Abstract
To evaluate diagnostic utility of tear meniscus height (TMH) measurement using anterior segment optical coherence tomography (AS-OCT) for lacrimal passage disorder (LPD) in patients scheduled for cataract surgery. Patients with cataracts were enrolled. Lacrimal irrigation test was used to diagnose LPD. TMH measurement was performed using prior to cataract surgery. A receiver operating characteristic curve analysis was performed to calculate the cutoff value of TMH to detect LPD. Correlation between TMH and age, sex, LPD, use of glaucoma eyedrops, and use of dry eye drops in patients with and without LPD was evaluated. Sixty-six patients (127 eyes) were included, of which 12 (9.4%) eyes had LPD. The mean TMH was significantly higher in the LPD group than in the non-LPD group (P = 0.007). Multiple regression analysis revealed a significant association of TMH with LPD (P = 0.000). With an area under the curve of 0.740, the optimal cut-off TMH value was 401.0 μm. The sensitivity and specificity of this cutoff value were 58.3% and 83.0%, respectively. These findings demonstrate the diagnostic utility of TMH, measured using AS-OCT, for LPD. This suggests that AS-OCT is useful for preoperative exclusion of LPD.
Similar content being viewed by others
Introduction
Lacrimal passage disorder (LPD) is a common disorder among elderly patients that can lead to discharge, epiphora, and acute dacryocystitis. Postoperative endophthalmitis is a serious sight-threatening complication following intraocular surgery, including cataract surgery, and occurs in 0.04–0.076% of patients with cataracts1,2,3,4,5,6. Patients with LPD have an increased incidence of postoperative endophthalmitis following cataract surgery. Lopez et al.7 reported that the incidence of postoperative endophthalmitis was 2%. Bacterial conjunctival contamination is common in patients with LPD. Kam et al.8 reported that the rate of LPD among patients with endophthalmitis after cataract surgery was 50%. Therefore, LPD needs to be evaluated before intraocular surgery to prevent postoperative endophthalmitis.
The lacrimal irrigation test is the most common diagnostic procedure for LPD and is among the essential examinations in dacryology9,10,11. However, this procedure is invasive, and requires clinical knowledge and practical experience. Other methods for detecting LPD include dacryocystography and dacryoendoscopy, which are valuable for direct visualization of LPD; however, these methods are more invasive than the lacrimal irrigation test12.
The tear meniscus is a crucial clinical feature for evaluating the lacrimal drainage system, which can be assessed through slit-lamp examination and the fluorescein dye disappearance test. There has been increasing interest in anterior segment optical coherence tomography (AS-OCT), a valuable tool for evaluating the anterior segment given its high-resolution visualization and objective as well as noninvasive measurement of the cornea, anterior chamber, anterior angle, and meniscus. AS-OCT allows quantitative measurement of the tear meniscus height (TMH), cross-sectional area, and depth. Several studies have performed quantitative TMH evaluation using AS-OCT in patients with dry eye disease as well as before and after lacrimal surgery13,14,15,16,17,18,19,20,21,22,23,24,25. However, studies on the quantitative evaluation of TMH using AS-OCT before cataract surgery are scarce. Therefore, the relationship between LPD and TMH remains unclear.
This study aimed to investigate the diagnostic utility of TMH measured using AS-OCT for LPD in patients scheduled for cataract surgery.
Results
We initially enrolled 148 eyes from 75 patients, of which 14,7 eyes were excluded due to incomplete data acquisition, canalicular obstruction/puncta atresia, respectively. None of the patients had complaints of epiphora. Ultimately, 127 eyes from 66 patients (mean age: 75.1 ± 9.6 years) were included in the irrigation test analyses. Based on the slit-lamp examination results, we excluded 13 eyes with conjunctivochalasis and 2 eyes with entropion. Accordingly, 112 eyes from 59 patients (mean age: 74.1 ± 9.5 years) were included in the AS-OCT analyses (Table 1; Fig. 1).
Incidence rate of LPD and TMH evaluation using AS-OCT
Among 127 eyes, 12 (9.4%) eyes from nine patients (mean age: 77.11 ± 3.07 years [range, 71–81 years]; four women, five men) had LPD based on the lacrimal irrigation test. None of the patients with conjunctivochalasis and entropion showed LPD. The mean TMH was significantly higher in the LPD group than in the non-LPD group (494.9 ± 263.7 μm vs. 292.8 ± 130.0 μm, P = 0.007; Table 2; Fig. 2).
