Abstract
Complex reef structure, built via calcium carbonate production by stony corals and other calcifying taxa, supports key ecosystem services. However, the decline in coral cover on reefs of the Florida Reef Tract (US), caused by ocean warming, disease, and other stressors, has led to erosion exceeding accretion, causing net loss of reef framework. Active coral restoration, aimed at rapidly increasing coral cover, is essential for recovering reef structure and function. Traditionally, restoration success focused on the survivorship and growth of transplanted corals. This is the first empirical study to examine the role of high-density outplants of the endangered staghorn coral, Acropora cervicornis, in restoring positive carbonate accretion on Florida reefs. Successful transplantation of staghorn corals contributed to positive net carbonate production. Restored plots yielded a mean net carbonate production rate of 3.06 kg CaCO3 m− 2 yr− 1, whereas control plots exhibited net erosive states. Staghorn restoration plots sustained positive net carbonate production at a threshold of ~ 2.96% coral cover. However, bleaching, storms, and disease challenge these reefs, highlighting the need for restoration strategies that enhance resilience to environmental stressors. Establishing Acroporid aggregations through outplanting, alongside climate adaptation strategies, could foster reef habitat growth and enhance the recovery of ecosystem services.
Similar content being viewed by others
Introduction
Florida’s Coral Reef (US) has experienced severe declines in coral cover and abundance, threatening local communities, ecological stability, and the tourism sector that depends on the benefits provided by these biodiverse, complex, and highly productive marine ecosystems1,2,3. Since the 1970s, branching species like Acropora cervicornis (staghorn coral) that naturally form dense thickets have been lost to anthropogenic impacts and climate stressors (e.g. rising ocean temperatures and disease outbreaks)4. The combination of these stressors has led to a decline in reef structural complexity as well as the key ecosystem services, such as shoreline protection and tourism, that coral reefs provide3,5,6. The loss of reef habitat has been shown to decrease fisheries productivity and diminish the ability of reefs to attenuate wave energy, exposing coastal communities to heightened risks of erosion and flooding6,7,8,9. Given these threats, there is a pressing need to not only protect coral reefs from the impacts of climate change but also to actively restore Florida’s Coral Reef ecosystems that support a large local economy10.
Restoration practitioners have grown and outplanted 10,000s of corals across Florida’s Coral Reef in the past two decades to recover staghorn coral populations and increase coral cover11. To date, the evaluation of restoration success has focused on measuring coral colony survivorship and growth12,13,14. Yet there still is a lack of understanding of the potential benefits that coral restoration can have at an ecosystem level15. While coral colony metrics are important for measuring species recovery, a more comprehensive approach that includes additional ecological metrics is needed to determine whether the goal of creating a self-sustaining, resilient reef can be achieved through active restoration16,17,18,19,20,21.
Reef function and resilience are dependent on the growth and maintenance of complex reef structure5,6. In this context, net carbonate production (NCP) is a critical indicator of reef health, representing the balance between the calcium carbonate deposited by calcifying organisms and that removed by bioeroding taxa. Positive net carbonate production (i.e., net growth) indicates that accretion by calcifiers exceeds erosion, contributing to the buildup and maintenance of reef structure. Conversely, negative net carbonate production (i.e., net loss) signifies that erosion processes dominate, leading to reef degradation. Calcium carbonate skeletons, formed primarily by reef-building coral species, contribute positively to the overall growth of the reef, whereas bioeroding taxa, such as parrotfish, sponges, and other endolithic organisms, break down and erode the reef framework22,23,24. Parrotfish in particular can play a major role in reef bioerosion as they consume coral tissue and skeletons during foraging25,26. Understanding the dynamics between these competing forces is crucial as they serve as an indicator of reef condition, providing insights into the balance between constructive and destructive forces shaping reef ecosystems. Furthermore, monitoring carbonate production not only illuminates pathways to enhancing reef resilience but also underscores the critical role of reefs in delivering essential ecosystem services such as coastal protection and fisheries habitat.
