, is strongly activated, the REA is increased both in presence and in absence of physical inputs, as already proposed by Prete and colleagues27.
Besides the main aim of the study, a further explorative hypothesis was that the propensity to experience non-clinical AHs could impact the results. To assess this possibility, we measured the tendency to experience unusual events, including AHs, by means of a standardized questionnaire, and we used the scores obtained in this measure as a between-subject factor. We found that the percentage of right ear responses was enhanced in male participants with higher propensity to experience AHs, as measured by means of the O-LIFE subscale42. This interaction seems to suggest that, at least in males, a higher susceptibility to non-clinical AHs could be linked to an enhanced REA, possibly suggesting a stronger activity of the left-linguistic area in this subsample, independently of the stimulation. This speculation must be further explored, but it seems in line with the enhanced activity of the left linguistic areas described in patients suffering from clinical AHs43. Furthermore, we hypothesized a stronger REA and/or a better performance in localizing the voices during the right-hemispheric stimulation in participants with higher proneness to AHs, mainly starting from the evidence of stronger right-hemispheric stimulation effects in affecting high salience AHs in patients31. Results revealed a complex interaction, showing that only in males and only when the voice was very ambiguous because presented at the lowest intensity, the performance was enhanced (correct localization of vocal stimuli) when TMS was applied over the right than the left hemisphere but in participants with low O-LIFE scores, and the opposite pattern emerged for participants with high O-LIFE scores (left-hemispheric TMS enhanced their performance with respect to right-hemispheric stimulation). In this regards only speculations can be proposed due to the very low numerosity of the subsamples called into question, but it is intriguing to hypothesize that, at least for males, on the one hand the inhibition of the left temporoparietal cortex leads to a better performance in correctly localizing ambiguous voices in participants with a high proneness to non-clinical AHs, confirming also in a WN speech illusion paradigm the direct involvement of the left hemisphere in the manifestation of AHs. On the other hand, the inhibition of the right homologue area would lead to a better performance in correctly localizing the same ambiguous voices in participants with a scarce proneness to AHs, confirming in the same WN speech illusion paradigm the main role of the right hemisphere in this kind of task for participants who do not show a propensity to AHs. This speculation must be further explored in future studies, but it could contribute to explain the complex relationship between hemispheric asymmetries and auditory perception/illusions: as specified above, in fact, it is quite surprising that the most acknowledged models of AHs attributed these phenomena to a hyperactivity of the left-linguistic cortex, but at the same time the absence of the (expected) REA is described in this clinical population. We can figure out that a different pattern of inter-hemispheric organization is the key for this inconsistency: maybe, the higher proneness to experience AHs makes the cerebral activity of this subgroup more similar to that of patients with clinical AHs, so that the inhibition of the left-linguistic area leads to a better performance in correctly localizing vocal stimuli (i.e., turning off aberrant perceptions and with a consequent better overall performance). This could be viewed as linked to the positive effects on AHs symptomatology described in the clinical population following 1-Hz rTMS applied over the left temporoparietal areas28,29,32, at least for low salience AHs, which could be considered similar to non-clinical AHs, being less intrusive both in thoughts and behaviours compared with high salience ones31. The fact that this result is present only in males makes it even more speculative, so that only hypothetical assumptions can be advanced and future research with both clinical and non-clinical samples is needed to shed light on these possibilities.
Conclusions
To conclude, the present results confirmed the expected perceptual and illusory Right Ear Advantage in a sample of healthy participants during a lateralized WN speech illusion task, also confirming their positive correlation, with a stronger perceptual bias positively correlated to a stronger illusory bias. Moreover, a clear effect of left vs right temporoparietal inhibition due to 1-Hz rTMS on the auditory performance did not emerge, confirming previous findings with electrical stimulation. This evidence showed that neither of the two hemispheres is solely responsible for the REA in the WN speech illusion task, but a complex relationship is suggested among the language-related activity of the two hemispheres and the proneness to experience AHs, at least in males, when the vocal stimuli are ambiguous and confused in a noise stream. This interaction must be explored in future studies, and it could help bridge the cerebral basis between clinical and non-clinical AHs.
