Abstract
In this study, we tested a novel approach of “repurposing” a biomarker typically associated with breast cancer for use in melanoma. HER2/neu is a well characterized biomarker in breast cancer for which effective anti-HER2/neu therapies are readily available. We constructed a lentivirus encoding c-erb-B2, an animal (rat) homolog to HER2/neu. This was used to transfect B16 melanoma in vitro for use in an orthotopic preclinical mouse model, which resulted in expression of rat c-erb-B2 as a neoantigen target for anti-c-erb-B2 monoclonal antibody (7.16.4). The c-erb-B2-expressing melanoma was designated B16/neu. 7.16.4 produced statistically significant in vivo anti-tumor responses against B16/neu. This effect was mediated by NK-cell antibody-dependent cell-mediated cytotoxicity. To further model human melanoma (which expresses < 5% HER2/neu), our c-erb-B2 encoding lentivirus was used to inoculate naïve (wild-type) B16 tumors in vivo, resulting in successful c-erb-B2 expression. When combined with 7.16.4, anti-tumor responses were again demonstrated where approximately 40% of mice treated with c-erb-B2 lentivirus and 7.16.4 achieved complete clinical response and long-term survival. For the first time, we demonstrated a novel strategy to repurpose c-erb-B2 as a neoantigen target for melanoma. Our findings are particularly significant in the contemporary setting where newer anti-HER2/neu antibody-drug therapies have shown increased efficacy.
Similar content being viewed by others
Introduction
Since the early 2010s, immune checkpoint blockade has vastly improved clinical outcomes for patients with locally advanced and metastatic melanoma1,2,3. However, up to 40–50% of these patients still die from melanoma or develop resistance to currently available immune checkpoint inhibitors4,5. While there are other targeted agents available for melanomas with BRAF mutations, durability of their response is limited6. Adverse events from immunotherapy or targeted therapies also limit their use, and there are populations of patients in whom immune checkpoint blockade is contraindicated, such as those with organ transplants7. Therefore, novel therapies and approaches are needed to continue to improve outcomes for melanoma patients and overcome emerging mechanisms of resistance.
For melanoma and other cancers, there is much investigation into uncovering neoantigen biomarkers and an array of strategies to therapeutically target such neoantigens, including antigen-based vaccines and adoptive effector cell therapy (such as the recent approval of lifileucel, a personalized autologous tumor-infiltrating lymphocytes, or TIL, therapy)8,9,10,11. While the goal of these innovative approaches is to develop mainstream, clinically effective treatments, this may not be the case for many early therapeutics or may take decades to become realized through the industrial pipeline.
While targeted agents specific for melanoma are limited (namely BRAF and MEK inhibitors), there are other cancers that have different targeted drugs with long-term durability. For primary, locally advanced, and metastatic HER2/neu + breast cancer, as examples, patients often benefit from anti-HER2 monoclonal antibodies (i.e., trastuzumab and pertuzumab). These monoclonal antibodies mediate response via a variety of mechanisms, including (1) direct neutralization of HER2 and subsequent inhibition of downstream intracellular signaling of proliferation pathways, and (2) promotion of natural killer (NK) cell antibody-dependent cell-mediated cytotoxicity (ADCC)12,13. Both mechanisms may work simultaneously to generate responses against HER2-expressing tumors.
In addition to anti-HER2/neu monoclonal antibodies, patients have also more recently derived benefit from the newer antibody-drug conjugates (ADCs), including ado-trastuzumab (T-DM1 or emtansine) and trastuzumab deruxtecan (T-DXd)14,15. These more contemporary agents have been shown to be effective even when HER2 expression is low because the chemotherapeutic component of the ADC is delivered to the tumor targets (via HER2 recognition) in order to generate their effect16.
In this preclinical study, the goal was to determine whether the animal homolog of HER2/neu (namely the c-erb-B2 oncoprotein derived from rat) could be “repurposed” as a neoantigen target for mouse melanoma. It has been shown that human melanoma overexpresses HER2/neu in less than 5% of cases, and thus anti-HER2 therapies are not currently approved or used for melanoma17,18. However, the rationale of this study was that if c-erb-B2 could be expressed in melanoma tumors, then anti-c-erb-B2 monoclonal antibodies would generate effective anti-tumor responses through recognition of repurposed anti-c-erb-B2 as a neoantigen target. Our primary hypothesis was that the use of a c-erb-B2-encoding lentivirus vector would be effective in expressing c-erb-B2 as a neoantigen for anti-c-erb-B2 monoclonal antibody and lead to effective anti-tumor responses in an orthotopic mouse model of melanoma. Herein, we investigated the potential role of a c-erb-B2-encoding lentivirus vector as a novel therapeutic strategy for a pre-existing anti-c-erb-B2 monoclonal antibody (7.16.4) and determined the mechanism of action (e.g., neutralization, ADCC, or both) by which 7.16.4 might exert anti-tumor effects in our model.
Materials and methods
Animals
Male and female C57BL/6 mice (6–8 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). Prior to use, mice were allowed to acclimate to the animal facility for 1 week.
Animal care and use were in accordance with institutional guidelines and approved under Mayo Clinic IACUC protocol A00005532-23: Expressing the oncoprotein neu on B16 melanoma as a therapeutic target for dynamic control of tumor vessels.
When animal experiments were completed, mice were humanely euthanized via a 30–70% per minute displacement of cage/chamber air with compressed CO2. This was confirmed with cervical dislocation per our IACUC approved protocol.
Tumor cell lines
B16 (subclone F10) melanoma cells were obtained from ATCC and authenticated. Cells were cultured in RPMI 1640 (Roswell Park Memorial Institute Medium) supplemented with 10% FCS (fetal calf serum), 2 mM L-glutamine, 100 U/ml penicillin, 50 µg/ml streptomycin, and 50 µM β-mercaptoethanol (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA).
Reagents
FCS was obtained from Gemini Bioproducts (Woodland, CA). RPMI 1640, PBS, penicillin-streptomycin, L-glutamine, and β-mercaptoethanol were obtained from Life Technologies Inc. (Grand Island, NY). The monoclonal antibody 7.16.4, a mouse IgG2a antibody reactive with the rat neu oncogene-encoded p185 molecule, was obtained from Invitrogen (Thermo Fisher Scientific, Carlsbad, CA) and has been previously described by our group19. This antibody can bind to both human and rat c-erb-B2/neu. The IgG2a isotype control antibody was also obtained from Invitrogen. Enhanced chemiluminescence reagents and enhanced chemiluminescence film were obtained from Amersham International (Oakville, Ontario, Canada).