Currently, the majority of reefs along Florida’s Coral Reef (> 70%), USA, especially those located in the northern region are experiencing a net loss of reef habitat due to reduced cover of reef calcifiers such as stony corals, a shift away from reef-building coral taxa towards more opportunistic coral taxa with limited carbonate production, and dominance by fleshy macroalgae3. Over the last decades, declines in coral cover on Florida’s reefs have been primarily caused by disease, storms, and repeated bleaching events2. In contrast to carbonate producers, several bioeroding taxa have been shown to benefit from changing environmental conditions, especially acidification, which could further shift the balance towards reef degradation3,27,28. Carbonate budget assessments quantify the abundance of reef calcifiers and bioeroders and use locally-adjusted calcification and bioerosion rates for the taxa encountered to provide an estimate of net carbonate production29,30,31. Here, we conducted carbonate budget surveys, following the ReefBudget methodology29, at Florida sites restored using the endangered staghorn coral Acropora cervicornis to determine whether the restoration of dense staghorn coral aggregations improves net carbonate production, reduces the impacts of bioerosion, and ultimately contributes to reef persistence.
Results
Staghorn coral cover was significantly different among plot types (Kruskal-Wallis’ chi-square = 31.02, df = 3, p ≤ 0.001). Staghorn coral cover in restored (9.97 ± 2.02%; mean ± Standard Error of the Mean (SEM)) and thicket plots (28.13 ± 7.58%) was significantly higher (p-value ≤ 0.05) than in control plots (unrestored control, 0%; thicket control, 0.10 ± 0.10%). These restored plots had reached high coral cover 2–4 years after transplantation.
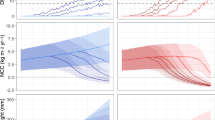
Accordingly, significant variation in net carbonate production was observed across plot types (Kruskal-Wallis’ chi-squared = 27.84, df = 3, p ≤ 0.001) (Fig. 1). Mean net carbonate production was highest for thicket (9.96 ± 3.16 kg CaCO3 m− 2 yr− 1) and restored (3.06 ± 0.86 kg CaCO3 m− 2 yr− 1) plots, whereas mean negative net carbonate production was reported for unrestored control (-1.62 ± 0.30 kg CaCO3 m− 2 yr− 1) and thicket control (-0.49 ± 0.17 kg CaCO3 m− 2 yr− 1) plots (Fig. 1; Table 1). Parrotfish erosion rates were highest in the unrestored control plots whereas thicket and thicket control plots recorded the lowest mean parrotfish bioerosion rates (Table 1). Sea urchin abundance was negligible in the survey sites resulting in urchin erosion rates that were predominantly zero. All plot types exhibited low levels of microbioerosion and sponge erosion (macrobioerosion). The bioeroding sponges observed included species in the genera Cliona and Siphonodictyon. Mean crustose coralline algae (CCA) carbonate production was negligible in all plot types but was highest in restored plots (Table 1).
A linear regression model identified A. cervicornis cover as a highly significant positive predictor of net carbonate production (p ≤ 0.001). The model accounted for approximately 99% of the variance in net carbonate production (Fig. 2). The linear regression model projected that a staghorn cover value of 2.96% is required to sustain a positive net carbonate budget at the scale of the plots surveyed (10 m in diameter). At a staghorn coral cover of 0%, the model predicted a net negative carbonate production of -1.21 kg CaCO3 m− 2 yr− 1 within plots.
A linear regression model revealed a significantly negative relationship between net carbonate production and parrotfish biomass (p = 0.022). However, although the p-value is significant, the low R-squared value (0.144) means that the predictor parrotfish biomass only explains a small portion of the variability in NCP (Fig. 2). In the unrestored control plots, the majority of carbonate production (52%) was accounted for by calcification of corals other than A. cervicornis. The largest contribution to bioerosion in both staghorn and unrestored control plots was that by parrotfish, which made up 73.0% and 82.84% of bioerosion within these sites, respectively.
Discussion
Coral restoration efforts focused on asexual propagation have shown promising results in terms of outplant survivorship and growth, highlighting the potential for active restoration to contribute to coral and reef recovery11,15. The staghorn restoration plots examined here exhibited high colony survivorship and maintained elevated coral cover 2–4 years after initial transplantation. However, the criteria for restoration success should extend beyond the immediate metrics of coral colony health and coral cover and encompass broader ecosystem processes, such as carbonate production, herbivory, and coral settlement to evaluate more wholistically how coral restoration may support and enhance overall reef structure and function. The increase in coral cover as a result of colony outplanting directly correlates with increased net carbonate production, with restored A. cervicornis plots showing significantly greater net carbonate production rates than control plots, which, in the absence of restoration, are in a state of net loss in Florida.