Materials and methods
Participants
A sample of 20 right-handed healthy participants took part in the study. Two participants were excluded from the analysis due to technical issues, so that the final sample was composed of 18 participants (10 females; same numerosity exploited in a previous TMS study with a healthy sample37). The mean age of the sample was 20.5 years (± 0.7 years) and all of the participants were right-handers, with a mean handedness score of 70.63 (± 4.38), as measured by means of the Edinburgh Handedness Inventory44 (-100 corresponds to a complete left preference and + 100 corresponds to a complete right preference). Participants completed the short version of the Unusual Experiences scale of the Oxford-Liverpool Inventory of Feelings and Experiences questionnaire (O-LIFE42), composed of 12 items exploring perceptual aberrations and hallucinations (see also24,25). The final score of the scale used can range between 0 and 12, with higher scores being related to the positive symptoms of psychosis, and the mean score of the sample was 5.5 (± 0.5). Participants were divided into two subgroups depending on the O-LIFE score: ten participants obtained a final score lower than the mean of the sample (Low O-LIFE: scores between 0 and 5; 6 females) and eight participants obtained a final score higher than the mean of the sample (High O-LIFE: scores between 6 and 9; 5 females).
All participants signed a written informed consent to take part in the study, and they were enrolled if they did not report any auditory impairment and/or psychiatric and neurological conditions. An audiometric assessment already used in previous studies with a similar paradigm27 was performed prior the beginning of the task: a female voice pronouncing the phoneme /a/ was presented repeatedly in each ear via earphones, with increased intensities (2 dB steps) and participants had to press a button when the voice became perceivable. Participants were recruited when no different hearing thresholds were present between left and right ear (Δ = 10 dB). The study conformed to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Psychology of the Department of Psychological, Health and Territorial Sciences – University “G. d’Annunzio” of Chieti-Pescara (protocol number: IRBP/22,011).
Transcranial magnetic stimulation
Each participant performed two separate sessions of 8-min repetitive Transcranial Magnetic Stimulation (rTMS): a left-hemispheric stimulation session (l-TMS) and a right-hemispheric stimulation session (r-TMS), and the order of sessions was balanced among participants. Two active sessions were used and compared to one another instead of an active session and a no-stimulation session (e.g., vertex stimulation or coil orientation), to avoid a difference between sessions due to the noise produced by TMS. Due to the auditory task exploited in the present study, in fact, the noise produced by the stimulation could affect the performance, indeed we preferred to compare left vs right active temporal stimulation. The repetitive TMS train was delivered with 8-min duration, 1-Hz frequency, and intensity set at 90% of the individual motor threshold, following previous TMS studies in patients suffering from AHs29, as well as safety guidelines45. This TMS setup had been shown to inhibit the target cortical area for a period corresponding at least to that of the stimulation46,47,48.
The location of the two stimulation sites was automatically identified on the participant’s scalp using the SofTaxic navigator system (E.M.S. Italy, www.emsmedical.net), which uses a set of digitized skull landmarks (nasion, inion, and two pre-auricular points), and about 40 scalp points entered with a Fastrak Polhemus digitizer system (Polhemus), and an averaged stereotaxic MRI atlas brain in Talairach space49. The average Talairach coordinates in the SofTaxic navigator system were transformed through a linear transformation to each individual participant’s scalp. This strategy has been successful in previous rTMS studies50,51,52. Target areas were chosen in accordance with previous studies on AHs using 1-Hz rTMS in clinical populations28, and were as follows for TP3: x = − 63.88, y = − 47.19, z = + 33.9553.
Stimuli
Stimuli were the same as those used in a previous study without brain stimulation27: a white noise stream (WN) was created by using GoldWave v5.25 software (GoldWave Inc., Canada), lasting 250 ms and presented at 60 dB. Four female and four male voices were recorded, and the phoneme /a/ was presented for 250 ms at 60 dB. A linear fade-in lasting 50 ms and a linear fade-out lasting 70 ms were added to both WN and vocal stimuli. Then, vocal stimuli were manipulated to obtain two other intensities lower than the original voice (60 dB), namely 48 dB and 42 dB.
