Abstract
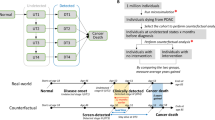
Lung cancer, the leading cause of cancer deaths globally, has better survival rates with early detection. Annual low-dose CT (LDCT) screenings are recommended for high-risk individuals due to age and smoking. These individuals are also at risk for other cancers. Our study explores gastrointestinal (GI) cancer mortality in lung cancer screening participants and the potential of LDCT screenings to detect pancreatic cancer. We utilized data from a prospective multi-institutional cohort study, the International Early Lung Cancer Action Project (I-ELCAP). We analyzed GI cancer deaths among participants in New York State (1992–2010), exploring demographics and GI cancer distribution. Radiologists retrospectively reviewed pancreatic cancer cases within 24 months post-LDCT, comparing findings with original reports. Among 10,150 participants, 189 died from GI cancers; mean age 75, mostly male smokers. Pancreatic cancer (41.8%) led, followed by esophageal (17.5%) and colon cancer (16.9%). Median time between baseline LDCT and death was 116 months (9.7 years). 82/189 (43.4%) participants died within 5 years of their last LDCT screening, with pancreatic cancer again prominent (45.1%). In 79 pancreatic cancer deaths, 17.7% occurred within 24 months post-LDCT. A re-review identified previously undetected pancreatic findings, with 4 out of 14 participants (28.6%) showing abnormalities. This underscores the potential of lung cancer screening programs to provide insights beyond lung health. This study of over 10,000 participants in a lung cancer screening program reveals that they are at risk for GI cancer deaths, particularly pancreatic cancer. Re-reviews of LDCT scans revealed previously undocumented pancreatic findings in a third of participants who died from pancreatic cancer, underscoring the need to identify, document, and follow up on these findings.
Similar content being viewed by others
Introduction
Lung cancer continues to be the leading cause of cancer-related deaths worldwide, but early detection significantly contributes to lowering lung cancer deaths1. The US Preventive Services Task Force recommends annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 50 to 80 years who have a 20 pack-year smoking history who are currently smoking or have quit within the past 15 years2. Lung cancer screening participants have an increased risk of other cancers due to shared factors like age and smoking3,4.
In the American population, the primary malignant conditions of the gastrointestinal (GI) tract, collectively referred to as GI cancers – including cancers of the esophagus, stomach, small intestines, colon, rectum, anal canal, liver, biliary tract, gallbladder, and pancreas – accounted for more lives lost than respiratory system cancers in 20235. While screening recommendations already exist for colorectal cancers2, gastric cancers in specific populations6, and hepatic cancers in high-risk patients7, there is currently no widely accepted protocol for pancreatic cancer screening, despite it being the third leading cause of cancer-related deaths in the United States. Pancreatic cancer accounts for approximately 62,200 new patients annually, leading to 48,800 deaths8. The most common type, pancreatic ductal adenocarcinoma, constitutes 90% of these cases, with an average diagnosis age of 658. Pancreatic cancer-specific survival rate is low but early-stage diagnosis (stage I) significantly improves five-year survival rates to around 80%9,10. Pancreatic cancers often develop from well-defined precursor lesions such as pancreatic intraepithelial neoplasms, intraductal papillary mucinous neoplasms, and mucinous cystic neoplasms11. These lesions can be detected on CT scans, suggesting a potential screening window12,13,14. However, due to its relative rarity, the US Preventive Services Task Force does not recommend general population screening, citing a grade D recommendation against it for asymptomatic individuals15,16.
The International Cancer of the Pancreas Screening consortium’s study of 2552 high-risk individuals (deemed at high risk for pancreatic cancer due to a strong family history or a pancreatic cancer gene mutation) revealed that 28 (1%) developed pancreatic cancer over a median follow-up of 29 months (Interquartile range of 40 months)17. Some participants in this study may be eligible for lung cancer screening (median age 60, 30% smokers)17.