Construction of the B16 c-erb-B2 cell line (B16/neu)
A cDNA PCR amplicon corresponding to the full length rat c-erb-B2 gene was inserted into pLenti6.3 (Thermo Fisher Scientific, Carlsbad, CA) by NEBuilder® HiFi seamless cloning (NEB, Ipswich, MA) in order to generate the plasmid pLenti6.3 c-erb-B2. The rat c-erb-B2 gene was utilized as the neoantigen target in this study. High quantities of our lentivirus were generated by transfection of 293FT cells with pLenti6.3 c-erb-B2 and ViraPower™ packaging mix at a ratio of 1:3 using Lipofectamine 3000 per the manufacturer’s instructions (Thermo Fisher Scientific, Carlsbad, CA). Filtered fresh virus was added to naïve (wild-type) B16 cells at a 1:5 ratio in the presence of 6 µg/ml polybrene for 24 h, and then media was replaced. At 72 h post infection, infected B16 cells were selected with 10 µg/ml blasticidin for 2 weeks to generate the stable B16 c-erb-B2 cell line, which was designated B16/neu.
Quantitative PCR
Two-step quantitative reverse transcriptase-mediated real-time PCR (qPCR) was used to measure the relative abundance of c-erb-B2 mRNA from equal amounts of cDNA (10 ng) of B16 and B16/neu using the TaqMan™ gene expression assay (Rn00566561_m1) and POLR2A (Mm00839502_m1) (Thermo Fisher Scientific, Carlsbad, CA). Data were normalized to the endogenous control POLR2A20, and mRNA abundance was calculated using the established ΔΔCT method20.
Western blotting
Total cell lysates (50 µg) were resolved on Bolt 8% Bis–Tris Plus gels, which were electrophoretically transferred to a PDVF membrane with the iBlot2 apparatus (Thermo Fisher Scientific, Carlsbad, CA). Following 1 h in blocking buffer [5% dry milk, TTBS (Tween Tris buffered saline, TBS + 0.05% Tween)], blots were stained with Cell Signaling (Danvers, MA) antibodies against HER2/c-erb-B2 (D8F12) and b-actin (13E5), followed by a goat anti-rabbit secondary antibody (7074). All blots were washed 3 times with TTBS after antibody staining. Bands were detected with the Amersham ECL Prime chemiluminescent system (Cytiva, Marlborough, MA) and visualized with film.
Flow cytometry
For tumors, single cell suspensions were generated using a mouse tumor dissociation kit in combination with the gentleMACS™ Octo Desiccator (Miltenyi Biotec, Auburn, CA) per manufacturer protocol. Cell lines were harvested with a cell stripper dissociation reagent (Corning, Manassa, VA). Single cell solutions were washed twice in PBS and resuspended in 1× FACS buffer at 1 × 107 cells/ml. The FACS buffer was comprised of 0.5% BSA and 1mM EDTA in PBS.
Prior to staining, the monoclonal antibody 7.16.4 and IgG2a isotype control were initially conjugated to DyLight™ 650 using the Lightning-Link antibody labeling kit (Novus Biologicals, Centennial, CO). Antibodies were added at 0.5 ug per 100 µl of cell sample and incubated for 30 min on ice, alongside a set of unstained samples. Samples were washed 4× with the FACS buffer with centrifugations at 400G @ 5 min each. After the final wash, samples were resuspended in 0.5 ml FACS buffer with the viability dye SYTOX Green (1:1000), except for selected controls. Fluorescence was detected using the Attune NxT Cytometer (Thermo Fisher Scientific, Carlsbad, CA) and gated based on viability. Plots and calculations were analyzed with the FCS Express software.
Cell proliferation in vitro
Cells were plated at 3000 cells per well in a total volume of 200 µl in quintuple per condition/time point, using black, clear bottom 96 well plates. At each time point, cell proliferation was measured using the CyQUANT direct cell proliferation assay (C3501) according to manufacturer instructions (Thermo Fisher Scientific, Carlsbad, CA) and a SpectraMax M5 spectrophotometer (Ther Devices, San Jose, CA). For baseline (time = 0), cells were allowed to attach for 3 h before measuring with the CyQuant assay. Background values of wells containing no cells (“no cell wells”) was subtracted from all data prior to plotting values.
Tumor cell injections in vivo
For experiments involving naïve (wild-type) B16 or B16/neu (derived in vitro), 1 × 105 tumor cells were injected subcutaneously into the dorsal skin of C57BL/6 mice in 0.1 ml of PBS using a 23-gauge needle. Treatments were initiated when tumors reached 5 mm3 in measured volume (approximately 7–10 days post-inoculation). Tumors typically measured 2.5 × 2 × 1 mm, or 5 mm3. The antibody 7.16.4 was administered intraperitoneally (ip) at 400 µg in 0.2 ml of PBS every 3 days until mice reached the predefined endpoints listed in the next section of the “Materials and methods”. As an antibody control, isotype IgG2a was used at the same frequency and dosage ip (400 µg). Tumor measurements were taken every 5–6 days.
To address scientific rigor and reproducibility, equal numbers of male and female C57BL/6 mice were used for each experiment. Specifically, each group within a given experiment was comprised of 4 mice, including 2 male and 2 female. Experiments were completed in triplicate to assess for reproducibility, and data were pooled for graphical representation and statistical analysis. With 4 mice per group per experiment, pooled analyses consisted of 12 mice (6 male and 6 female).
Lentivirus injections in vivo
To obtain the optimal dose of in vivo pLenti6.3 c-erb-B2, we tested a linear set of viral doses from 6 × 105 pfu to 1 × 107 pfu. These doses were injected into naïve B16 tumors in C57BL/6 when the tumors grew to a minimum volume of 5 mm3 (example tumor measurement = 2.5 × 2.0 × 1.0 mm). Virus was injected every 3 days. Aliquots of the different viral dosages were delivered in 50 µl PBS. At smaller tumor sizes (5–10 mm3), 1–2 injections into the tumor were performed. As tumors grew beyond 10 mm3, 3–5 injections were performed to cover as much of the tumor volume as possible. Virus injections were performed for a total of 4 intratumoral inoculations (or over 12 days).
Combination experiments with pLenti6.3 c-erb-B2 and 7.16.4 in vivo
For C57BL/6 mice bearing naïve B16 (non c-erb-B2-expressing) tumors, 7.16.4 (400 µg ip) was added to pLenti6.3 c-erb-B2 intratumoral injections after the first 2 doses (6 days) of virus (either pLenti6.3 control or pLenti6.3 c-erb-B2). Virus and 7.16.4 injections were given every 3 days until endpoints were met. Similar to our prior experiments, equal numbers of male and female mice were used, and experiments were completed in triplicate, again to meets standard for rigor and reproducibility.
Assessment of tumor responses in vivo
Three perpendicular axes of the tumors were measured approximately every 5–6 days using external digital calipers (Control Co.). Tumor volume was calculated using the formula ½ × length × width × height. Mice that died or were euthanized due to morbidity, tumor ulceration, or tumor reaching the size endpoint (2000 mm3) were classified as events. Measurements were performed by a lab member who was blinded to the treatment group. All in vivo experiments were performed in triplicate for replication. Group/treatment randomization was performed on the basis of cage position on the rack(s) within our vivarium. Cages were assigned a numerical designation. For each group, a cage was selected randomly from the pool of all cages. To establish blinding within each experiment involving tumor response in animals, tasks were completed by different members of the lab. These tasks included tumor cell preparation, tumor cell inoculation, treatment administration, and measurement of tumor response (tumor growth and survival). Following experiment completion, groups were unblinded in order to analyze the data.
