Abstract
This article is devoted to the synthesis of a new magnetic palladium catalyst that has been immobilized on A-TT-Pd coated-magnetic Fe3O4 nanoparticles. Such surface functionalization of magnetic particles is a promising method to bridge the gap between heterogeneous and homogeneous catalysis approaches. The structure, morphology, and physicochemical properties of the particles were characterized through different analytical techniques, including TEM, FT-IR, XRD, SEM, EDS, TGA-DTG, ICP, and VSM techniques. The obtained Fe3O4@SiO2@A-TT-Pd performance can show excellent catalytic activity for the synthesis of diaryl ethers and oxidation of sulfides, and the corresponding products were obtained with high yields. The advantages of this catalyst include a simple test method, green reaction conditions, no use of dangerous solvents, short reaction time, low catalyst loading, and reusability. Also, the nanocatalyst was easily separated from the reaction mixture with the help of a bar magnet and recovered and reused several times without loss of stability and activity.
Similar content being viewed by others
Introduction
Nanomaterials that can be magnetically separated are considered one of the most significant material classes due to their distinct physicochemical properties. They have garnered interest from a diverse group of researchers1,2. With their potential as green heterogeneous catalysts in diverse organic functional group transformations and as catalytic supports, spinel ferrite compounds show great promise for use in industry and technology3. Over the last ten years, magnetic nanoparticles have been extensively used as a support in the production of magnetic nanocatalysts due to their simple preparation and easy retrieval using a magnetic field4. The easy separation of these nanoparticles from the reaction mixture using an external magnet is one of the benefits of magnetic nanoparticles5. The field of catalysis science plays a central role in numerous crucial organic reactions. Many essential organic functional group transformations necessitate the presence of a catalyst in the reaction environment to enable the selective conversion of reagents and synthons into the desired products with high efficiency6. The use of diverse nanomaterials as catalysts has globally captivated attention because of their distinctive ability to transform manufacturing processes into environmentally friendly, more sustainable, and cost-effective methods7. Among heterogeneous catalysts, Fe3O4 has been greatly favored since they are simply synthesized and surface-modified using a magnet8,9. Different catalysts can be supported on Fe3O4 nanoparticles due to easy separation after several reuses. Over the past decade, scientists have achieved a significant milestone with the discovery of the C-O coupling reaction facilitated by palladium-containing complexes10,11. Transition metal catalyst systems have greatly transformed the synthesis of organic structures through carbon-heteroatom coupling reactions12,13,14. These model reactions are extensively utilized in the synthesis of pharmaceutical, agricultural, and chemical compounds15,16,17. Diaryl ethers are ubiquitous in targeted synthetic and natural products, including agricultural and pharmaceutical chemicals, pharmaceuticals, fragrances, and flavorings18,19.
The targeted oxidation of organic sulfur compounds holds significant importance in biological, synthetic, and industrial contexts20. Sulfides play crucial roles in both biological and industrial processes21. They serve as significant reagents in organic synthesis, acting as protective agents for thiol, facilitating sulfonylation of enolates and other anions, and enabling the synthesis of organo-sulfur compounds through C–S bond formation22. Moreover, they are essential starting materials for the preparation of sulfenyl and sulfinyl reagents23. The selective oxidation of sulfides to sulfoxides holds great significance in organic chemistry24. Some biologically active sulfoxides are crucial as therapeutic agents, serving as antifungal, antibacterial, anti-atherosclerotic, anti-ulcer, antihypertensive, psychotropic, and vasodilator compounds25. A variety of methods, such as different catalysts and various oxidants, have been employed for the selective oxidation of organic sulfides and thiols. In this context, the use of H2O2 as a green oxidant has garnered significant interest due to its environmental implications, accessibility, high atom efficiency, and relatively lower costs compared to other oxidizing agents26.