Boxplots of the lacrimal irrigation test results and the tear meniscus height in patients scheduled for cataract surgery. Lacrimal passage disorder (LPD) was defined based on the lacrimal irrigation test results. Mann–Whitney U test was applied to compare the tear meniscus height (TMH) between groups. The LPD group showed a significantly higher TMH than the non-LPD group (P = 0.007).
The area under the curve (AUC), which was calculated using ROC analysis, revealed an acceptable sensitivity and specificity of the diagnostic variables for LPD. The cutoff TMH value was 401.0 μm, with an AUC value of 0.740 (95% confidence interval: 0.574–0.905). The sensitivity and specificity were 58.3 and 83.0%, respectively; further, the positive and negative predictive values were 26.2 and 95.0%, respectively (Fig. 3).
Receiver-operating characteristics curve to calculate diagnostic variables for lacrimal passage disorder. Diagnostic variables for lacrimal passage disorder (LPD) were analyzed using the receiver-operating characteristics (ROC) curve. The area under the curve (AUC) values calculated using the ROC technique revealed an acceptable sensitivity and specificity of diagnostic variables for LPD. The cutoff value of TMH for predicting LPD was 401.0 μm, in which the AUC value was 0.740. The sensitivity and specificity were 58.3 and 83.0%, respectively. Further, the positive and negative predictive values were 26.2 and 95.0%, respectively.
Relationship between preoperative parameters and TMH
Multiple linear regression analysis revealed a significant association of TMH with LPD (β = 1.984 × 10− 1; P = 0.000), but not with male sex (β = 4.756 × 10− 2; P = 0.105), age (β =-2.740 × 10− 4; P = 0.863), eyedrops for glaucoma (β =-3.850 × 10− 2; P = 0.354), and eyedrops for dry eye (β = 2.141 × 10− 2; P = 0.675) (Table 3).
Discussion
Our findings demonstrated the rate of LPD in patients before cataract surgery; furthermore, the measurement of using AS-OCT revealed significantly higher TMH in the LPD group than in the non-LPD group. The optimal cutoff value of TMH for detecting LPD was 401.0 μm. Lastly, the preoperative TMH of patients undergoing cataract surgery was significantly associated with LPD.
The incidence rate of LPD identified using the lacrimal irrigation test before cataract surgery is reportedly 3.3–11.8%5,7,8. This is consistent with our finding of a 9.4% incidence rate; however, it varies according to race, population, and clinical background.
The mean TMH in patients with and without LPD were 494.9 ± 263.7 μm and 292.8 ± 130.0 μm, respectively. Recent studies have used SS AS-OCT to measure TMH and detect LPD. TMH before and after lacrimal surgery has been reported to be 448–707 μm and 253–340 μm, respectively, consistent with our findings18,19,25. Specifically, TMH in the non-LPD group did not significantly differ from that after lacrimal duct surgery in previous reports. We found that TMH was significantly higher in the LPD group than in the non-LPD group (Fig. 4), which is consistent with previous reports that TMH was significantly higher in patients with LPD than in healthy participants14,25,26,27. We observed a significant association of TMH with LPD, which suggests that TMH measurement using AS-OCT before cataract surgery can facilitate the prediction of the presence of LPD.
In our study, the cutoff value of TMH for predicting LPD was 401.0 μm, and the AUC value was 0.740. The sensitivity and specificity were 58.3 and 83.0%, respectively. Further, the positive and negative predictive values were 26.2 and 95.0%, respectively. These findings suggest that, in the case of TMH > 401.0 μm, LPD should be suspected and confirmed through an irrigation test. Accordingly, noninvasive measurement of TMH using AS-OCT can facilitate predicting the presence of LPD.
Two previous studies have assessed the cutoff value of TMH measured using AS-OCT in LPD. Park et al.26 compared the epiphora group (23 patients with LPD and 21 patients with functional nasolacrimal duct obstruction) with healthy participants and reported that the cutoff value of the TMH in patients with epiphora measured by AS-OCT was 342 μm, with a sensitivity and specificity of 92 and 96%, respectively, in which the AUC value was 0.842. Ishikawa et al.28 compared 72 patients in the LPD group and 89 patients in the non-LPD group among patients with complaints of epiphora and reported that the cutoff value of the TMH in patients with epiphora measured by AS-OCT was 280 μm, with a sensitivity and specificity of 93 and 60%, respectively, in which the AUC value was 0.79. The cutoff values differ between the present study and previous reports, probably owing to different subject populations. The present study was conducted in preoperative cataract surgery patients without epiphora, whereas the previous reports were conducted on patients with epiphora, which may have contributed to the difference in results.