Immunohistochemistry
B16 tumor histologic sections were stained with standard hematoxylin and eosin and for NK cells [anti-mouse NK1.1 Monoclonal Antibody (PK136)—1:50 dilution, Invitrogen, Thermo Fisher Scientific]. Stained sections were scanned with an Aperio Scanscope XT (Leica Microsystems Inc., Buffalo Grove, IL) and evaluated using the Aperio Spectrum software.
Statistical analysis
Cyquant cell viability was assessed by a two-tailed Student t test. Tumor growth in vivo was assessed by two-way ANOVA (GraphPad Prism, Version 6). Error bars represent standard errors of the mean unless otherwise noted. Standard Kaplan–Meier methods (Log-Rank Mantel-Cox analysis) were used to perform the time to event analysis (GraphPad Prism, Version 6). For in vivo tumor growth experiments, a sample size of 4 mice per group would yield at least 80% power to detect a difference of at least 1.3 standard deviations (std) in tumor volume, using a 2-sample t-test at the 0.05 significance level per experiment. For all experiments, values of p < 0.05 were considered significant.
Results
Construction of c-erb-B2 lentivirus vector and confirmation of transfected wild-type B16 (B16/neu)
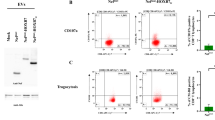
Successful construction of the c-erb-B2 lentivirus (Fig. 1A) and transfection into naïve (wild-type) B16 melanoma cells were confirmed via q-PCR (Fig. 1B), Western blot (Fig. 1C), and flow cytometry (Fig. 1D). Original Western blots with a positive neu control using the c-erb-B2 expressing mouse cell line MMC (derived from mammary cells of female BALB/c mice) are included in the supplementary materials (Supplementary Fig. 1). Figure 1C shows a cropped version of this Western blot, which highlights the c-erb-B2 expression in our newly created c-erb-B2-expressing cell line compared to naïve B16. Similar to human melanoma, naïve B16 showed only approximately 1% endogenous c-erb-B2 (neu) expression on flow cytometry. This was significantly increased with pLenti6.3 c-erb-B2 transfection to over 95% surface expression of c-erb-B2. The c-erb-B2-expressing B16 was designated “B16/neu.” We also confirmed via gene sequencing that the c-erb-B2 insert represented the oncogenic variant of neu, as opposed to the wild-type phenotype (Fig. 1E)21,22. Expression of the oncogenic c-erb-B2 variant was important for our model in order to better replicate human breast cancer, where oncogenic HER2 overexpression is the target of anti-HER2 therapies23.
(A) pLenti6.3 c-erb-B2 construct showing the insertion of the rat c-erb-B2 (neu) gene into the lentivirus DNA. The c-erb-B2-expressing B16 melanoma cell line was designated B16/neu. c-erb-B2 expression by B16/neu was confirmed by qPCR (B), Western blot (C), and flow cytometry (D), which altogether showed high levels (> 95%) of c-erb-B2 transcription and surface expression by the newly created B16/neu cell line. In contrast, naïve or wild-type B16 showed only about 1% c-erb-B2 expression. (E) The oncogenic variant of c-erb-B2 was inserted into pLenti6.3 and confirmed through gene sequencing, which demonstrated a valine to glutamine mutation. The oncogenic variant differs from wild-type c-erb-B2, which contains valine at this position.
Anti-neu monoclonal antibody (7.16.4) has no effect on B16/neu in vitro
Compared to naïve B16, the newly created B16/neu cell line showed increased growth and cell viability in vitro as measured by the Cyquant assay. This was observed throughout all time points up to 96 h.
We investigated whether 7.16.4 had a neutralizing effect on B16/neu in vitro. When 7.16.4 was added to the cell culture at increasing dose concentrations, there was no significant effect on B16/neu growth in vitro (Fig. 2). As expected, there was also no effect from 7.16.4 on naïve (non-c-erb-B2-expressing wild-type) B16.
Naïve B16 and B16/neu were grown in vitro, and cell viability was assessed via the Cyquant assay. Overall, B16/neu showed higher cell viability at all time points compared to naïve B16. When the anti-c-erb-B2 monoclonal antibody 7.16.4 was added in increasing doses to B16/neu, no statistically significant effects were observed on cell growth at any of the tested doses.
Interestingly, the absence of effect of 7.16.4 on B16/neu was contrary to our group’s previous investigation of 7.16.4 in c-erb-B2 + mouse breast cancer (using the c-erb-B2 + MMC cell line)19. However, naïve B16 does not normally express oncogenic c-erb-B2 (as shown in Fig. 1D), and its oncogenicity is driven by other melanoma-specific mutations24,25. Thus, neutralization of B16/neu growth by 7.16.4 in vitro was not highly anticipated.
Anti-neu 7.16.4 generates in vivo responses against B16/neu
To test our hypothesis that 7.16.4 could generate anti-tumor responses against B16/neu in vivo, B16/neu tumors were grown orthotopically within the dorsal skin of both male and female C57BL/6 mice. Although prior studies including our own have not demonstrated sex-based differences in B16 growth26,27, we accounted for possible sex-based differences in the setting of the newly transfected c-erb-B2, which is most often associated with breast cancer cells in female subjects. Equal numbers of male and female mice were included per group (male n = 2 and female n = 2, total mice per group per experiment = 4), and experiments were repeated in triplicate (pooled total n = 12 for analysis).
Figure 3 shows the pooled (n = 12) tumor growth (A) and survival analysis (B) for the control and experimental groups. As expected, there was no effect of 7.16.4 on naïve (non c-erb-B2-expressing) B16. There was slower tumor growth observed with the B16/neu controls (PBS or isotype) compared to naïve B16, but these differences were not statistically significant (p = 0.08 for PBS control, p = 0.09 for isotype control). When compared to isotype controls, B16/neu tumors treated with 7.16.4 showed statistically significant decreased tumor growth (p ≤ 0.0001) and improved median survival (55 days vs. 35 days, p ≤ 0.0001) with 5/12 (41.2%) tumors showing complete tumor response and long-term regression (p ≤ 0.0001). Table 1 shows the number of mice that had complete clinical responses and long-term (90 day) survival per experiment based on sex, which were equivalent across the 3 replicated experiments. Mice that obtained complete tumor response, of which 2 were female and 3 were male, did not have tumor volumes that exceeded 10 mm3 during the course of the experiment. This suggested that tumor growth kinetics beyond 10 mm3 was sufficient to overcome the anti-tumor effects of 7.16.4. Antibody therapy was stopped 60 days after tumor inoculation, and the mice with complete response showed long-term survival (90 days) and were then humanely euthanized.