Considering the interesting benefits of heterogeneous catalysts with the use of novel and green materials, herein, we reported the synthesis of an efficient and heterogeneous novel A-TT-Pd coated on Fe3O4 MNPs and its application in the synthesis of diaryl ethers and oxidation of sulfides in high yields under mild conditions.
Experimental
Preparation of Fe3O4@SiO2@A-TT-Pd
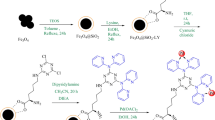
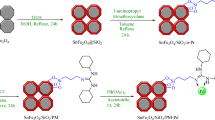
For the synthesis of Fe3O4 nanoparticles, a mixture of FeCl2.3H2O (2 g) and FeCl3.6H2O (4 g) in 25 mL ethanol at room temperature was added to a round bottom flask. After adding 2 g of NaOH to the reaction container, the solution was vigorously stirred at 80 °C for 48 h. Subsequently, the prepared magnetic nanoparticles were separated using a magnet, washed with deionized water, and dried at 60 °C for 10 h. Following this, 1 gram of the obtained Fe3O4 was dispersed in a mixture of 40 mL of ethanol, 5.0 mL of ammonia solution, and 20 mL of H2O. Then, 2 g of PEG-400 and 3 mL of tetraethyl orthosilicate (TEOS) were added to the mixture, which was stirred for 24 h at room temperature. The resulting product (Fe3O4@SiO2) was then separated using an external magnet, subjected to multiple washes with ethanol and H2O, and dried at 25 ℃ (Scheme 1)27,28. To synthesize the Fe3O4@SiO2@A complex, 1 g of the prepared Fe3O4@SiO2 was dispersed in 30 mL EtOH by sonication for 30 min. After that, 3 mmol of 3-aminopropyltriethoxysilane (A) was introduced into the reaction container and stirred under reflux conditions for 24 h. Following the completion of the reaction, the Fe3O4@SiO2@A product was isolated using a magnet and subsequently cleansed with ethyl acetate and EtOH, before being dried at 50 °C in an oven for 15 h. The initial step in the preparation of Fe3O4@SiO2@A@T involved dispersing 1 g of Fe3O4@SiO2@A samples in 20 mL of toluene. Subsequently, 2.5 mmol of trimethylamine (Et3N) and 2.5 mmol of 1,3,5-trichloro triazine were added as a cross-linking reagent to the reaction mixture, which was then refluxed for 24 h. The Fe3O4@SiO2@A@T were subsequently separated with the help of an external magnet, cleaned with EtOH, and dried. In the continuation of the nanocatalyst synthesis, Fe3O4@SiO2@A@T (1 g) was reacted with tris(hydroxymethyl)amino methane (0.6 g) and Et3N (1.5 mmol) in dry toluene. The mixture was stirred for 24 h at a temperature of 70 °C. Following this duration, the separation, washing, and drying process was carried out as the final step. Finally, to prepare Fe3O4@SiO2@A@TT-Pd organometallic catalytic, a mixture of Fe3O4@SiO2@A@TT (1.0 g), Palladium (II) acetate (2.5 mmol) and 50 ml ethanol was added into the flask, and it was stirred at reflux condition for 24 h. Also, 2 mmol of NaBH4 was added to the reaction mixture and stirred for 4 h. After the completion of the reaction, the Fe3O4@SiO2@A-TT-Pd catalyst was separated and washed with H2O and ethanol and dried in vacuum.
Aromatic ethers formation catalyzed by Fe3O4@SiO2@A-TT-Pd
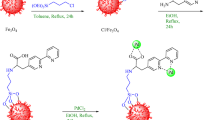
Aryl halide (1 mmol), KOH (5 mmol), phenol (1 mmol), and Fe3O4@SiO2@A-TT-Pd (30 mg) were stirred in H2O at 70 °C and the progression of the reaction was seen by TLC. After completing the reaction, the reaction mixture was cooled to room temperature. Subsequently, water was added to dilute the mixture, and the residual catalyst was removed using an external magnet and then rinsed with ethyl acetate. The resulting solution was filtered and then separated into ethyl acetate and water layers. The Na2SO4 (2 g) was used to dry the solution, after which the solvent was evaporated, yielding pure ether derivatives (Scheme 2).