The final set of stimuli consisted of WN at 60 dB presented binaurally, to which a voice could be superimposed either in the left or in the right channel: each of the eight voices was superimposed to the WN trace (always presented binaurally at 60 dB), at each of the 3 intensities (42 dB, 48 dB, 60 dB) in the left and in the right channel, separately (8 vocal stimuli × 3 intensities × 2 channels = 48 stimuli). Moreover, WN was also presented in isolation (60 dB, binaural presentation).
Procedure
Participants were tested in isolation in a quiet room, in which no external auditory inputs could be heard. They were asked to wear headphones, to seat in front of a computer screen and to gaze at a fixation cross presented in the center of the screen for the whole duration of the task. In each trial, after 500 ms in which only the cross was present, an auditory stimulus was delivered (duration: 250 ms). Participants were instructed to localize the voice as perceived in the left ear or in the right ear, by pressing “z” with the left hand and “m” with the right hand respectively, after which the following trial started. Participants were informed that a female or male voice would be presented lateralized together with a noise presented in both ears, and that the voice could be presented at different volumes so that in some trials it could be difficult to distinctively hear it, but that they had anyway to localize the voice in each trial. They were also asked to be fast and to give the first response they believed to be correct.
The task was controlled by means of E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA) and it was composed of 72 stimuli, repeated 4 times, for a total of 288 trials. Among the original 72 stimuli, 24 were WN presented in both ears without any superimposed voice (WN condition), 24 were binaural WN superimposed to a voice presented in the Left Ear (LE condition), and 24 were binaural WN superimposed to a voice presented in the Right Ear (RE condition). In both LE and RE conditions, voices were presented at each of the three intensities (42 dB, 48 dB, 60 dB). The order of stimuli was randomized within and among participants. Each participant took part in two distinct sessions spaced at least 20 min apart, in which rTMS was applied either over the left or the right temporoparietal cortex, and the order of the sessions was balanced among participants.
Prior to the beginning of each session, four trials were presented to familiarize with the procedure. After the end of the task, participants were invited to complete the Unusual Experiences scale and the Edinburg Handedness Inventory, and then they were debriefed.
Data availability
The datasets generated during and/or analysed during the current study are available in the Supplementary file.
References
Broca, P. Sur le principe des localizations cérébrale. Bulletin de la Société d’Anthropologie 2, 190–204 (1861).
Tervaniemi, M. & Hugdahl, K. Lateralization of auditory-cortex functions. Brain Res. Rev. 43, 231–246 (2003).
Finch, K. H., Seery, A. M., Talbott, M. R., Nelson, C. A. & Tager-Flusberg, H. Lateralization of ERPs to speech and handedness in the early development of Autism Spectrum Disorder. J. Neurodevel. Disord. 9, 4 (2017).
Ocklenburg, S., Güntürkün, O., Hugdahl, K. & Hirnstein, M. Laterality and mental disorders in the postgenomic age—–A closer look at schizophrenia and language lateralization. Neurosci. Biobehav. Rev. 59, 100–110 (2015).
Kimura, D. Functional asymmetry of the brain in dichotic listening. Cortex 3, 163–178 (1967).
Kimura, D. Some effects of temporal-lobe damage on auditory perception. Can. J. Psychol. 15, 156–165 (1961).
Hirnstein, M., Westerhausen, R., Korsnes, M. S. & Hugdahl, K. Sex differences in language asymmetry are age-dependent and small: A large-scale, consonant-vowel dichotic listening study with behavioral and fMRI data. Cortex 49, 1910–1921 (2013).
Eichele, T., Nordby, H., Rimol, L. M. & Hugdahl, K. Asymmetry of evoked potential latency to speech sounds predicts the ear advantage in dichotic listening. Cognitive Brain Res. 24, 405–412 (2005).
Prete, G., D’Anselmo, A., Tommasi, L. & Brancucci, A. Modulation of the dichotic right ear advantage during bilateral but not unilateral transcranial random noise stimulation. Brain Cogn. 123, 81–88 (2018).
D’Anselmo, A., Prete, G., Tommasi, L. & Brancucci, A. The dichotic right ear advantage does not change with transcranial direct current stimulation (tDCS). Brain Stimul. 8, 1238–1240 (2015).