In parallel, the 2023 PANDA (PANcreatic cancer Detection with Artificial intelligence) study demonstrated AI’s impressive capability to detect and diagnose pancreatic ductal adenocarcinoma and non-malignant lesions on non-contrast CT scans with high accuracy18. However, while AI holds promise, there is still a lack of substantial data, particularly on large cohorts without family risk.
Our study aimed to investigate the occurrence of deaths attributed to GI cancer, with a specific focus on pancreatic cancer, and examine early detection possibilities within the International Early Lung Cancer Action Project (I-ELCAP). Initiated in 1992, the project focused on diagnosing lung cancer using annual LDCT screenings, involving over 90,000 participants. Our objective is to enhance our understanding of the usefulness of LDCT screening in identifying and addressing pancreatic cancer among I-ELCAP participants in New York State, thereby maximizing the benefits of participating in a lung cancer screening program.
Materials and methods
We searched all reported deaths due to GI cancer-related deaths among participants who underwent LDCT screening in I-ELCAP 1992 to January 31, 20101. These participants include all those enrolled in the initial Early Lung Cancer Action Program19, NY-ELCAP20, and all subsequent participants enrolled the Early Lung Cancer Action Program at Weill Cornell Medical College between 1999 and 2010. The cause of death was coded according to the International Classification of Diseases, 10th Revision (ICD-10)21. We have conducted a search for deaths related to malignant neoplasms of digestive organs with the following codes, C15-C26: C15 (malignant neoplasm of esophagus), C16 (malignant neoplasm of stomach), C17 (malignant neoplasm of small intestine), C18 (malignant neoplasm of colon), C19 (malignant neoplasm of rectosigmoid junction), C20 (malignant neoplasm of rectum), C21 (malignant neoplasm of anus and anal canal), C22 (malignant neoplasm of liver and intrahepatic bile ducts), C23 (malignant neoplasm of gallbladder), C24 (malignant neoplasm of other and unspecified parts of biliary tract), C25 (malignant neoplasm of pancreas), and C26 (malignant neoplasm of other and ill-defined digestive organs).
All participants consented to the LDCT screening according to the approved institutional review board protocols at each participating institution. Each participant’s demographics, smoking history, height and weight, body mass index (BMI), comorbidities of diabetes, chronic obstructive pulmonary disease (COPD) or emphysema, hypertension, cardiovascular disease (CVD) were self-reported and documented at time of baseline enrollment.
All patients were followed annually by the principal investigator and by the study coordinator at each participating institution, who submitted the information required by the protocol to the coordinating center. Updates for each participant were regularly maintained and presented at each of the International Conferences on Screening for Lung Cancer held since 199922. In cases of known participant death, details including date and cause of death were obtained from their physician, family, and the National Death Index in the United States. Follow-up from date of last CT to death, the last contact, or January 15th, 2020, whichever came first, was documented for each participant.
We focused on patients who had died within 5 years after their last LDCT. We have opted for a 5-year timeline with the aim of encompassing precursor lesions associated with some slow-growing GI cancer23,24. We explored the demographic characteristics of these patients and examined the distribution of GI cancer among them. We reviewed the original structured reports of their last LDCT to identify any findings that could have been signs of GI cancer.
To specifically explore pancreatic cancer, experienced radiologists retrospectively re-reviewed the most recent and baseline LDCT scans of the participants who succumbed to pancreatic cancer within 24 months after their last LDCT, to identify any pancreatic findings such as calcification(s), atrophy/fatty infiltration, mass, pancreatic edema and pancreatic duct dilatation. We have chosen a 24-month time frame, considering the rapid development of pancreatic cancers and the duration of their preclinical phase17. Radiologists were blinded to the cause of death and vital status. These results were then compared with the original radiological report.
All methods and procedures carried out in this study were in strict compliance with the relevant guidelines and regulations, ensuring the highest standards of research integrity and participant safety.