A general procedure for the oxidation of sulfides
A combination of sulfide (2 mmol) and H2O2 33% (0.3 mL) was poured into the round-bottomed flask containing Fe3O4@SiO2@A-TT-Pd (0.02 g). The mixture was stirred at room temperature, and the progress of the reaction was monitored by TLC. At the end of the reaction, the catalyst was removed by a magnet, and the products were extracted by water and ethyl acetate. The organic solvents were dried over anhydrous Na2SO4. Then, the organic solvent was evaporated, and pure products were obtained in high yields (Scheme 3).
Selected NMR data
Oxydibenzene:1H NMR (400 MHz, DMSO): δH = 7.04 (m, 5 H), 6.83 (m, 5 H) ppm.
1-Methoxy-4-phenoxybenzene:1H NMR (400 MHz, DMSO): δH = 7.47 (d, 1 H), 7.34 (d, 5 H), 7.18 (s, 3 H), 7.18 (s, 3 H) ppm.
1-Nitro-4-phenoxybenzene:1H NMR (MHz, DMSO): δH = 7.95 (d, 3 H), 7.39 (d, 4 H), 7.07 (s, 2 H) ppm
(Sulfinylbis(methylene))dibenzene:1H NMR (400 MHz, DMSO): δH = 7.56 (d, 5 H), 7.28 (d, 5 H), 4.07 (s, 4 H) ppm.
(Ethylsulfinyl)ethane:1H NMR (400 MHz, DMSO): δH = 2.71 (m, 4 H), 1.14 (d, 6 H) ppm.
(Benzylsulfinyl)benzene:1H NMR (400 MHz, DMSO): δH = 2.34 (m, 10 H), 4.17 (d, 2 H) ppm.
Catalyst characterizations
The FTIR spectra of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2@A, Fe3O4@SiO2@A@T, Fe3O4@SiO2@A@TT, and Fe3O4@SiO2@A-TT-Pd are shown in Fig. 1. A sharp peak at 678 cm−1 in the FT-IR spectrum of bare Fe3O4 is related to the Fe–O vibrations (Fig. 1a). After encapsulation of Fe3O4 MNPs, one new peak appears at 1121 cm−1 in the FTIR spectrum, which is related to the stretching vibration of Si–O-Si, which is not indicated in the FTIR spectrum of bare Fe3O4. Furthermore, the stretching vibration of the surface O–H appeared as a broad peak above 3400 cm−1 in the FT-IR spectra (Fig. 1b). The surface modification of Fe3O4@SiO2 by APTMS was confirmed by the emergence of multiple new peaks at > 3000 cm−1, corresponding to the vibration of aliphatic CH2 groups in the propyl chain. This characteristic was not observed in the FT-IR spectrum of Fe3O4@SiO2 (Fig. 1c). After modifying the nanoparticles with cyanuric chloride, three bands at 1709, 1479, and 1419 cm−1 appeared, possibly corresponding to the aromatic ring of cyanuric chloride (Fig. 1d). Following the immobilization of tris(hydroxymethyl)amino methane on nanoparticles, a distinctive peak emerges at 1579 cm−1, potentially indicative of the bending vibration of N–H groups. Also, the peak appearing in the region of 2500–3400 is related to the hydroxy ligand group of tris(hydroxymethyl)amino methane (Fig. 1e). More importantly, the change in the intensity of the peaks of Fe3O4@SiO2@A@TT-Pd confirms the coordination of the nitrogen atom of the amino groups to Pd (Fig. 1f).