Hugdahl, K. et al. Auditory verbal hallucinations in schizophrenia as aberrant lateralized speech perception: evidence from dichotic listening. Schizophr. Res. 140, 59–64 (2012).
Beaton, A. A. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender, and dyslexia: A review of the evidence. Brain Language 60, 255–322 (1997).
Larøi, F. et al. The characteristic features of auditory verbal hallucinations in clinical and nonclinical groups: State-of-the-art overview and future directions. Schizophrenia Bull. 38, 724–733 (2012).
Hill, K. & Linden, D. E. J. Hallucinatory Experiences in Non-clinical Populations. In The Neuroscience of Hallucinations (ed. Jardri, R.) (Springer, 2013).
Beavan, V. Towards a definition of “hearing voices”: A phenomenological approach. Psychosis 3, 63–73 (2011).
Beavan, V., Read, J. & Cartwright, C. The prevalence of voice-hearers in the general population: A literature review. J. Mental Health 20, 281–292 (2011).
Daalman, K. et al. The same or different? A phenomenological comparison of auditory verbal hallucinations in healthy and psychotic individuals. J. Clin. Psychiatry 72, 320–325 (2011).
Raij, T. T. & Riekki, T. J. J. Poor supplementary motor area activation differentiates auditory verbal hallucination from imagining the hallucination. Neuroimage Clin. 1, 75–80 (2012).
van de Ven, V. & Linden, D. E. J. The role of mental imagery in aberrant perception: A neurobiological perspective. J. Exp. Psychopathol. 3, 274–296 (2012).
Hugdahl, K. ‘Hearing voices’: auditory hallucinations as failure of top-down control of bottom-up perceptual processes. Scand. J. Psychol. 50, 553–560 (2009).
Hunter, M. D. et al. Neural activity in speech-sensitive auditory cortex during silence. Proc. Natl. Acad. Sci. U. S. A. 103, 189–194 (2006).
Kraemer, D. J. M., Macrae, C. N., Green, A. E. & Kelley, W. M. Musical imagery: sound of silence activates auditory cortex. Nature 434, 158 (2005).
Prete, G., Marzoli, D., Brancucci, A. & Tommasi, L. Hearing it right: Evidence of hemispheric lateralization in auditory imagery. Hear Res. 332, 80–86 (2016).
Prete, G., Tommasi, V. & Tommasi, L. Right news, good news! The valence hypothesis and hemispheric asymmetries in auditory imagery. Lang. Cogn. Neurosci. 35, 409–419 (2020).
Altamura, M. et al. Do patients with hallucinations imagine speech right?. Neuropsychologia 146, 107567 (2020).
Moseley, P., Fernyhough, C. & Ellison, A. The role of the superior temporal lobe in auditory false perceptions: A transcranial direct current stimulation study. Neuropsychologia 62, 202–208 (2014).
Prete, G., D’Anselmo, A., Brancucci, A. & Tommasi, L. Evidence of a Right Ear Advantage in the absence of auditory targets. Sci. Rep. 8, 15569 (2018).
Hoffman, R. E. et al. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet 355, 1073–1075 (2000).
Hoffman, R. E. et al. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol. Psychiatry 58, 97–104 (2005).
Hoffman, R. E. et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch. General Psychiatry 60, 49–56 (2003).
Hoffman, R. E. et al. Transcranial magnetic stimulation of Wernicke’s and Right homologous sites to curtail ‘voices’: A randomized trial. Biol. Psychiatry 73, 1008–1014 (2013).
Hoffman, R. E. et al. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices”. Biol. Psychiatry 46, 130–132 (1999).
Hoffman, R. E. & Cavus, I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. Am. J. Psychiatry 159, 1093–1102 (2002).
Hoffman, R. E., Pittman, B., Constable, R. T., Bhagwagar, Z. & Hampson, M. Time course of regional brain activity accompanying auditory verbal hallucinations in schizophrenia. Br. J. Psychiatry 198, 277–283 (2011).