Recent studies have revealed a concerning decline in net carbonate production across Florida’s Coral Reef from 1996 to 2019 3,32,33. This decline, driven by the loss of stony corals due to multiple local and global drivers, can negatively impact reef structural complexity and result in a significant reduction in surface rugosity within just one year, especially in regions with heightened human disturbance34. Furthermore, reef sites in the Southeast Florida region specifically, encompassing all sites in this study, are almost exclusively in a state of net habitat loss3. Of the 9 reef sites surveyed in this study, all plots with wild and restored Acropora were in a state of net growth, while all control, unrestored plots were in a state of net loss. This indicates that the restoration of high-density staghorn coral aggregations can facilitate positive carbonate budgets and promote reef growth. Unrestored reef areas in Florida, characterized presently by low coral cover35, are stuck in a state of degradation as sources of physical and biological erosion exceed reef accretion3. Patterns of decline and bioerosion are only projected to increase in severity as Florida experienced its worst coral bleaching event on record in 2023, making reef restoration a crucial tool in the persistence of this degraded ecosystem until the climate driven factors of decline are mitigated8. Our results reflect these concerns but also document a pathway for mitigation through active restoration. The implications of these findings are profound, indicating that even a modest increase in A. cervicornis cover can shift a restored study plot within the Florida Reef Tract towards a state of positive net growth. The loss of reef habitat underscores the need for effective restoration strategies, such as enhancing coral cover through asexual propagation of fast-growing species, that can mitigate reef degradation and recover lost reef structure at small but expandable scales as proposed in the large-scale restoration of seven reefs in Florida as part of the Mission Iconic Reefs Program8,36.
Unsurprisingly, our carbonate budget surveys revealed a significant positive relationship between A. cervicornis cover and net carbonate production. In fact, the net carbonate production values recorded for well-developed A. cervicornis thickets in Florida (up to 23 kg CaCO3 m− 2 yr− 1) are among the highest recorded in the literature37,38. These values are due to the high complexity of staghorn colonies in mature thickets that often exceed 75 cm in height. The net carbonate production values recorded for restored staghorn plots in our study are also high but lower than those recorded for fully restored sites in Indonesia (20.6 ± 0.6 kg CaCO3 m− 2 yr− 1)38 and the Great Barrier Reef (25.3 ± 1.0 kg CaCO3 m− 2 yr− 1)37. In all of these cases, restoration was completed by using fast-growing branching corals that achieved high coral cover rapidly. If high coral cover and a robust thicket framework are achieved through active restoration, reefs could be more effective in protecting coastlines against storm damage, particularly in the face of sea-level rise8,9. Our findings also indicate that reef restoration using fast-growing branching species like A. cervicornis significantly reduces the minimum coral cover threshold necessary for a positive carbonate budget, from the 10% observed in reef communities3,31 to approximately 2.96% cover. This lower threshold is attributed to the high calcification rates and structural complexity of this branching species. Rather than focusing on achieving higher outplant densities at individual plots or sites, resources could be more effectively spread across multiple reef sites, achieving the necessary coral cover needed to promote net growth with lower coral biomass over a larger footprint and at reduced costs.
While focusing propagation and restoration efforts on fast-growing branching corals like A. cervicornis can boost carbonate budgets and quickly build habitat for associated taxa38, it is crucial to consider the inherent physical fragility and ephemeral nature of their skeletons compared to massive or encrusting species as well as their susceptibility to bleaching and disease39, requiring a more comprehensive approach to restoration that includes a variety of coral morphologies40,41. In contrast, massive or encrusting corals, with their dense skeletons and robust morphologies, integrate more permanently into the reef framework, enhancing structural stability and long-term resilience31,42. The resilience of massive coral species to thermal stress highlights their critical role in maintaining ecosystem stability through environmental fluctuations42. Therefore, incorporating slower-growing species is important for restoration efforts to fully recover ecosystem function and resilience. Even after significant loss in coral cover was seen following a bleaching event in 2015–2016, a reef in the Maldives showed resilience, particularly due to its dominance by massive corals43. Similarly, studies in the central Mexican Pacific have underscored the effectiveness of direct outplanting of both branching and massive coral species in enhancing coral carbonate production and overall reef function44. Multi-species coral restoration could be particularly advantageous in restoring the reef framework and associated ecosystem services, offering a balanced approach to enhancing both the rapid habitat formation provided by branching corals and the structural integrity formed by massive and encrusting species.