The diffraction patterns of Fe3O4@SiO2@A-TT-Pd are depicted in Fig. 2. A study using powder X-ray diffraction (XRD) was conducted to analyze the phase behavior and crystallinity of the catalyst. The initial phases in the 2θ region up to 30°, 36°, 45°, 54°, 57°, and 64° were identified as the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0) planes of the highly crystalline Fe3O4@SiO2@A-TT-Pd nanostructure. The XRD pattern of the catalyst indicates that the Fe3O4 phase remained unchanged following the modifications with a distinct organic functional group (Fig. 2).
The TGA-DTG curve of Fe3O4@SiO2@A-TT-Pd indicated a weight loss of 9% below 200 °C which corresponds to desorption of physically adsorbed solvents. The most significant weight loss, about 19%, occurs within the temperature range of 200 to 600 °C, and it is associated with the elimination of organic compounds from Fe3O4 (see Fig. 3). which shows the thermal stability of the mentioned catalyst up to a temperature of 600 °C. Also, the successful attachment of A-TT-Pd to the surface of Fe3O4 MNPs was confirmed through the results of TGA analysis.
EDX is one of the best approaches to determining elements present in nanoparticles and the purity of nanoparticles (Fig. 4). Figure 4 illustrates the EDX spectrum of Fe3O4@SiO2@A-TT-Pd MNPs, confirming the presence of C, N, Fe, O, Si, and Pd in the catalyst and providing evidence for the successful synthesis of nanoparticles.
The SEM analysis revealed the morphology and dimensions of Fe3O4@SiO2@A-TT-Pd. The resulting SEM image illustrated that the nanoparticles were spherical and within the nano-size range (Fig. 5).
To demonstrate the particle size distribution of these catalysts, Fig. 6 displays the histogram of particle sizes extracted from SEM images. As shown, the particle size of Fe3O4@SiO2@A-TT-Pd shows homogeneous diameters in the obtained histogram SEM images.
Also, the morphology of the Fe3O4@SiO2@A-TT-Pd was investigated by TEM, as shown in Fig. 7. The images revealed the modified Fe3O4 nanoparticles with TT-Pd as an organic shell covered. Images from scanning electron microscopy reveal that the catalyst particles are within the nanometer range and exhibit a spherical structure. This was further confirmed by the data obtained from transmission electron microscopy images (Fig. 7).
Furthermore, ICP-OES analysis was conducted to determine the quantity of Pd in Fe3O4@SiO2@A-TT-Pd. According to the analysis, the catalyst was determined to contain 2.4 × 10−4 mol of Pd per gram based on ICP-OES. The Pd leaching amount after recycling the catalyst was investigated through ICP-OES analysis. Based on this analysis, the reused catalysts contain 2.3 × 10−4 mol. g−1 of Pd, indicating minimal leaching of Pd from the Fe3O4@SiO2@A-TT-Pd framework.
The magnetic behavior of Fe3O4 (Fig. 8a), and Fe3O4@SiO2@A-TT-Pd (Fig. 8b) was investigated using VSM techniques. As expected, the decrease in saturation magnetization from about 61 emu/g to about 43 emu/g, is related to the newly coated layer (Fig. 8).
Catalytic studies
The catalytic activity of Fe3O4@SiO2@A-TT-Pd was studied in the C-O coupling reaction for producing diaryl ether derivatives. In the production of diaryl ethers, the pairing of phenol with iodobenzene using the catalytic potential of Fe3O4@SiO2@A-TT-Pd has been selected as a model reaction to determine the optimized conditions. Initially, the pattern reaction was tested without Fe3O4@SiO2@A-TT-Pd, resulting in the pattern reaction not proceeding. Then, the pattern reaction was carried out using the variant value of the catalyst which was completed with 99% of yield when 0.02 g of Fe3O4@SiO2@A-TT-Pd was used (Table 1). The reaction pattern was studied under a wide range of temperatures, with a focus on the effects of different solvents and bases. The best results for diaryl ether synthesis were achieved using H2O as the solvent and KOH as the base at 70 ºC, as shown in Table 1.