Bais, L. et al. Short and Long Term Effects of Left and Bilateral Repetitive Transcranial Magnetic Stimulation in Schizophrenia Patients with Auditory Verbal Hallucinations: A Randomized Controlled Trial. PLoS One 9, e108828 (2014).
Bais, L. et al. Effects of low frequency rTMS treatment on brain networks for inner speech in patients with schizophrenia and auditory verbal hallucinations. Progress Neuro-Psychopharm. Biol. Psychiatry 78, 105–113 (2017).
Hirnstein, M., Westerhausen, R. & Hugdahl, K. The right planum temporale is involved in stimulus-driven, auditory attention–evidence from transcranial magnetic stimulation. PLoS One 8, e57316 (2013).
Heimrath, K., Spröggel, A., Repplinger, S., Heinze, H.-J. & Zaehle, T. Transcranial static magnetic field stimulation over the temporal cortex modulating the right ear advantage in dichotic listening. Neuromodulation 23, 335–340 (2020).
Rivadulla, C., Foffani, G. & Oliviero, A. Magnetic field strength and reproducibility of neodymium magnets useful for transcranial static magnetic field stimulation of the human cortex. Neuromodulation 17, 438–441 (2014).
Prete, G., Malatesta, G. & Tommasi, L. Facial gender and hemispheric asymmetries: A hf-tRNS study. Brain Stimul. 10, 1145–1147 (2017).
Prete, G., Fabri, M., Foschi, N. & Tommasi, L. Asymmetry for symmetry: right-hemispheric superiority in bi-dimensional symmetry perception. Symmetry 9, 76 (2017).
Mason, O. & Claridge, G. The Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE): further description and extended norms. Schizophr. Res. 82, 203–211 (2006).
Hugdahl, K., Løberg, E.-M. & Nygård, M. Left temporal lobe structural and functional abnormality underlying auditory hallucinations in schizophrenia. Front. Neurosci. 3, 34–45 (2009).
Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Rossi, S., Hallett, M., Rossini, P. M. & Pascual-Leone, A. Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 120, 2008–2039 (2009).
Pascual-Leone, A. et al. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. 15, 333–343 (1998).
Capotosto, P., Babiloni, C., Romani, G. L. & Corbetta, M. Resting-state modulation of α rhythms by interference with angular gyrus activity. J. Cogn. Neurosci. 26, 107–119 (2014).
Altomare, E. C., Committeri, G., Di Matteo, R., Capotosto, P. & Tosoni, A. Automatic coding of environmental distance for walking-related locomotion in the foot-related sensory-motor system: A TMS study on macro-affordances. Neuropsychologia 150, 107696 (2021).
Talairach, J. & Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System : An Approach to Cerebral Imaging. (G. Thieme, 1988).
Prete, G. et al. The role of the right supramarginal gyrus in time estimation: A TMS study. Clin. Neurophysiol. 156, 16–18 (2023).
Spadone, S., Sestieri, C., Baldassarre, A. & Capotosto, P. Temporal dynamics of TMS interference over preparatory alpha activity during semantic decisions. Sci. Rep. 7, 2372 (2017).
Capotosto, P., Sulpizio, V., Galati, G. & Baldassarre, A. Visuo-spatial attention and semantic memory competition in the parietal cortex. Sci. Rep. 13, 6218 (2023).
Wang, Y. et al. Heterogeneous brain abnormalities in schizophrenia converge on a common network associated with symptom remission. Schizophr. Bull. 50, 545–556 (2024).
Funding
This project is funded by the BIAL foundation (grant number 20/22 attributed to GP for the project “From psychiatric disorder to spiritual gift: Phenomenology and cerebral correlates of hearing voices”).
Author information
Authors and Affiliations
Contributions
GP, BR, IC, RP designed the paradigm and analyzed the data. GP and PC wrote the manuscript. ADD, NM, LT supervised the study. LT reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
There are no competing interests for the present manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Prete, G., Rollo, B., Palumbo, R. et al. Investigating the effect of rTMS over the temporoparietal cortex on the Right Ear Advantage for perceived and imagined voices. Sci Rep 14, 24930 (2024). https://doi.org/10.1038/s41598-024-75671-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75671-z