(A) 7.16.4 generated statistically significant responses on B16/neu growth compared to B16 and isotype (IgG2a) controls. The bar shows statistical significance between the two groups of particular interest: (1) B16/neu + 7.16.4 and (2) Naïve (wild-type) B16 + 7.16.4. (B) Survival was also significantly improved with 5/12 (41.2%) of C57BL/6 mice bearing B16/neu tumors achieving a complete clinical response and long-term survival. Numbers at risk are shown. Mice that achieved complete clinical response (cure) were euthanized at day 90.
Anti-neu 7.16.4 responses are mediated by NK-cell antibody-dependent cell-mediated cytotoxicity
Whereas 7.16.4 did not demonstrate a neutralizing effect on B16/neu in vitro (as shown in Fig. 2), we hypothesized that the mechanism by which in vivo c-erb-B2 expression facilitated anti-tumor responses from 7.16.4 (as shown in Fig. 3) was obtained by NK-cell antibody-dependent cell-mediated cytotoxicity (ADCC). There has been an increasing body of evidence to suggest that anti-HER2/neu antibodies exert their anti-tumor effects via NK-cell ADCC28,29. To test this hypothesis, we performed separate in vivo experiments (n = 4 per group, 2 male and 2 female) with anti-NK cell monoclonal antibody (NK 1.1) at 200 µg injected every 3 days ip for C57BL/6 mice bearing B16/neu tumors. The NK 1.1 injections started 1 week before B16/neu inoculation to deplete NK cells prior to tumor growth and 7.16.4 treatment.
The addition of NK 1.1 inhibited the effect of 7.16.4 on B16/neu tumors (Fig. 4). Mice treated with NK 1.1 and 7.16.4 or NK 1.1 alone (control) showed similar growth rates (Fig. 4A) and survival (Fig. 4B) to the negative (PBS) controls. Again, mice treated with 7.16.4 only demonstrated superior statistically significant responses for tumor growth (p ≤ 0.0001 compared to 7.16.4 + NK 1.1). While there was not an observed difference in median survival (p = ns), there was statistical significance achieved with long-term survival (p = 0.0289) with 33.3% achieving complete clinical response. Table 2 shows the survival per experiment based on sex, which were again equivalent across the 3 replicated experiments.
We hypothesized that 7.16.4 generated anti-tumor responses via NK-cell antibody-dependent cell-mediated cytotoxicity. The addition of anti-NK cell monoclonal antibody (NK1.1) inhibited and essentially reversed the anti-tumor effects of 7.16.4 on both tumor growth (A) and survival (B). In (A), the bar shows statistical significance between the two groups of particular interest: (1) mice that received 7.16.4, which had the lowest tumor growth and (2) mice that received 7.16.4 + NK1.1, which showed similar growth to the negative control group and the NK1.1 control. In (B), complete response was again obtained for 4/12 mice treated with 7.16.4 alone. These mice were euthanized at day 90 post-tumor inoculation. Resected tumors from mice treated with NK1.1 showed little to no presence of NK cells on immunohistochemistry (C). In contrast, tumors resected from non-responders treated with only 7.16.4 showed a significantly higher number of stained NK cells on tumor sections (D), providing pathological evidence that NK1.1 effectively eliminated NK cells from infiltrating into B16/neu tumors. IHC staining of NK cells within naïve spleens (obtained from non-tumor bearing C57BL/6 mice) was used as a control (E).
Whole tumors were removed from mice treated with NK 1.1 +/− 7.16.4 and from non-responders that were treated with 7.16.4. Resected tumors were sectioned and analyzed by IHC for NK cells. Mice treated with 7.16.4 only (7.16.4 control) showed a qualitatively significant, higher number of stained NK cells within the harvested B16/neu tumors (Fig. 4D) compared to mice treated with NK 1.1 +/− 7.16.4 (Fig. 4C), which showed essentially no NK cells within the tumor parenchyma. This provided pathological confirmatory evidence that NK 1.1 effectively inhibited NK cell activity via NK cell depletion, and that at least one in vivo anti-tumor mechanism of action of 7.16.4 was NK-cell ADCC. IHC for NK cells was also performed on normal (non-tumor bearing) C57BL/6 splenic tissue as a control, showing expectedly high levels of NK cells within the spleen parenchyma (Fig. 4E).
In vivo transfection of naïve B16 melanoma tumors with c-erb-B2 lentivirus combined with 7.16.4 generates anti-tumor responses
The presented experiments have thus far utilized B16/neu that was developed in vitro using our c-erb-B2 lentivirus (pLenti6.3 c-erb-B2). To better model human melanoma that does not normally express high levels of HER2/neu, we tested the in vivo injection of naïve B16 tumors with pLenti6.3 c-erb-B2. We hypothesized that in vivo transfection of naïve B16 tumors with pLenti6.3 c-erb-B2 would generate c-erb-B2 as a neoantigen target for 7.16.4 and allow for the repurposing of anti-c-erb-B2 (7.16.4) therapy.
In vivo surface expression of c-erb-B2 as a neoantigen target for 7.16.4 was quantified by flow cytometry after resecting and processing the tumors. c-erb-B2 expression using this approach was shown to be dose dependent, with the highest dose (1 × 107 pfu) yielding approximately 45% successful expression of c-erb-B2 in vivo. This was the selected dose of pLenti6.3 c-erb-B2 used in this set of experiments. As a virus control, normal or “blank” pLenti6.3 was used at the same intratumoral dose and schedule.
Figure 5A and B show the tumor growth and survival data, respectively. Similar to our experiments with B16/neu (derived in vitro) and 7.16.4, the in vivo inoculation of naïve (non-c-erb-B2) B16 tumors generated c-erb-B2 as a neoantigen target for 7.16.4. Naïve B16 tumors treated with pLenti6.3 c-erb-B2 and 7.16.4 showed statistically significant responses compared to isotype controls. These responses were demonstrated by decreased tumor growth (p ≤ 0.0001) and improved long-term survival (p ≤ 0.0001), although there was no statistically significant difference in median survival (p = ns). Similar to our experiments with B16/neu derived in vitro, our in vivo approach yielded a 41.2% complete clinical response rate (5/12 mice, 3 female and 2 male). Table 3 shows the survival per experiment based on sex, which were again equivalent across the 3 replicated experiments. These mice showed long-term survival to 90 days. Similar to the prior experiments, tumors in these mice did not progress beyond 10 mm3 during the treatment period.
Naïve (wild-type) B16 tumors were inoculated with pLenti6.3 c-erb-B2 to generate c-erb-B2 as a neoantigen target for 7.16.4 in vivo. The addition of 7.16.4 to pLenti6.3 c-erb-B2 resulted in statistically significant decreased tumor growth (A) and improved long-term survival (B), again with 41.2% of mice showing complete clinical response (mice euthanized at day 90). In (A), the bar shows statistical significance between the two groups of particular interest: (1) naïve B16 + pLenti6.3 c-erb-B2 and (2) naïve B16 + pLenti6.3 c-erb-B2 + 7.16.4.