Akiyama et al.29 reported that the cutoff value of the TMH measured by AS-OCT for predicting dry eye disease was 191 μm, with a sensitivity and specificity of 66.7 and 88.5%, respectively, in which the AUC value was 0.846. Shinzawa et al.30 reported that a cutoff TMH value of 197 μm predicted dry eye disease with a sensitivity and specificity of 74 and 78%, respectively, in which the AUC value was 0.85. These reports indicated that TMH has high sensitivity and specificity for diagnosing dry eye disease. Taken together, TMH measurement using AS-OCT may facilitate the detection of LPD in patients scheduled for cataract surgery.
We showed that TMH was not significantly associated with male sex, which is consistent with previous reports of no sex differences in TMH among healthy participants31,32.
We observed no significant association between TMH and age, inconsistent with previous reports that TMH measured by AS-OCT was negatively or positively correlated with age in healthy individuals31,32,33,34,35. This inconsistency could be attributed to differences in the study population. Specifically, we did not include individuals aged < 40 years, while the previous studies did. Moreover, our patients, who were scheduled for cataract surgery, were older and presented with conditions that can increase (eyelid laxity and meibomian gland dysfunction) or decrease (dry eye conditions) TMH.
We found that using eye drops for glaucoma was not associated with TMH, which is inconsistent with previous reports. Specifically, some reports indicated that TMH measured by AS-OCT was as low in patients with medically controlled glaucoma as in patients with dry eye disease. These reports demonstrated that medical treatment of glaucoma significantly contributed to secondary evaporative dry eye, with meibomian gland dysfunction being a crucial factor; further, patients on multi-therapy showed a relatively higher reduction in tear meniscus parameters34,36,37. Our study investigated the association of TMH with the use of at least one or no glaucoma eyedrops, which could have contributed to the inconsistency with previous reports.
Similarly, we found that using dry eye drops was not associated with TMH, which is inconsistent with previous reports of an increase in TMH following eyedrops-based treatment for dry eye disease24,38. This inconsistency could be attributed to the fact that the use of dry eye drops was assessed based on a medical interview rather than a diagnosis of dry eye disease. Moreover, some patients who used dry eye drops also used glaucoma drops.
This study had some limitations. First, the sample size was small, which may have impeded the detection of small between-group differences. Second, dry eye disease was not evaluated, which may affect the TMH. Although we assessed the history of dry eye drop use, undiagnosed and untreated patients with dry eye may have still been included. Third, we did not evaluate other conditions that might have affected TMH, including allergic conjunctivitis, meibomian gland dysfunction, and eyelid laxity. In addition, given the lack of a standard measurement method for TMH, it is difficult to accurately evaluate TMH measured under different conditions given the potential influence of various factors such as temperature, wind, blinking, and humidity39. Fourth, although we used SS AS-OCT, the obtained TMH measurements are not absolute values due to interdevice differences.
In conclusion, our findings demonstrated the predictive utility of TMH measured using AS-OCT before cataract surgery for the presence of LPD. Furthermore, we calculated the cutoff TMH value and demonstrated its potential as a diagnostic marker for LPD. AS-OCT can be used for preoperative exclusion of LPD, which is a significant risk factor for postoperative endophthalmitis.
Materials and methods
This study adhered to the Declaration of Helsinki and was approved by the Institutional Review Board of the Japanese Red Cross Society Osaka Hospital (J-0513). Written informed consent was obtained from all patients.
Study participants
For this prospective study, we reviewed the medical records of consecutive patients who underwent preoperative examinations before cataract surgery between February and March 2023 at the Department of Ophthalmology of the Japanese Red Cross Society Osaka Hospital (Osaka, Japan). The exclusion criteria were as follows: punctum atresia, canalicular obstruction, history of congenital nasolacrimal obstruction, previous lacrimal surgery, and incomplete ophthalmic measurements. Patients with conjunctivochalasis, ptosis, entropion, and ectropion based on the slit-lamp examination results were excluded. Previous lacrimal surgery, complaints of epiphora, and use of eye drops for dry eye and glaucoma were determined through medical interviews.