The optimizing conditions mentioned were explored for various aryl halide derivatives to broaden the catalytic scope of Fe3O4@SiO2@A-TT-Pd (Table 2). Aryl halide derivatives with different functional groups, whether electron-withdrawing or electron-donating in nature, were effectively coupled with phenol in high yields using this catalyst. As indicated in Table 2, aryl iodides exhibit a higher reaction rate compared to aryl bromides, while aryl chlorides demonstrate the lowest reaction rate when coupling phenol using the Fe3O4@SiO2@A-TT-Pd catalyst. This suggests that the C–Cl bond is stronger than the C–I bond, as the carbon and chlorine orbitals share similar size, energy, and symmetry, whereas the iodine and carbon orbitals differ in size and energy. Furthermore, the C–I bond is longer and weaker than the C–Cl bond, requiring less energy to break and resulting in a faster coupling rate compared to the shorter C–Cl bond. For instance, the coupling of phenol with 4-nitrobromobenzene surpasses that of 4-nitrochlorobenzene. This pattern is also evident in the coupling of phenol with iodobenzene, bromobenzene, and chlorobenzene using the Fe3O4@SiO2@A-TT-Pd catalyst.
The Carbon-Oxygen cross-coupling reaction’s proposed mechanism, as depicted in Scheme 4, is based on previous findings. In the initial step, iodobenzene undergoes oxidative addition with Pd, yielding intermediate (1). Subsequently, intermediate (1) engages with phenol to generate intermediate (2), which ultimately undergoes reductive elimination to yield ether while liberating the Pd nanoparticle.
To optimize the reaction conditions, we investigated the oxidation process of methyl phenyl sulfide as a representative compound using H2O2 under different reaction parameters, including time and product yield (see Table 3). As shown in Table 3, the reaction was incomplete in the absence of Fe3O4@SiO2@A-TT-Pd even after 12 h. Under solvent-free conditions at room temperature, utilizing a catalytic amount of Fe3O4@SiO2@A-TT-Pd (0.01 g), H2O2 was determined to be the optimal reagent for the complete conversion of methyl phenyl sulfide to methyl phenyl sulfoxide.
The generality of this approach has been demonstrated by facile oxidation of aryl, cyclic, benzylic, and linear as shown in Table 4. The sulfoxides were quickly obtained with high yields. To demonstrate the chemoselectivity of the protocol, sulfides containing oxidation-prone and acid-sensitive functional groups such as CHO, OH, and CO2CH3 were used in the sulfoxidation reaction. Importantly, these functional groups remained unaffected during the sulfide to sulfoxide conversion, as shown in Table 4. Catalyst evaluation heavily relies on selectivity, which plays a crucial role in determining their effectiveness. Chemoselectivity specifically refers to the reactivity of a functional group when other susceptible functional groups are present and subject to the same reaction. An example of this is the oxidation of sulfide in the presence of a hydroxyl group, which can also be oxidized to form a carbonyl. The chemoselectivity of Fe3O4@SiO2@A-TT-Pd was investigated in the oxidation of 2-phenylthioethanol. This catalyst shows good chemoselectivity in synthesizing sulfoxides in the oxidation of 2-phenylthioethanol (Table 4, entry 9).
Based on previous studies, a suggested and possible mechanism for the oxidation of sulfides catalyzed by Fe3O4@SiO2@A-TT-Pd has been presented in Scheme 5. In Fe3O4@SiO2@A-TT-Pd, Palladium plays a crucial role as a magnetic nanocatalyst by forming the active oxidant complex. This mechanism facilitates the transfer of oxygen to sulfur, resulting in the formation of sulfoxide.
Hot filtration
In order to determine any leaching of palladium in the reaction mixture and to demonstrate the heterogeneous nature of Fe3O4@SiO2@A-TT-Pd catalyst, a hot filtration test was conducted during the synthesis of diaryl ethers of iodobenzene with phenol. According to the study, the yield of the product reached 58% in half of the reaction time. Subsequently, the experiment was replicated, and at the midpoint of the reaction, the catalyst was separated, allowing the filtrate to continue reacting. The yield at this stage amounted to 60%, thus confirming the absence of palladium leaching.