All participants involved in the study provided informed consent to undergo low-dose CT screening. This process was part of a Health Insurance Portability and Accountability Act (HIPAA)–compliant, Institutional Review Board (IRB)–approved cohort study (Mount Sinai, STUDY-12-00212), conducted in accordance with the protocols set forth by the IRBs at each participating institution. The study adhered to both HIPAA regulations and the principles of the Declaration of Helsinki. The IRBs rigorously reviewed and sanctioned the procedures to ensure that the rights, safety, and well-being of all participants were upheld throughout the study.
Statistical analyses
We conducted a comprehensive analysis of the data, which included frequencies and descriptive statistics for all variables. Crude mortality rates, mortality of GI, and pancreatic cancer per 100,000 person-years were calculated. Baseline characteristics among participants who died from different causes of GI cancers were compared using the Student t-test or Kruskal-Wallis test for continuous variables, and chi-square or Fisher’s exact test for categorical variables. To compare the re-review and the original radiological reports, we employed chi-square or Fisher’s exact test. These analyses were executed using Medistica. pvalue.io, a Graphic User Interface to the R statistical analysis software, 2019-2425. All statistical tests were two-sided, and a p-value less than 0.05 was considered statistically significant.
Results
Among the 10,150 participants, 2909 (28.7%) died at the end of follow-up yielding a mortality rate of 149.95 per 100,000 person-years. Of these 2909 deaths, 189 (6.5%) died from malignant neoplasms of digestive organs (ICD-10, C15-C26); a mortality rate of 9.74 per 100,000 person-years. Mean age was 67 years, the majority were male (117/189, 61.9%), nearly all were smokers (187/189, 98.9%), with a high average pack-year of 53.9. The most prevalent comorbidity reported at baseline was COPD or emphysema (30/189, 15.9%), followed by hypertension (19/189, 10.1%), asthma (14/189, 7.4%), and diabetes (12/189, 6.3%). Five participants already presented with another cancer at baseline, and 26/189 (13.8%) had a family history of lung cancer. Characteristics of participants who died from GI cancers are detailed in Table 1.
The most frequent cause of GI cancer deaths was pancreatic cancer (C25) (79 participants of 189, constituting 41.8%), followed by esophageal cancer (C15) (33/189, 17.5%), colon cancer (C18) (32/189, 16.9%), liver cancer (C22) (18/189, 9.5%), stomach cancer (C16) (16/189, 8.5%), biliary tract cancers (C24) (6/189, 3.2%), rectal cancer (C20) and gallbladder cancer (C23) (1/189, 0.5%) (Fig. 1).
Distribution of gastrointestinal cancer mortality in 189 participants. This chart illustrates the distribution of GI cancer mortality in participants of a lung cancer screening program, highlighting the relative impact of each type on overall cancer-related deaths. Pancreatic cancer is the most frequent cause of GI cancer deaths and lacks a screening strategy. These data underscore the unmet need and the importance of maximizing participation in a lung cancer screening program to conduct a comprehensive health check.
The median time between baseline LDCT scan and death was 116 months (9.7 years). The median age at death was 75 years. The median time between the last LDCT scan performed as part of the lung cancer screening program and death was 72 months (6 years).
Pancreatic cancer consistently stands out as the leading cause of GI cancer mortality across age brackets at the time of death (60–69, 70–79, 80–89 years) (Fig. 2). Irrespective of smoking intensity, pancreatic cancer remains the predominant cause of GI cancer death, even among patients with lower smoking exposure (Fig. 3). GI cancer related deaths are more frequent among those with over 40 pack-years of smoking history. Ages at death and tobacco history do not significantly influence the distribution of mortality among different GI cancers.
Characteristics of participants who died from GI cancer within 5 years of the last LDCT screening
As shown in Tables 2, 82/189 (43.4%) participants died from GI cancer within 5 years of their last LDCT screening. Again pancreatic cancer emerged as the most prevalent (37 participants out of 82, constituting 45.1%), followed by esophageal cancer (14/82, 17.1%), colon cancer (13/82, 15.9%), liver cancer (7/82, 8.5%), stomach cancer (6/82, 7.3%), rectal cancer (2/82, 2.4%), biliary tract cancers (2/82, 2.4%) and gallbladder (1/82, 1.2%).