Discussion
Herein, we demonstrated the feasibility and efficacy of repurposing c-erb-B2 as a neoantigen target via a c-erb-B2-encoding lentivirus vector. To our knowledge, this represents the first preclinical approach to utilize c-erb-B2 as a neoantigen in melanoma. Importantly, there were no sex-based differences even with c-erb-B2 most commonly being associated with breast cancer in females (Tables 1, 2 and 3; Figs. 3, 4 and 5). c-erb-B2 expression was successfully generated both in vitro and in vivo, and anti-c-erb-B2 antibody produced highly significant responses with approximately 30–40% complete clinical response against B16, a highly aggressive melanoma tumor cell line. Responses appeared to be mediated via NK-cell ADCC, which is a well-established mechanism of antibody-mediated tumor killing.
Our preclinical model holds promise for future clinical relevance, particularly given the success of targeted anti-HER2/neu treatments. Anti-HER2/neu monoclonal antibodies (trastuzumab and pertuzumab) are part of the standard of care for all stages of breast cancer, which was derived from landmark trials30,31. Targeted anti-HER2 therapy is also beneficial for neoadjuvant treatment in resectable T2 primary tumors with or without lymph node metastases32. Contemporary studies have demonstrated continued benefit of anti-HER2/neu monoclonal antibodies in real world applications, which are also now being combined with newer therapies33,34. Within the last decade, novel HER2-based antibody-drug conjugates (including ado-trastuzumab and trastuzumab deruxtecan) have been developed and shown to be even more effective than the monoclonal antibodies alone. These drugs have been approved for metastatic disease and adjuvant therapy35,36, and in the future may be utilized in the neoadjuvant setting37,38,39. Newer studies are showing more promise that these anti-HER2/neu ADCs may indeed improve long-term outcomes in the neoadjuvant approach40. Importantly, the anti-HER2/neu ADCs have been shown to effective in low HER2-expressing cancers because the chemotherapeutic component of the ADCs exerts its anti-tumor effect after targeted delivery to the HER2 + tumors, even if the HER2 expression is low41,42,43. Altogether, this results in the bystander effect that is characteristic of ADCs, but not of monoclonal antibodies44. Our model aims to take advantage of these currently available therapies and repurposes them for cancers that do not express HER2/neu as a therapeutic biomarker, such as melanoma. If successful, our novel approach may establish anti-HER2/neu therapies for cancers that have limited effective systemic options and open up known effective treatments to a larger number of cancer patients.
The mechanism by which 7.16.4 functions to generate anti-tumor responses appeared to be mediated by NK-cell ADCC. Unlike prior work by members of our group in c-erb-B2 + mouse breast cancer cell lines (i.e. MMC)19, 7.16.4 did not appear to have a neutralizing effect on B16/neu (Fig. 2). It may be the case that other driver mutations with B16 (which is a melanoma cell line) accounted for the inability of 7.16.4 to inhibit its growth in vitro even with the expression of c-erb-B2, as opposed to c-erb-B2 + MMC where c-erb-B2 is a main driver of cell proliferation. However, depletion of NK cells in vivo reversed the positive effects of 7.16.4 in mice bearing B16/neu tumors (Fig. 4). Our findings contribute to a growing body of preclinical and clinical studies supporting NK-cell ADCC as a mechanism of action for anti-HER2 monoclonal antibodies12,13,28,29.
The application of our approach to melanoma is fitting because most human melanomas to not express HER217,45. Melanoma subtypes other than cutaneous melanoma, such as mucosal, uveal, and acral lentiginous melanomas, may have slightly higher expression of HER2 than the more common cutaneous melanomas18,46,47. In addition, a few case reports have showcased rare occurrences of metastatic melanoma clones that were susceptible to anti-HER2 therapy due to unusually high levels of HER2 expression48,49. Still, anti-HER2 therapy is not currently approved or part of the standard of care for the vast majority of patients diagnosed with cutaneous melanoma, and analysis of HER2 expression is not routinely performed for melanoma (unlike for breast cancer where HER2 status is assessed via IHC and FISH). Other potential biomarkers for melanoma, however, are emerging and may represent new candidates for targeted therapies50. HER3 has been increasingly investigated as a biomarker with increased expression in human melanoma and can be associated with a worse prognosis45,51,52,53. Other biomarkers of interest include AXL, EGFR, and MET, whereby preclinical studies offer promising results of possible future applications of novel targeted therapeutics54,55,56,57,58,59,60,61.
While we have demonstrated the effectiveness of our approach in a preclinical animal model, additional adjustments would need to be considered when translated to human therapy. We utilized the oncogenic (as opposed to wild-type) variant of c-erb-B2, comprising the entire gene sequence. Applying a similar approach to human therapy may increase the oncogenicity or metastatic potential of human cancers, which would be a counter-productive consequence of our approach. Thus, our future investigations will determine whether attenuated c-erb-B2 expression could generate similar responses. c-erb-B2 consists of superficial, transmembrane, and intracellular domains, which are altogether required to promote tumor growth62,63,64. However, utilization of just the extracellular domain may be sufficient to repurpose anti-c-erb-B2 targeted therapy. The extracellular domain has been shown in breast cancer models to generate immune responses without increasing tumorigenicity, which occurs through signal transduction mediated by the transmembrane and intracellular components65. Therefore, one of our future investigations will determine whether a virus vector encoded with only the extracellular c-erb-B2 sequence can yield similar anti-tumor responses. Refining our strategy in this manner may provide the most optimal combination of neoantigen recognition without increasing tumorigenicity, which will be critically important when translating our approach to human cancer studies.
Our chosen viral vector was the lentivirus pLenti6.3. While our recombinant plasmid pLenti6.3 c-erb-B2 was able to generate the B16/neu cell line in vitro (Fig. 1) and produce a high level of transfection and anti-tumor effect in vivo in naïve B16 tumors (Fig. 5), it is important to note that this vector does not result in cell lysis66,67. As a non-oncolytic plasmid, pLenti6.3 has the advantage of limiting c-erb-B2 expression within the injected tumor targets and thereby minimizes neoantigen spread to bystander tissues or to distant tissues should the plasmid enter the bloodstream during intratumoral inoculations. While there is an intrinsically subjective component to the technique of intratumoral injections or vaccinations (where precision may vary from provider to provider), use of pLenti6.3 likely restricts c-erb-B2 expression to the site of intratumoral injection.