The ability to reuse catalysts is a crucial advantage, making them valuable for commercial use. We discovered that Fe3O4@SiO2@A-TT-Pd was rapidly recovered and displayed outstanding recyclability. To explore this, we examined the catalyst’s recyclability in the oxidation of methyl phenyl sulfide. Following the reaction, the catalyst was isolated, rinsed with ethanol to eliminate any remaining product, and dried. Fresh substrates were then introduced to the remaining catalyst for the subsequent reaction. As depicted in Fig. 9, this catalyst can be reused for up to 5 cycles without experiencing significant loss of catalytic activity or Pd leaching.
Comparison of catalyst
To explain the catalytic activity of Fe3O4@SiO2@A-TT-Pd, we analyzed the outcomes of methylphenyl sulfide oxidation using this catalyst and compared them with previously documented methods (Table 5). Notably, in contrast to other catalysts, the preparation of Fe3O4@SiO2@A-TT-Pd is straightforward using cost-effective and readily available materials, and it can be reused up to five times with no significant decline in activity. This catalyst results in a favorable reaction time and higher yield when compared to others. As a result, this novel catalyst demonstrates comparable or even superior characteristics in terms of cost, non-toxicity, stability, and ease of separation.
Conclusion
In summary, we have reported Fe3O4@SiO2@A-TT-Pd as a green, efficient, and reusable catalyst for the oxidation of sulfides to sulfoxides and the synthesis of a wide range of diaryl ether derivatives. Both activity and selectivity in Fe3O4@SiO2@A-TT-Pd are much higher than other previous catalysts. These protocols offer numerous benefits, including the utilization of readily available, cost-effective, environmentally friendly, and chemically stable materials, as well as straightforward operation and high to excellent yields. The simple procedure for preparation, use of non-toxic solvents, short reaction time, excellent tolerance of our method towards different functional groups, and the ability to recycle and reuse the catalyst using an external magnet up to five times with only a minor decrease in product yields are several advantages of this method. Furthermore, the Fe3O4@SiO2@A-TT-Pd is more cost-effective and environmentally friendly due to its low Pd leaching.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Nikoorazm, M. et al. Synthesis of a new complex of lanthanum on MCM-41 as an efficient and reusable heterogeneous catalyst for the chemoselective synthesis of sulfoxides and tetrahydrobenzo[b]pyrans. J. Porous Mater. 31, 511–526 (2024).
Emad-Abbas, N., Naji, J., Moradi, P. & Kikhavani, T. 3-(Sulfamic acid)-propyltriethoxysilane on biochar nanoparticles as a practical, biocompatible, recyclable and chemoselective nanocatalyst in organic reactions. RSC Adv. 14, 22147–22158 (2024).
Rostami, A. et al. Silica sulfuric acid-coated Fe3O4 nanoparticles as high reusable nanocatalyst for the oxidation of sulfides into sulfoxides, protection and deprotection of hydroxyl groups using HMDS and Ac2O. J. Saudi Chem. Soc. 21, 399–407 (2017).
Jabbari, A., Nikoorazm, M. & Moradi, P. Two Schiff-base complexes of cadmium and manganese on modified MCM-41 as practical, recyclable and selective nanocatalysts for the synthesis of sulfoxides. J. Porous Mater. 30, 1395–1402 (2023).
Zhang, J. et al. Preparation of core/shell-structured ZnFe2O4@ZnIn2S4 catalysts and its ultrafast microwave catalytic reduction performance for aqueous cr(VI). Chem. Eng. J. 451, 138182 (2023).
Yang, B. et al. The roles of ZnFe2O4 and α-Fe2O3 in the biphasic catalyst for the oxidative dehydrogenation of n-butene. J. Catal. 381, 70–77 (2020).