The median time between the participants’ baseline LDCT screening and death was 117 months (9.8 years). The median time between the participants’ last LDCT screening and death was 27.7 months (2.3 years).
According to the original documented reports of their last LDCT scans, 55 out of these 82 (67.1%) participants had lung nodules and 38 out of 82 (46.3%) had one or more abdominal findings. These abdominal findings were diverse: 11 participants with gallbladder findings, 5 with spleen findings, 13 with liver findings, 3 with pancreas findings, 9 with adrenal findings, and 16 with kidney findings. These findings were generally benign, but 17/82 (20.7%) exhibited findings that could be identified retrospectively as signs of GI cancer on their last LDCT. Eight participants had hepatic cysts (possible metastasis), two had suspicious pancreatic findings, two presented suspicious esophageal findings, two had suspicious finding for lung metastases, one had ascites, one had a suspicious gastric findings and one had a suspicious lymph node. For 7 out of 17 (41.2%) participants, additional investigations were recommended in the original report. 11 out of these 17 (64.7%) had findings that can be retrospectively interpret as signs of already advanced disease.
Characteristics of participants who died from pancreatic cancer
Among the 10,150 participants, 79 (0.8%) died of pancreatic cancer (mortality rate of 4.07 per 100,000 person-years). The baseline characteristics of these 79 patients were generally similar to those of the remaining GI cancer-related deaths (Table 1). Median time between baseline LDCT screening and death was 110 months (range: 66-1453 months); it was 67.1 months, (range 30.6–107.9 months) from their last LDCT screening.
Of the 79 participants who died of pancreatic cancer, two had pancreatic lesions identified on the original report of their last LDCT screening. These included a cystic structure in the tail of the pancreas (discovered 49 months before death), and a dilatation of the main pancreatic duct (noted 58 months before death). Both of these signs are indicators that should have raised suspicion of pancreatic cancer in the 2 participants26,27. The other 77 (97.5%) participants who died of pancreatic cancer had no reported pancreatic lesions.
Three of the 79 participants who passed away from pancreatic cancer, had cystic hepatic lesions identified in their last LDCT screening which may have been pancreatic cancer metastases, 9.5 months, 18 months, and 26 months respectively before death.
Original vs. re-review of baseline and last LDCT in lung cancer screening participants who died of pancreatic cancer
Of the 79 participants who died of pancreatic cancer, 14 (17.7%) died due to pancreatic cancer within 24 months following their last LDCT screening. No pancreatic findings were reported in the original baseline LDCT reports of these participants, but in 3 of 14 (21.4%) further investigation were recommended on their last LDCT screening: two for suspicious lung lesions that could indicate metastases, and one for the presence of ascites.
On re-review of the baseline LDCTs for these 14 participants (median time 31.5 months, IQR 20–75, before death), 4 participants (28.6%) (compared to 0/14 in the original report, p = 0.098) were found to have pancreatic lesions: one with calcifications, two with atrophy, and one with both calcifications and atrophy. Among them, 3/4 had stable pancreatic findings on the last LDCT (median 26 months later, IQR 14-37.5), while one showed worsening atrophy.
Overall, upon re-review of the last available LDCTs for these 14 participants (median 15.5 months after their baseline LDCT, IQR 12–65), two additional participants showed findings (one with calcification, one with atrophy), bringing the total to 6/14 (42.9%) with pancreatic abnormalities (compared to 0/14 in the original reports, p < 0.05) (Table 3).
Discussion
All I-ELCAP screening participants who had their baseline low-dose CT screening performed in New York City and State between 1992 and 2010 are included in this prospectively collected cohort. Among 10,150 participants, deaths from GI cancer -namely cancers of esophagus, stomach, small intestines, colon, rectum, anal canal, liver, biliary tract, gallbladder, and pancreas- were documented in 189 (1.9%). The distribution of these GI cancers across various primary sites is similar to that reported by recent studies which all showed that pancreatic cancer was the most common cause of GI cancer-related deaths5. These participants, on average, died 9.2 years after their entry into the program, suggesting a potential window for early diagnosis. Most of the participants who died from GI cancer were men and heavy smokers, with an average of over 50 pack-years of smoking.