We recognize that our data are early and are derived from a preclinical animal model of primary melanoma. Much of the morbidity and mortality for human melanoma stems from locally advanced, in-transit, and metastatic disease that are unresectable and unresponsive to current immunotherapies and targeted therapies68,69. Whether our biomarker repurposing approach can be applied to regional or distant metastases was not addressed by these experiments. However, intratumoral injection of immunogenic viruses comprise a feasible, effective, and approved method for treating melanoma. Specifically, Talimogene Laherparepvec (T-VEC) is a herpes simplex virus oncolytic therapy that selectively replicates within melanoma and produces granulocyte macrophage colony-stimulating factor (GM-CSF) to enhance systemic anti-tumor immune responses70,71. T-VEC can be injected into cutaneous melanoma tumors, but it can also be used for regional and distant metastases that can be accessed via image-guided percutaneous injections72. Thus, there is an established precedent for intratumoral virus injections for melanoma at both cutaneous (superficial) and metastatic (deep) anatomical locations. While the mechanisms differ between our anti-HER2 repurposing approach and T-VEC, the use of viral injection therapy has been demonstrated to be safe without increased risk of local-regional tumor spread or metastases from the virus injections themselves.
We recognize other limitations with our approach. Our strategy did not produce complete clinical responses in all animal subjects. However, the complete response rate was still quite high (> 40% in replicated experiments) and appeared to be associated with tumor growth kinetics. If tumor growth was able to plateau (at approximately 10 mm3), then the success of our approach was significantly higher than if tumors grew more rapidly and outpaced any potential anti-tumor responses. The observed tumor growth kinetics and its association with complete long-term response rates was reflected by the fact that not all of the median survival rates among experiments were statistically significant. However, improved statistically significant survival was obtained long term (i.e., 90 days), accounting for the later split in the survival curves. This observation would represent another potential barrier of our approach to human clinical translation, where advanced disease tends to be larger or bulkier than small primary tumors. In addition, the extent to which the injection of pLenti6.3 c-erb-B2 may inoculate normal (non-cancerous) peri-tumoral tissues is unknown. Expression of c-erb-B2 in the surrounding tumor tissues would be an unintended side effect of our approach and may increase the risk of local toxicity. Quantification of c-erb-B2 expression in peri-tumoral tissues will be investigated in future studies planned by our group. Lastly, although our results demonstrated efficacy of our repurposing approach, we were unable to include a true positive control in the form of an intrinsically expressing c-erb-B2 + mouse melanoma cell line because such a cell line is not available. Despite these limitations, our mouse model represents a promising starting point for our novel strategy and demonstrates proof of principle that c-erb-B2 can be repurposed as a neoantigen target for effective anti-c-erb-B2 therapies.
In conclusion, our approach to repurpose HER2/neu may be a timely, innovative strategy for melanoma and potentially other cancers that do not intrinsically overexpress oncogenic HER2/neu. Because of persistent limitations and emerging mechanisms of resistance to current therapies for melanoma, novel treatments are needed. In this study, the innovation of our approach is highlighted by the repurposing of contemporary effective therapies to malignancies for which they are not currently approved. Future preclinical studies are in development to determine whether our approach can be successful for regional or metastatic melanoma, larger primary tumors, and other cancers that do not normally express c-erb-B2.
Data availability
Data will be made available upon request. The corresponding author, Dr. Emmanuel Gabriel, can be contacted via telephone at 1-(904)-953-2523 and via email at Gabriel.Emmanuel@mayo.edu.
Abbreviations
- ADC:
-
Antibody-drug conjugates
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- ANOVA:
-
Analysis of variance
- FCS:
-
Fetal calf serum
- IHC:
-
Immunohistochemistry
- KM:
-
Kaplan-Meier
- Ip:
-
Intraperitoneal
- mm:
-
Millimeter
- NK:
-
Natural killer
- PBS:
-
Phosphate buffered saline
- pfu:
-
Plaque-forming units
- RPMI:
-
Roswell Park Memorial Institute Medium
- T-DM1:
-
Ado-trastuzumab
- T-DXd:
-
Trastuzumab deruxtecan
- TTBS:
-
Tween Tris buffered saline
- µg:
-
Microgram
- µm:
-
Micrometer
- µM:
-
Micromolar
References
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723. https://doi.org/10.1056/NEJMoa1003466 (2010).
Ribas, A. et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 16, 908–918. https://doi.org/10.1016/s1470-2045(15)00083-2 (2015).
Robert, C. et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 372, 2521–2532. https://doi.org/10.1056/NEJMoa1503093 (2015).
Wolchok, J. D. et al. Overall survival with combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 377, 1345–1356. https://doi.org/10.1056/NEJMoa1709684 (2017).
Marei, H. E., Hasan, A., Pozzoli, G. & Cenciarelli, C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 23, 64. https://doi.org/10.1186/s12935-023-02902-0 (2023).
Grob, J. J. et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol. 16, 1389–1398. https://doi.org/10.1016/s1470-2045(15)00087-x (2015).
Aguirre, L. E., Guzman, M. E., Lopes, G. & Hurley, J. Immune Checkpoint inhibitors and the risk of allograft rejection: a comprehensive analysis on an emerging issue. Oncologist. 24, 394–401. https://doi.org/10.1634/theOncologist.2018-0195 (2019).
Hall, M. S. et al. Neoantigen-specific CD4(+) tumor-infiltrating lymphocytes are potent effectors identified within adoptive cell therapy products for metastatic melanoma patients. J. Immunother. Cancer. 11 https://doi.org/10.1136/jitc-2023-007288 (2023).
Zeng, T. et al. Carrier-Free Nanovaccine: an innovative strategy for Ultrahigh Melanoma Neoantigen Loading. ACS nano. 17, 18114–18127. https://doi.org/10.1021/acsnano.3c04887 (2023).
Chesney, J. et al. Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts of the C-144-01 study. J. Immunother. Cancer. 10 https://doi.org/10.1136/jitc-2022-005755 (2022).
Keam, S. J. & Lifileucel First approval. Mol. Diagn. Ther. https://doi.org/10.1007/s40291-024-00708-y (2024).
Santana-Hernández, S. et al. NK cell-triggered CCL5/IFNγ-CXCL9/10 axis underlies the clinical efficacy of neoadjuvant anti-HER2 antibodies in breast cancer. J. Exp. Clin. cancer Res.: CR 43, 10. https://doi.org/10.1186/s13046-023-02918-4 (2024).
Muntasell, A. et al. Interplay between natural killer cells and Anti-HER2 antibodies: perspectives for breast Cancer immunotherapy. Front. Immunol. 8, 1544. https://doi.org/10.3389/fimmu.2017.01544 (2017).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380, 617–628. https://doi.org/10.1056/NEJMoa1814017 (2019).
Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401, 105–117. https://doi.org/10.1016/s0140-6736(22)02420-5 (2023).
Giugliano, F., Curigliano, G. & Tarantino, P. HER2-low expression in breast oncology: treatment implications in the smart chemotherapy era. Eur. J. Cancer Prevention 32, 149–154. https://doi.org/10.1097/cej.0000000000000781 (2023).
Kluger, H. M. et al. Her2/neu is not a commonly expressed therapeutic target in melanoma -- a large cohort tissue microarray study. Melanoma Res. 14, 207–210. https://doi.org/10.1097/01.cmr.0000130874.33504.2f (2004).