Yao, L. et al. A rational design of CdS/ZnFe2O4/Cu2O core-shell nanorod array photoanode with stair-like type-II band alignment for highly efficient bias-free visible-light-driven H2 generation. Appl. Catal. B 268, 28, (2020).
Wu, H., Hao, L., Chen, C. & Zhou, J. Superhydrophobic Fe3O4/OA magnetorheological fluid for removing oil slick from water surfaces effectively and quickly. ACS Omega. 5, 27425–27432 (2020).
Pinto, V. H. A. et al. Mn porphyrins immobilized on non-modified and chloropropyl-functionalized mesoporous silica SBA-15 as catalysts for cyclohexane oxidation. Appl. Catal. A. 526, 9–20 (2016).
Jammi, S. et al. CuO nanoparticles catalyzed C – N, C – O, and C – S cross-coupling reactions: scope and mechanism. J. Org. Chem. 74, 1971–1976 (2009).
Singh, P., Mishra, S., Sahoo, A. & Patra, S. A magnetically retrievable mixed-valent Fe3O4@SiO2/Pd0/PdII nanocomposite exhibiting facile tandem Suzuki coupling/transfer hydrogenation reaction. Sci. Rep. 11, 9305 (2021).
Ashraf, M. A., Liu, Z., Zhang, D. & Alimoradi, A. L-lysine‐Pd complex supported on Fe 3 O 4 MNPs: a novel recoverable magnetic nanocatalyst for Suzuki C‐C Cross‐Coupling reaction. Appl. Organomet. Chem. 34, e5668 (2020).
Kanchana, U. S., Diana, E. J., Mathew, T. V. & Anilkumar, G. Palladium-catalyzed cross-coupling reactions of coumarin derivatives: an overview. Appl. Organomet. Chem. 34, (2020).
Lakshmidevi, J., Naidu, B. R. & Venkateswarlu, K. CuI in biorenewable basic medium: three novel and low E-factor Suzuki-Miyaura cross-coupling reactions. Mol. Catal. 522, 112237 (2022).
Chen, J., Zhang, J., Zhang, Y., Xie, M. & Li, T. Nanoporous phenanthroline polymer locked pd as highly efficient catalyst for Suzuki-Miyaura Coupling reaction at room temperature. Appl. Organomet. Chem. 34, e5310, (2020).
Lei, D. et al. Oxalate enhanced synergistic removal of chromium(VI) and arsenic(III) over ZnFe2O4/g-C3N4: Z-scheme charge transfer pathway and photo-Fenton like reaction. Appl. Catal. B 282, 119578, (2021).
Han, C. et al. Enhanced support effects in single-atom copper-incorporated carbon nitride for photocatalytic suzuki cross-coupling reactions. Appl. Catal. B. 320, 121954 (2023).
Li, C. J. Reflection and perspective on green chemistry development for chemical synthesis - daoist insights. Green Chem. 18, 1836–1838 (2016).
Johansson Seechurn, C. C. C., Kitching, M. O., Colacot, T. J. & Snieckus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 nobel prize. Angewandte Chemie - Int. Ed. 51, 5062–5085 (2012).
Jia, Y. et al. Recent advances in doping strategies to improve Electrocatalytic Hydrogen Evolution performance of Molybdenum Disulfide. ACS Catal. 14, 4601–4637 (2024).
Geissel, F., Lang, L., Husemann, B., Morgan, B. & Deponte, M. Deciphering the mechanism of glutaredoxin-catalyzed roGFP2 redox sensing reveals a ternary complex with glutathione for protein disulfide reduction. Nat. Commun. 15, 1733 (2024).
Karami, M. & Aghabarari, B. The Advancement of Molybdenum Disulfide Quantum dots nanoparticles as Nanocarrier for Drug Delivery systems: cutting-edge in dual therapeutic roles. J. Mol. Struct. 139149 https://doi.org/10.1016/j.molstruc.2024.139149 (2024).