Focusing on the 82 participants who died within 5 years, pancreatic cancer remains the most lethal GI cancer. This mirrors trends in the general population, where pancreatic cancer leads GI cancer mortality and paradoxically, it remains one of the cancers where most at-risk individuals do not meet the strictly reserved screening criteria28. To effectively leverage lung cancer screening programs for GI cancer detection and prevent mortality, concentrating on the pancreas for enhanced monitoring and early detection efforts seems strategic. The shared mechanism between pancreatic lesion and associated lung adenocarcinoma could potentially stem from smoking and the overconsumption of diets high in total fat29,30,31. There may also be other mechanisms at play, such as genetic and epigenetic factors, which still need to be explored.
Of the 82 participants who died from GI cancer within 5 years of their last LDCT, 17 (20.7%) had lesions that, upon retrospective re-review could have been considered as signs of cancer (median time before deaths: 27.8 months). However, it remains uncertain whether these findings, and their thorough exploration, could have influenced the course of events as over half of the cases had suspicious findings of already advanced disease.
We identified a cohort of 79/189 (41.8%) participants who passed away due to pancreatic cancer, primarily comprising elderly individuals and heavy smokers. Most of these individuals died long after exiting the screening program, limiting the scope of our conclusions. Among them, two (2.5%) displayed suspicious pancreatic lesions on the original report of their last screening LDCT scans, years before their deaths. However, these findings did not prompt further investigation. This suggests the importance of not only reporting these findings, but monitoring them over time, and conducting additional examinations if necessary.
Because of the rapid tumor progression of pancreatic cancer, we then focused on the participants who died from pancreatic cancer within 24 months after their last available CT. The original reports of their last scan, and their baseline LDCT, did not indicate any pancreatic findings. Additional investigations were originally recommended for three participants due to extra-pancreatic abdominal lesions on their last available LDCT, suggesting that the disease was already advanced. Reviewing their baseline LDCT showed that about a third of participants had pancreatic lesions that could have hypothetically necessitated additional investigations and perhaps changed their outcomes. This underscores the importance of careful scrutiny of the pancreas and timely management of suspicious pancreatic findings due to the extremely limited window of opportunity.
The American Gastroenterological Association guidelines suggest that pancreatic cancer screening should be considered for patients at high risk, including first-degree relatives of individuals with pancreatic cancer who have at least 2 genetically related affected relatives32. Studies are now focusing on well-defined high-risk cohorts. A meta-analysis of pancreatic cancer screening covering 16 studies and 1,551 high-risk participants found a pooled proportion of 1.4% in achieving screening goal33. These goals were defined as any diagnosis of resectable pancreatic cancer, pancreatic intraepithelial neoplasia-3, or high-grade dysplasia intraductal papillary mucinous neoplasm. These findings underscore the potential benefits of targeted pancreatic cancer screening in high-risk groups.
Our findings suggest that participants in a lung cancer screening program are suitable candidates for testing AI tools to detect and diagnose pancreatic cancer on non-contrast CT scans. Future feasibility studies should adjust imaging protocols to account for the rarity but rapid progression of pancreatic cancer in this population.
LDCT screenings have the potential to identify additional findings beyond lung cancer, including significant conditions like pancreatic cancer. Expanding LDCT’s role could make screening more comprehensive, improving early cancer detection and patient outcomes.