Lacrimal irrigation test
The presence of LPD was determined based on the lacrimal irrigation test, which was performed by trained clinicians. The patient was placed in a supine position and received topical anesthesia before the examination. A blunt-tipped 23-G lacrimal cannula (G-15267; M.E. Technica, Tokyo, Japan) attached to a 2.5-mL syringe containing saline solution was inserted through the upper and lower punctum; syringing was performed under minimal pressure. The patient was asked whether the fluid had reached the pharynx. LPD was defined as complete and/or partial obstruction, while non-LPD was defined as the absence of regurgitation. Complete obstruction was considered if the saline completely refluxed and did not irrigate into the pharynx. Partial obstruction was characterized by partial irrigation into the pharynx with some extent of regurgitation. Regurgitation from the contralateral punctum to the injecting side was considered an obstruction in the common canaliculus or more nasal structures. Contrastingly, direct saline regurgitation from the injecting punctum was indicative of canalicular obstruction.
Imaging analyses
All patients underwent routine preoperative examinations, including visual acuity and intraocular pressure tests, slit-lamp examination, AS-OCT, and lacrimal irrigation test. The slit-lamp examination results were used to diagnose conjunctivochalasis, ptosis, entropion, and ectropion.
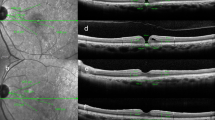
TMH measurements were performed using swept-source (SS) AS-OCT (CASIA2; Tomey, Tokyo, Japan). Vertical line scans through the corneal center were performed before lacrimal irrigation and pupil dilation. TMH was manually evaluated and defined as the distance between the lower eyelid–meniscus junction and the upper cornea–meniscus junction on the AS-OCT images. TMH was automatically calculated using built-in software (Fig. 5).
Statistical analyses
Mann–Whitney U test was applied for between-group comparison of TMH. Multiple linear regression analysis was performed to evaluate the association between TMH and parameters such as age, sex, LPD, and use of eyedrops for glaucoma and dry eye. Statistical significance was set at P < 0.05. Diagnostic variables for LPD were analyzed based on receiver-operating characteristic (ROC) curve analysis. Furthermore, ROC curve analysis was performed to calculate the optimal cutoff value of TMH for predicting LPD. Moreover, the sensitivity and specificity of the cutoff value were calculated as the proportion of patients with LPD and non-LPD who were positive and negative for the variable, respectively. All statistical analyses were conducted using R software version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/).
Data availability
The data that support the findings of this study are available from the corresponding author, H.M., upon reasonable request.
References
Wong, T. Y. & Chee, S. P. The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology. 111, 699–705. https://doi.org/10.1016/j.ophtha.2003.07.014 (2004).
Lalitha, P. et al. Post-cataract endophthalmitis in South India: Incidence and outcome. Ophthalmology. 112, 1884–1889. https://doi.org/10.1016/j.ophtha.2005.05.020 (2005).
Miller, J. J. et al. Acute onset endophthalmitis after cataract surgery (2000–04) incidence, clinical settings and visual acuity outcomes, after treatment. Am. J. Ophthalmol. 139, 983–987. https://doi.org/10.1016/j.ajo.2005.01.025 (2005).
Hartikainen, J., Lehtonen, O. P. & Saari, K. M. Bacteriology of lacrimal duct obstruction in adults. Br. J. Ophthalmol. 81, 37–40. https://doi.org/10.1136/bjo.81.1.37 (1997).
Hayashi, Y. et al. Bacteriology of the conjunctiva in pre-cataract surgery patients with occluded nasolacrimal ducts and the operation outcomes in Japanese patients. BMC Ophthalmol.https://doi.org/10.1186/s12886-017-0410-x (2017).
Speaker, M. G., Milch, F. A., Shah, M. K., Eisner, W. & Kreiswirth, B. N. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 98, 639–649. https://doi.org/10.1016/s0161-6420(91)32239-5 (1991). discussion 650.
Lopez, P. F. et al. Pneumococcal endophthalmitis associated with nasolacrimal obstruction. Am. J. Ophthalmol. 116, 56–62. https://doi.org/10.1016/s0002-9394(14)71744-1 (1993).
Kam, J. K., Cheng, N. M., Sarossy, M., Allen, P. J. & Brooks, A. M. Nasolacrimal duct screening to minimize post-cataract surgery endophthalmitis. Clin. Exp. Ophthalmol. 42, 447–451. https://doi.org/10.1111/ceo.12244 (2014).
Kim, U. et al. Regurgitation on pressure over the lacrimal sac versus lacrimal irrigation in determining lacrimal obstruction prior to intraocular surgeries. Indian J. Ophthalmol. 70, 3833–3836. https://doi.org/10.4103/ijo.IJO_1722_22 (2022).