Sekar, P. et al. Regiodivergent synthesis of 4- and 5-Sulfenyl oxazoles from Alkynyl Thioethers. Chem. – Eur. J. n/a, 40, e202401465 (2024).
Li, X. R., Zhang, R. J., Xiao, Y., Tong, Q. X. & Zhong, J. J. N-Sulfenyl phthalimide enabled Markovnikov hydrothiolation of unactivated alkenes via ligand promoted cobalt catalysis. Org. Chem. Front. 11, 646–653 (2024).
Wang, P. et al. Thiosuccinimide enabled S–N bond formation to access N-sulfenylated sulfonamide derivatives with synthetic diversity. Org. Biomol. Chem. 22, 990–997 (2024).
Balajirao Dapkekar, A., Naveen, J. & Satyanarayana, G. Electrochemical Annulation of Ortho-Alkynylbiphenyls to Fused Sulfenyl Phenanthrenes and Spiro Cyclohexenone Indenes. Adv. Synth. Catal. 366, 18–23 (2024).
Zhang, F., Shen, B., Jiang, W., Yuan, H. & Zhou, H. Hydrolysis extraction of diosgenin from Dioscorea Nipponica Makino by sulfonated magnetic solid composites. J. Nanopart. Res. 21, 12 (2019).
Dindarloo Inaloo, I., Majnooni, S., Eslahi, H. & Esmaeilpour, M. N-Arylation of (hetero)arylamines using aryl sulfamates and carbamates via C–O bond activation enabled by a reusable and durable nickel(0) catalyst. New J. Chem. 44, 13266–13278 (2020).
Ghorbani-Choghamarani, A., Moradi, P. & Tahmasbi, B. Nickel(II) immobilized on dithizone–boehmite nanoparticles: as a highly efficient and recyclable nanocatalyst for the synthesis of polyhydroquinolines and sulfoxidation reaction. J. Iran. Chem. Soc. 16, 511–521 (2019).
Nikoorazm, M., Rezaei, Z. & Tahmasbi, B. Two Schiff-base complexes of copper and zirconium oxide supported on mesoporous MCM-41 as an organic–inorganic hybrid catalysts in the chemo and homoselective oxidation of sulfides and synthesis of tetrazoles. J. Porous Mater. 27, 671–689 (2020).
Islam, S. M. et al. Selective oxidation of sulfides and oxidative bromination of organic substrates catalyzed by Polymer anchored Cu(II) complex. Tetrahedron Lett. 53, 127–131 (2012).
Hussain, S., Talukdar, D., Bharadwaj, S. K. & Chaudhuri, M. K. VO2F(dmpz)2: a new catalyst for selective oxidation of organic sulfides to sulfoxides with H2O2. Tetrahedron Lett. 53, 6512–6515 (2012).
Thurow, S. et al. Base-free oxidation of thiols to disulfides using selenium ionic liquid. Tetrahedron Lett. 52, 640–643 (2011).
Acknowledgements
The authors extend their appreciation to King Saud University, Saudi Arabia, for funding this work through Researchers Supporting Project number (RSP2024R397), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Durgesh Singh. Kamini Singh. Pawan Sharma. Yashwantsinh Jadeja. Johar MGM. Priyanka Singh. Kiranjeet Kaur. M. Atif. Mohammed A. El-Meligy and Beneen Husseen Funding acquisition, Conceptualization, Resources, Supervision, Writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, D., Singh, K., Sharma, P. et al. In situ decorated pd NPs on Triazin-encapsulated Fe3O4/SiO2-NH2 as magnetic catalyst for the synthesis of diaryl ethers and oxidation of sulfides. Sci Rep 14, 25261 (2024). https://doi.org/10.1038/s41598-024-75681-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75681-x
Keywords
This article is cited by
-
Copper complex of 1-(benzo[d]thiazol-2-yl)urea on mesoporous SBA-15 as a green and robust catalyst for production of tetrazoles
Journal of Porous Materials (2025)