At present, our emphasis is solely on encouraging radiologists to identify pancreatic findings and to document these lesions. When pancreatic lesions are identified, an attempt should be made to localize, measure and describe them, along with noting other changes when identified, and follow them on the next scan. Suspicious pancreatic lesions should trigger additional investigation, even if recommendations for monitoring specific pancreatic findings in lung cancer screening participants are still lacking. For now, it is advisable to adhere to guidelines established for the general population34,35,36. Among these, MRI may be considered when fatty infiltration deviates from the common patterns of lipomatosis34,37. A 3.5 mm threshold is reasonable for diagnosing ductal dilatation and guiding further investigation38,39. Pancreatic cysts larger than 2.5 cm, or those presenting high-risk features-such as a thickened wall, lymphadenopathy, or changes in the main duct caliber- should be further evaluated35,40.
This underscores the need to follow a well-defined protocol for detecting and interpreting pancreatic findings, along with providing appropriate follow-up recommendations, especially for participants with a significant smoking history and diabetes; associated with an increased risk of pancreatic cancer41. The retrospective nature of this study, despite the prospective data collection, presents an inherent limitation. Relying on mortality data may introduce a bias, especially for pancreatic cancer, which, while relatively uncommon, is exceptionally deadly. This bias could lead to a distortion in the apparent frequency of pancreatic cancer compared to other cancers within our cohort. The entire pancreas may not be fully visualized in LDCT scans performed for lung cancer screening. Furthermore, image quality and soft tissue resolution may be affected by the use of LDCT. However, our study demonstrates that pancreatic findings can still be detected using LDCT. Future integration of AI tools may further improve detection and allowing for further evaluation using high-resolution imaging when necessary. Another potential bias arises from the radiologists’ re-review, which could notably impact results. Such a re-review may introduce detection bias, as radiologists might have focus more on the lungs in the initial report, potentially resulting in a less thorough examination or reporting of pancreatic and other gastrointestinal findings. Nevertheless, this influence is mitigated by the fact that radiologists were blinded to both the patient’s vital status and the study objectives. Another limitation is the small sample size, and the fact that we did not include CT scans of participants who did not die from pancreatic cancer as a comparative group in our re-review. Furthermore, compliance with the annual screening protocol was variable among the participants; that may impact validity and introduce uncertainties. Lastly, it is crucial to note that the study focuses on a diverse but American population based in New York City and State, limiting the generalizability of findings to other populations.
The significance of this study lies in its pioneering exploration of a critical avenue—leveraging thoracic LDCT scans conducted within the framework of lung cancer screening for the prevention of pancreatic cancer. The substantial inclusion of scans within our cohorts adds to the robustness of our findings.
Conclusion
This study of over 10,000 participants in an annual LDCT lung cancer screening program highlights the risk of death from GI cancers, particularly pancreatic cancer. A re-review of LDCT scans revealed undocumented pancreatic findings in baseline and last available LDCT scans of participants who died from pancreatic cancer within 2 years. It is crucial to carefully examine, document, and monitor the pancreas during lung cancer screenings. This study underscores the potential of lung cancer screening programs to provide valuable insights beyond lung health. Incorporating technological advancements, such as AI tools, could significantly enhance the effectiveness of these screenings and pave the way for including pancreatic cancer detection in lung cancer protocols, providing a comprehensive health check.
References
Henschke, C. I. et al. A 20-year follow-up of the International Early Lung Cancer Action Program (I-ELCAP). Radiology. 309(2), e231988 (2023).
US Preventive Services Task Force et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 325(10), 962–970 (2021).
Jacob, L., Freyn, M., Kalder, M., Dinas, K. & Kostev, K. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: a retrospective study of 422,010 patients followed for up to 30 years. Oncotarget. 9(25), 17420–17429 (2018).
White, M. C. et al. Age and cancer risk: a potentially modifiable relationship. Am. J. Prev. Med.46(3 Suppl 1), S7–15 (2014).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73(1), 17–48 (2023).
Kim, G. H., Liang, P. S., Bang, S. J. & Hwang, J. H. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest. Endosc. 84(1), 18–28 (2016).
Frenette, C. T., Isaacson, A. J., Bargellini, I., Saab, S. & Singal, A. G. A practical Guideline for Hepatocellular Carcinoma Screening in patients at risk. Mayo Clin. Proc. Innov. Qual. Outcomes. 3(3), 302–310 (2019).