Gottesdiener, L. S. et al. Rates of ERBB2 alterations across melanoma subtypes and a complete response to trastuzumab emtansine in an ERBB2-amplified acral melanoma. Clin. Cancer Res. 24, 5815–5819. https://doi.org/10.1158/1078-0432.ccr-18-1397 (2018).
Knutson, K. L., Almand, B., Dang, Y. & Disis, M. L. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Res. 64, 1146–1151. https://doi.org/10.1158/0008-5472.can-03-0173 (2004).
Radonić, A. et al. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 313, 856–862. https://doi.org/10.1016/j.bbrc.2003.11.177 (2004).
Guy, C. T. et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. U.S.A. 89, 10578–10582. https://doi.org/10.1073/pnas.89.22.10578 (1992).
Castagnoli, L. et al. Pathobiological implications of the d16HER2 splice variant for stemness and aggressiveness of HER2-positive breast cancer. Oncogene 36, 1721–1732. https://doi.org/10.1038/onc.2016.338 (2017).
Freudenberg, J. A. et al. The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies. Exp. Mol. Pathol. 87, 1–11. https://doi.org/10.1016/j.yexmp.2009.05.001 (2009).
Danciu, C. et al. A characterization of four B16 murine melanoma cell sublines molecular fingerprint and proliferation behavior. Cancer Cell Int. 13 https://doi.org/10.1186/1475-2867-13-75 (2013).
Nakamura, K. et al. Characterization of mouse melanoma cell lines by their mortal malignancy using an experimental metastatic model. Life Sci. 70, 791–798. https://doi.org/10.1016/s0024-3205(01)01454-0 (2002).
Simon, S. R. & Ershler, W. B. Hormonal influences on growth of B16 murine melanoma. J. Natl Cancer Inst. 74, 1085–1088 (1985).
Gabriel, E. M. et al. Dynamic control of tumor vasculature improves antitumor responses in a regional model of melanoma. Sci. Rep. 10, 13245. https://doi.org/10.1038/s41598-020-70233-5 (2020).
Mandó, P. et al. A different approach to HER2 breast cancer in the immunotherapy era. Breast 60, 15–25. https://doi.org/10.1016/j.breast.2021.08.007 (2021).
Li, F. & Liu, S. Focusing on NK cells and ADCC: a promising immunotherapy approach in targeted therapy for HER2-positive breast cancer. Front. Immunol. 13, 1083462. https://doi.org/10.3389/fimmu.2022.1083462 (2022).
Baselga, J. et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 366, 109–119. https://doi.org/10.1056/NEJMoa1113216 (2012).
Schneeweiss, A. et al. Pertuzumab plus Trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24, 2278–2284. https://doi.org/10.1093/annonc/mdt182 (2013).
Huang, L. et al. Neoadjuvant-adjuvant pertuzumab in HER2-positive early breast cancer: final analysis of the randomized phase III PEONY trial. Nat. Commun. 15, 2153. https://doi.org/10.1038/s41467-024-45591-7 (2024).
Debien, V. et al. The impact of initial tumor response on survival outcomes of patients with HER2-Positive advanced breast cancer treated with docetaxel, trastuzumab, and pertuzumab: an exploratory analysis of the CLEOPATRA trial. Clin. Breast Cancer. 24, 421–430e423. https://doi.org/10.1016/j.clbc.2024.02.012 (2024).
Kuemmel, S. et al. heredERA breast cancer: a phase III, randomized, open-label study evaluating the efficacy and safety of giredestrant plus the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with previously untreated HER2-positive, estrogen receptor-positive locally advanced or metastatic breast cancer. BMC Cancer 24, 641. https://doi.org/10.1186/s12885-024-12179-9 (2024).
Hurvitz, S. A. et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial. ESMO Open 9, 102924. https://doi.org/10.1016/j.esmoop.2024.102924 (2024).
Fehm, T. et al. Trastuzumab Deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): patient-reported outcomes from a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 25, 614–625. https://doi.org/10.1016/s1470-2045(24)00128-1 (2024).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791. https://doi.org/10.1056/NEJMoa1209124 (2012).
Tolaney, S. M. et al. Adjuvant trastuzumab emtansine versus paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT): a randomized clinical trial. J. Clin. Oncol. 39, 2375–2385. https://doi.org/10.1200/jco.20.03398 (2021).
Mosele, F. et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat. Med. 29, 2110–2120. https://doi.org/10.1038/s41591-023-02478-2 (2023).
Takano, T. et al. Long-term outcomes of neoadjuvant trastuzumab emtansine + pertuzumab (T-DM1 + P) and docetaxel + carboplatin + trastuzumab + pertuzumab (TCbHP) for HER2-positive primary breast cancer: results of the randomized phase 2 JBCRG20 study (Neo-peaks). Breast Cancer Res. Treat. 207, 33–48. https://doi.org/10.1007/s10549-024-07333-7 (2024).
Narayan, P. et al. US Food and Drug Administration approval Summary: fam-trastuzumab deruxtecan-nxki for human epidermal growth factor receptor 2-Low unresectable or metastatic breast Cancer. J. Clin. Oncol. 41, 2108–2116. https://doi.org/10.1200/jco.22.02447 (2023).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20. https://doi.org/10.1056/NEJMoa2203690 (2022).
Li, W. et al. Combined therapy of dabrafenib and an anti-HER2 antibody-drug conjugate for advanced BRAF-mutant melanoma. Cell. Mol. Biol. Lett. 29 https://doi.org/10.1186/s11658-024-00555-z (2024).
Nicolò, E., Zagami, P. & Curigliano, G. Antibody-drug conjugates in breast cancer: the chemotherapy of the future? Curr. Opin. Oncol. 32, 494–502. https://doi.org/10.1097/cco.0000000000000656 (2020).
Liu, S., Geng, R., Lin, E., Zhao, P. & Chen, Y. ERBB1/2/3 expression, prognosis, and Immune Infiltration in cutaneous melanoma. Front. Genet. 12, 602160. https://doi.org/10.3389/fgene.2021.602160 (2021).
Forsberg, E. M. V. et al. HER2 CAR-T cells eradicate uveal melanoma and T-cell therapy-resistant human melanoma in IL2 Transgenic NOD/SCID IL2 receptor knockout mice. Cancer Res. 79, 899–904. https://doi.org/10.1158/0008-5472.can-18-3158 (2019).
Shu, W. et al. Lapatinib dysregulates HER2 signaling and impairs the viability of human uveal melanoma cells. J. Cancer 14, 3477–3495. https://doi.org/10.7150/jca.88446 (2023).
Cui, X. & Iwenofu, O. H. HER2-positive metastatic melanoma: a cautionary tale! Appl. Immunohistochem. Mol. Morphol. AIMM 28, e73–e75. https://doi.org/10.1097/pai.0000000000000836 (2020).