Thomas, R., Thomas, S., Braganza, A. & Muliyil, J. Evaluation of the role of syringing prior to cataract surgery. Indian J. Ophthalmol. 45, 211–214 (1997).
Shenoy, P. et al. Comparison of post-cataract surgery endophthalmitis rates using syringing or regurgitation on pressure over the lacrimal sac as a preoperative screening tool for nasolacrimal duct obstruction: An impact assessment of protocol alteration due to the COVID-19 pandemic. Indian J. Ophthalmol. 69, 2824–2827. https://doi.org/10.4103/ijo.IJO_1218_21 (2021).
Nakamura, J., Kamao, T., Mitani, A., Mizuki, N. & Shiraishi, A. Accuracy of the lacrimal syringing test in relation to dacryocystography and dacryoendoscopy. Clin. Ophthalmol. 17, 1277–1285. https://doi.org/10.2147/OPTH.S409662 (2023).
Czajkowski, G., Kaluzny, B. J., Laudencka, A., Malukiewicz, G. & Kaluzny, J. J. Tear meniscus measurement by spectral optical coherence tomography. Optom. Vis. Sci. 89, 336–342. https://doi.org/10.1097/OPX.0b013e318242042b (2012).
Johnson, M. E. & Murphy, P. J. The agreement and repeatability of tear meniscus height measurement methods. Optom. Vis. Sci. 82, 1030–1037. https://doi.org/10.1097/01.opx.0000192352.78935.e0 (2005).
Srinivasan, S., Chan, C. & Jones, L. Apparent time-dependent differences in inferior tear meniscus height in human subjects with mild dry eye symptoms. Clin. Exp. Optom. 90, 345–350. https://doi.org/10.1111/j.1444-0938.2007.00174.x (2007).
Savini, G., Barboni, P. & Zanini, M. Tear meniscus evaluation by optical coherence tomography. Ophthalmic Surg. Lasers Imaging. 37, 112–118. https://doi.org/10.3928/1542-8877-20060301-06 (2006).
Fujimoto, M. et al. Evaluation of dacryocystorhinostomy using optical coherence tomography and rebamipide ophthalmic suspension. Clin. Ophthalmol. 8, 1441–1445. https://doi.org/10.2147/OPTH.S65654 (2014).
Kamao, T., Zheng, X. & Shiraishi, A. Outcomes of bicanalicular nasal stent inserted by sheath-guided dacryoendoscope in patients with lacrimal passage obstruction: A retrospective observational study. BMC Ophthalmol. 21, 103. https://doi.org/10.1186/s12886-020-01678-5 (2021).
Hoshi, S., Tasaki, K., Hiraoka, T. & Oshika, T. Improvement in contrast sensitivity function after lacrimal passage intubation in eyes with epiphora. J. Clin. Med. 9, 2761. https://doi.org/10.3390/jcm9092761 (2020).
Stahl, U., Francis, I. C. & Stapleton, F. Prospective controlled study of vapor pressure tear osmolality and tear meniscus height in nasolacrimal duct obstruction. Am. J. Ophthalmol. 141, 1051–1056. https://doi.org/10.1016/j.ajo.2005.12.051 (2006).
Ibrahim, O. M. A. et al. Application of Visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology. 117, 1923–1929. https://doi.org/10.1016/j.ophtha.2010.01.057 (2010).
Shen, M. et al. Upper and lower tear menisci in the diagnosis of dry eye. Invest. Ophthalmol. Vis. Sci. 50, 2722–2726. https://doi.org/10.1167/iovs.08-2704 (2009).
Chan, H. H., Zhao, Y., Tun, T. A. & Tong, L. Repeatability of tear meniscus evaluation using spectral-domain Cirrus® HD-OCT and time-domain Visante® OCT. Cont. Lens Anterior Eye. 38, 368–372. https://doi.org/10.1016/j.clae.2015.04.002 (2015).
Cui, L. et al. Age-related changes in tear menisci imaged by optical coherence tomography. Optom. Vis. Sci. 88, 1214–1219. https://doi.org/10.1097/OPX.0b013e3182271297 (2011).
Ohtomo, K. et al. Tear meniscus volume changes in dacryocystorhinostomy evaluated with quantitative measurement using anterior segment optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 55, 2057–2061. https://doi.org/10.1167/iovs.13-12692 (2014).