Stoffel, E. M., Brand, R. E. & Goggins, M. Pancreatic Cancer: changing Epidemiology and New approaches to Risk Assessment, early detection, and Prevention. Gastroenterology. 164(5), 752–765 (2023).
Dbouk, M. et al. The Multicenter Cancer of pancreas Screening Study: Impact on Stage and Survival. J. Clin. Oncol. 40(28), 3257–3266 (2022).
Singhi, A. D., Koay, E. J., Chari, S. T. & Maitra, A. Early detection of pancreatic Cancer: opportunities and challenges. Gastroenterology. 156(7), 2024–2040 (2019).
Hruban, R. H., Maitra, A., Kern, S. E. & Goggins, M. Precursors to pancreatic cancer. Gastroenterol. Clin. North. Am. 36(4), 831–849 (2007).
Toshima, F. et al. CT abnormalities of the pancreas Associated with the subsequent diagnosis of clinical stage I pancreatic ductal Adenocarcinoma more than 1 year later: a case-control study. AJR Am. J. Roentgenol. 217(6), 1353–1364 (2021).
Jabłońska, B., Szmigiel, P. & Mrowiec, S. Pancreatic intraductal papillary mucinous neoplasms: current diagnosis and management. World J. Gastrointest. Oncol. 13(12), 1880–1895 (2021).
Scott, J., Martin, I., Redhead, D., Hammond, P. & Garden, O. J. Mucinous cystic neoplasms of the pancreas: imaging features and diagnostic difficulties. Clin. Radiol. 55(3), 187–192 (2000).
Wada, K., Takaori, K. & Traverso, L. W. Screening for pancreatic Cancer. Surg. Clin. North. Am. 95(5), 1041–1052 (2015).
US Preventive Services Task Force et al. Screening for pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA. 322(5), 438–444 (2019).
Overbeek, K. A. et al. Timeline of Development of Pancreatic Cancer and implications for successful early detection in high-risk individuals. Gastroenterology. 162(3), 772–785e4 (2022).
Cao, K. et al. Large-scale pancreatic cancer detection via non-contrast CT and deep learning. Nat. Med. 29(12), 3033–3043 (2023).
Henschke, C. I. et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 354(9173), 99–105 (1999).
New York Early Lung Cancer Action Project Investigators. CT screening for lung cancer: diagnoses resulting from the New York Early Lung Cancer Action Project. Radiology. 243(1), 239–249 (2007).
SteindelSJ International classification of diseases, 10th edition, clinical modification and procedure coding system: descriptive overview of the next generation HIPAA code sets. J. Am. Med. Inf. Assoc. 17(3), 274–282 (2010).
IELCAP [Internet]. (2024). https://www.ielcap.org/home/ielcap/events/
Hisabe, T., Hirai, F. & Matsui, T. Development and progression of colorectal cancer based on follow-up analysis. Dig. Endosc. 26(Suppl 2), 73–77 (2014).
Choi, S. J., Kim, H. S., Ahn, S. J., Jeong, Y. M. & Choi, H. Y. Evaluation of the growth pattern of carcinoma of colon and rectum by MDCT. Acta Radiol. 54(5), 487–492 (2013).
Medistica pvalue.io, a Graphic User Interface to the R statistical analysis software for scientific medical publications. 2019–24. Available on: [Internet]. https://www.pvalue.io
Tanaka, S. et al. Main pancreatic duct dilatation: a sign of high risk for pancreatic cancer. Jpn J. Clin. Oncol. 32(10), 407–411 (2002).
Chandwani, R. & Allen, P. J. Cystic neoplasms of the pancreas. Annu. Rev. Med. 67, 45–57 (2016).
Waleleng, B. J. et al. Screening of pancreatic cancer: target population, optimal timing and how? Ann. Med. Surg. (Lond). 84, 104814 (2022).
Yang, J. J. et al. Dietary Fat Intake and Lung Cancer risk: a pooled analysis. J. Clin. Oncol. 35(26), 3055–3064 (2017).