Gams, P., Dolenc Stražar, Z., Šoštarič, M., Bošnjak, M. & Kšela, J. Cardiac melanoma metastasis with ERBB2 gene amplification: a potential for future targeted therapy. Case Rep. Oncol. 14, 622–627. https://doi.org/10.1159/000514981 (2021).
Sukniam, K., Manaise, H. K., Popp, K., Popp, R. & Gabriel, E. Role of surgery in metastatic melanoma and review of melanoma molecular characteristics. Cells 13 https://doi.org/10.3390/cells13060465 (2024).
Reschke, M. et al. HER3 is a determinant for poor prognosis in melanoma. Clin. cancer Research: Official J. Am. Association Cancer Res. 14, 5188–5197. https://doi.org/10.1158/1078-0432.ccr-08-0186 (2008).
Wimmer, E., Kraehn-Senftleben, G. & Issing, W. J. HER3 expression in cutaneous tumors. Anticancer Res. 28, 973–979 (2008).
Shteinman, E. R. et al. Molecular and clinical correlates of HER3 expression highlights its potential role as a therapeutic target in melanoma. Pathology 55, 629–636. https://doi.org/10.1016/j.pathol.2023.03.007 (2023).
Liu, Z. et al. Recent discovery and development of AXL inhibitors as antitumor agents. Eur. J. Med. Chem. 272 https://doi.org/10.1016/j.ejmech.2024.116475 (2024).
Shao, H., Teramae, D. & Wells, A. Axl contributes to efficient migration and invasion of melanoma cells. PloS One 18, e0283749. https://doi.org/10.1371/journal.pone.0283749 (2023).
Nyakas, M. et al. AXL inhibition improves BRAF-targeted treatment in melanoma. Sci. Rep. 12, 5076. https://doi.org/10.1038/s41598-022-09078-z (2022).
Zhang, W. N. et al. Comprehensive analysis of the novel omicron receptor AXL in cancers. Comput. Struct. Biotechnol. J. 20, 3304–3312. https://doi.org/10.1016/j.csbj.2022.06.051 (2022).
Pietraszek-Gremplewicz, K. et al. Expression level of EGFR and MET receptors regulates invasiveness of melanoma cells. J. Cell. Mol. Med. 23, 8453–8463. https://doi.org/10.1111/jcmm.14730 (2019).
Boone, B. et al. EGFR in melanoma: clinical significance and potential therapeutic target. J. Cutan. Pathol. 38, 492–502. https://doi.org/10.1111/j.1600-0560.2011.01673.x (2011).
Schartl, M. et al. A mutated EGFR is sufficient to induce malignant melanoma with genetic background-dependent histopathologies. J. Invest. Dermatol. 130, 249–258. https://doi.org/10.1038/jid.2009.213 (2010).
Rákosy, Z. et al. EGFR gene copy number alterations in primary cutaneous malignant melanomas are associated with poor prognosis. Int. J. Cancer 121, 1729–1737. https://doi.org/10.1002/ijc.22928 (2007).
Kanthala, S., Mill, C. P., Riese, D. J., Jaiswal, M., Jois, S. & nd, & Expression and purification of HER2 extracellular domain proteins in Schneider2 insect cells. Protein Exp. Purif. 125, 26–33. https://doi.org/10.1016/j.pep.2015.09.001 (2016).
Lee, J., Dull, T. J., Lax, I., Schlessinger, J. & Ullrich, A. HER2 cytoplasmic domain generates normal mitogenic and transforming signals in a chimeric receptor. EMBO J. 8, 167–173. https://doi.org/10.1002/j.1460-2075.1989.tb03361.x (1989).
Hudziak, R. M. & Ullrich, A. Cell transformation potential of a HER2 transmembrane domain deletion mutant retained in the endoplasmic reticulum. J. Biol. Chem. 266, 24109–24115 (1991).
Fendly, B. M. et al. The extracellular domain of HER2/neu is a potential immunogen for active specific immunotherapy of breast cancer. J. Biol. Response Modif. 9, 449–455 (1990).
Luis, A. The Old and the New: prospects for non-integrating Lentiviral Vector Technology. Viruses 12, 859. https://doi.org/10.3390/v12101103 (2020).
Hamilton, A. M., Foster, P. J. & Ronald, J. A. Evaluating nonintegrating lentiviruses as safe vectors for noninvasive reporter-based molecular imaging of multipotent mesenchymal stem cells. Hum. Gene Ther. 29, 1213–1225. https://doi.org/10.1089/hum.2018.111 (2018).
Huang, J. et al. Global incidence, mortality, risk factors and trends of melanoma: a systematic analysis of registries. Am. J. Clin. Dermatol. https://doi.org/10.1007/s40257-023-00795-3 (2023).
Gabriel, E. & Skitzki, J. The role of Regional therapies for in-transit melanoma in the era of improved systemic options. Cancers (Basel) 7, 1154–1177. https://doi.org/10.3390/cancers7030830 (2015).
Andtbacka, R. H. et al. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. ASCO Annual Meeting. J. Clin. Oncol. 31, LBA9008 (2013).
Andtbacka, R. H. et al. Talimogene Laherparepvec improves durable response rate in patients with Advanced Melanoma. J. Clin. Oncology: Official J. Am. Soc. Clin. Oncol. 33, 2780–2788. https://doi.org/10.1200/jco.2014.58.3377 (2015).
Ferrucci, P. F., Pala, L., Conforti, F. & Cocorocchio, E. Talimogene Laherparepvec (T-VEC): an Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers (Basel) 2021, 13. https://doi.org/10.3390/cancers13061383 (2021).
Acknowledgements
We acknowledge Davitte Cogen for assistance with purchasing of the mice and materials used in each of the experiments. We also thank Brandy Edenfield for performing the IHC staining.
Funding
This work was supported by the following grants to Dr. Gabriel: (1) the Eagles 5th District Cancer Telethon – Cancer Research Fund Pilot Project Opportunities for New Investigators, (2) the Mayo Clinic Florida Research Accelerator for Clinicians Engaged in Research (RACER) Program, (3) Clinical and Translational Science Awards (CTSA) Grant Number KL2 TR002379 from the National Center for Advancing Translational Science (NCATS), and (4) the Mayo Clinic K2R Research Pipeline Award.
Author information
Authors and Affiliations
Contributions
E.G., B.N., S.B., and K.K contributed to the study conception and design. E.G., B.N., D.B., and S.V. performed the experiments and acquired the data. E.G., B.N., S.V., and B.S. analyzed and interpreted the data. B.S. performed the statistical analysis. E.G. drafted the initial manuscript. Each author participated in the critical revision and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gabriel, E.M., Necela, B., Bahr, D. et al. Expression of c-erb-B2 oncoprotein as a neoantigen strategy to repurpose anti-neu antibody therapy in a model of melanoma. Sci Rep 14, 24545 (2024). https://doi.org/10.1038/s41598-024-76209-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76209-z