Park, D. I., Lew, H. & Lee, S. Y. Tear meniscus measurement in nasolacrimal duct obstruction patients with Fourier-domain optical coherence tomography: Novel three-point capture method. Acta Ophthalmol. 90, 783–787. https://doi.org/10.1111/j.1755-3768.2011.02183.x (2012).
Loureiro, T. et al. Corneal epithelial thickness changes after topical treatment of dry eye disease in primary Sjögren syndrome. Clin. Ophthalmol. 17, 993–1005. https://doi.org/10.2147/OPTH.S375505 (2023).
Ishikawa, S., Shoji, T., Yamada, N. & Shinoda, K. Efficacy of strip meniscometry for detecting lacrimal obstructive diseases among patients with epiphora. Transl Vis. Sci. Technol. 8, 8. https://doi.org/10.1167/tvst.8.6.8 (2019).
Akiyama, R., Usui, T. & Yamagami, S. Diagnosis of dry eye by tear meniscus measurements using anterior segment swept source optical coherence tomography. Cornea. 34, S115–S120. https://doi.org/10.1097/ICO.0000000000000583 (2015).
Shinzawa, M., Dogru, M., Miyasaka, K., Shimazaki, J. & Sekiryu, T. Application of CASIA SS-1000 optical coherence tomography tear meniscus imaging in testing the efficacy of new strip meniscometry in dry eye diagnosis. Eye Contact Lens. 44, S44–S49. https://doi.org/10.1097/ICL.0000000000000312 (2018).
Singh, S., Srivastav, S., Mohamed, A. & Basu, S. Non-invasive tear film assessment in normal population: Effect of age, sex, and interparametric relationship. Front. Med. (Lausanne). 9, 894184. https://doi.org/10.3389/fmed.2022.894184 (2022).
Zheng, X. et al. New method for evaluation of early phase tear clearance by anterior segment optical coherence tomography. Acta Ophthalmol. 92, e105–e111. https://doi.org/10.1111/aos.12260 (2014).
Hasan, I. Y. Dry eye syndrome risk factors: A systemic review. Saudi J. Ophthalmol. 35, 131–139. https://doi.org/10.4103/1319-4534.337849 (2022).
Agnifili, L. et al. Tear meniscus imaging by anterior segment-optical coherence tomography in medically controlled glaucoma. J. Glaucoma. 29, 374–380. https://doi.org/10.1097/IJG.0000000000001469 (2020).
Qiu, X., Gong, L., Sun, X. & Jin, H. Age-related variations of human tear meniscus and diagnosis of dry eye with Fourier-domain anterior segment optical coherence tomography. Cornea. 30, 543–549. https://doi.org/10.1097/ICO.0b013e3181fb84ea (2011).
Savini, G., Goto, E., Carbonelli, M., Barboni, P. & Huang, D. Agreement between Stratus and Visante optical coherence tomography systems in tear meniscus measurements. Cornea. 28, 148–151. https://doi.org/10.1097/ICO.0b013e31818526d0 (2009).
Baudouin, C. et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: Human and animal studies. Ophthalmology. 106, 556–563. https://doi.org/10.1016/S0161-6420(99)90116-1 (1999).
Mastropasqua, R., Agnifili, L. & Mastropasqua, L. Structural and molecular tear film changes in glaucoma. Curr. Med. Chem. 26, 4225–4240. https://doi.org/10.2174/0929867325666181009153212 (2019).
Palakuru, J. R., Wang, J. & Aquavella, J. V. Effect of blinking on tear dynamics. Invest. Ophthalmol. Vis. Sci. 48, 3032–3037. https://doi.org/10.1167/iovs.06-1507 (2007).
Acknowledgements
We thank the Japanese Red Cross Society Osaka Hospital for assistance in examinations.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Study conception and design: H.M. and Y.H.; Analysis and interpretation: H.M. and S.K.; Writing of the article: H.M. and S.K.; Preparing all figures: H.M.; Data collection: H.M., Y.H., and M.A.; Supervision: M.A.; Final approval of the article: all authors.
Corresponding author
Ethics declarations
Compliance with ethical standards
This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the Japanese Red Cross Society Osaka Hospital (J-0513). Written informed consent was obtained from all patients.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matsuyama, H., Kadomoto, S., Hosoda, Y. et al. Diagnostic utility of tear meniscus height measurement using anterior segment optical coherence tomography for lacrimal passage disorder before cataract surgery. Sci Rep 14, 25866 (2024). https://doi.org/10.1038/s41598-024-75979-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75979-w