Castiñeira-Alvariño, M. et al. The role of high fat diet in the development of complications of chronic pancreatitis. Clin. Nutr. 32(5), 830–836 (2013).
Ye, X., Lu, G., Huai, J. & Ding, J. Impact of smoking on the risk of pancreatitis: a systematic review and meta-analysis. PLoS One. 10(4), e0124075 (2015).
Aslanian, H. R., Lee, J. H. & Canto, M. I. AGA clinical practice update on Pancreas Cancer Screening in High-Risk individuals: Expert Review. Gastroenterology. 159(1), 358–362 (2020).
Paiella, S. et al. Screening/surveillance programs for pancreatic cancer in familial high-risk individuals: a systematic review and proportion meta-analysis of screening results. Pancreatology. 18(4), 420–428 (2018).
Fung, C. I. et al. Recommendations for the management of incidental pancreatic findings in adults by the Canadian Association of Radiologists Incidental Findings Working Group. Can. Assoc. Radiol. J. 73(2), 312–319 (2022).
Megibow, A. J. et al. Management of incidental pancreatic cysts: a White Paper of the ACR Incidental findings Committee. J. Am. Coll. Radiol. 14(7), 911–923 (2017).
Aziz, H., Acher, A. W., Krishna, S. G., Cloyd, J. M. & Pawlik, T. M. Comparison of Society Guidelines for the management and surveillance of pancreatic cysts: a review. JAMA Surg. 157(8), 723–730 (2022).
Takahashi, M. et al. Fatty pancreas: a possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 109(10), 3013–3023 (2018).
Stamm, B. H. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Hum. Pathol. 15(7), 677–683 (1984).
Tirkes, T. et al. Reporting standards for Chronic Pancreatitis by using CT, MRI, and MR Cholangiopancreatography: the Consortium for the study of chronic pancreatitis, diabetes, and pancreatic Cancer. Radiology. 290(1), 207–215 (2019).
Tanaka, M. et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 17(5), 738–753 (2017).
Ben, Q. et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur. J. Cancer. 47(13), 1928–1937 (2011).
Acknowledgements
Preliminary results were presented as a poster at the 2023 Multidisciplinary Thoracic Cancers Symposium (Nov 30 - Dec 2, New Orleans, LA). Further details are in the International Journal of Radiation Oncology (Vol. 118, Issue 1e, Jan 2024) at www.redjournal.org.
Funding
This research was funded by Foundation Nelia and Amadeo Barletta (FNAB).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: L Gros, CI Henschke, DF Yankelevitz, R Yip (II) Administrative support: CI Henschke, DF Yankelevitz, R Yip(III) Provision of study materials or patients: CI Henschke, DF Yankelevitz, R Yip(IV) Collection and assembly of data: All authors(V) Data analysis and interpretation: All authors(VI) Manuscript writing: L Gros, CI Henschke, R Yip(VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
Dr. Yankelevitz is a named inventor on a number of patents and patent applications related to the evaluation of chest diseases including measurements of chest nodules. Dr. Yankelevitz has received financial compensation for the licensing of these patents. In addition, he is a consultant and co-owner of Accumetra, a private company developing tools to improve the quality of CT imaging. He is on the advisory board and owns equity in HeartLung, a company that develops software related to CT scans of the chest. He is on the medical advisory board of Median Technology that is developing technology related to analyzing pulmonary nodules and is on the medical advisory board of Carestream, a company that develops radiography equipment. He is also on the advisory board for LungLife AI.• Dr. Claudia Henschke is also an inventor of the patents and pending patents owned by Cornell Research Foundation (CRF). As of April 2009, she has divested herself of all royalties and other interests arising from these. She is on the medical advisory board for LungLife AI. • The other authors have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gros, L., Yip, R., Zhu, Y. et al. GI cancer mortality in participants in low dose CT screening for lung cancer with a focus on pancreatic cancer. Sci Rep 14, 29851 (2024). https://doi.org/10.1038/s41598-024-76322-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76322-z