In the near absence of Diadema sea urchins in Florida after the mass mortality events of 1983–1984 and 2022 45,46,47, parrotfish emerged as the dominant drivers of bioerosion in our survey region. Previous studies found parrotfish responsible for approximately 80% of total bioerosion at sites along Florida’s Coral Reef48. This highlights the contrasting role these fish play on reef community dynamics49,50,51. In reefs where macroalgal overgrowth has resulted in declines in coral health and overfishing has reduced the abundance of fish grazers52, the protection and recovery of parrotfish populations has become a management priority53. While parrotfish play a crucial role in preventing the overproliferation of macroalgae and promoting successful coral recruitment54, their preference for feeding on the reef framework55 can have negative effects on reefs with sparse coral cover by exacerbating bioerosion. Additionally, parrotfish create a restoration bottleneck as they commonly target newly outplanted corals and can cause rapid tissue removal and mortality25,56. In our study, parrotfish biomass was lowest in wild thickets and restored plots, indicating an additional benefit of Acropora restoration. Parrotfish are not known to consume Acropora, and the branching morphology of the wild and restored corals may prevent parrotfish from grazing the substrate under these colonies. Therefore, restored Acropora thickets can foster a balance between herbivory and erosion, enhancing reef resilience.
While our study from Florida as well as the recent projects by Lange et al. from Indonesia38 and Nuñez Lendo et al. in the Great Barrier Reef37 show promising results of coral restoration through asexual propagation of fast-growing branching coral species in enhancing coral cover and net carbonate production within a relatively short amount of time (2–4 years), it is crucial to contextualize these successes within the broader scale of habitat formation, particularly considering the impacts of global change drivers like thermal anomalies that pose significant challenges to the long-term viability of restoration efforts. Recent bleaching events, including the severe episode experienced in Florida in 2023, underscore the vulnerability of coral ecosystems to thermal stress8,57. These events not only threaten immediate coral survival but also jeopardize the decadal-scale process of habitat formation critical for the sustained provision of ecosystem services. Therefore, our carbonate production estimates, while indicative of the potential for habitat recovery through restoration, must be viewed as part of a process that requires enhanced resilience against, and adaptation to, the increasing frequency and severity of bleaching events as well as other drivers of decline like disease and storms31. This perspective highlights the need for restoration strategies that not only focus on immediate gains in coral cover and structural complexity but also foster the long-term persistence and genetic diversity of coral populations through the implementation of restoration strategies such as sexual reproduction interventions as well as engineering approaches to mitigate bleaching impacts58. Enhancing coral resilience to bleaching, through the selection and breeding of thermally tolerant genotypes becomes imperative for ensuring that habitat formation continues in the face of climate change59. The integration of such adaptive measures is vital for maintaining the trajectory towards a positive carbonate budget and, ultimately, the structural and functional recovery of coral reef ecosystems.
Methods
Study sites
Reef carbonate budget surveys were conducted between October 2021 and December 2022 at all 9 sites in Miami-Dade County, Florida, USA (Table 2) (Fig. S1). These sites included: (1) six “restoration” sites where transplantation of A. cervicornis was conducted in 2017–2019 and (2) three “thicket” sites where natural thickets of wild A. cervicornis grow in dense aggregations (Fig. 3). Within each restoration site, two “restored” plots and two “unrestored control” plots were surveyed (n = 12 plots of each type). Within thicket sites, two “thicket” plots and two “thicket control” (no staghorn colonies) plots were surveyed (n = 6 plots of each type). Control plots were established at a minimum of 10 m from restored/thicket plots and from each other. Coral gardening techniques were used to grow staghorn corals for restoration and the restoration plots were established 2–4 years prior to this study60. Staghorn corals were grown in offshore nurseries and outplanted using cement onto circular plots with a diameter of ~ 10 m and a density of 2–4 corals per square meter. For the carbonate budget surveys, two replicate transects (10 m in length) were placed parallel to each other (at a distance of 2 m from each other) within each plot type following the methods described by Perry and Lange (2019)29. The data for each transect were averaged per plot to calculate plot-based values. Each plot was treated as independent units for analyses.
Visual comparison of unrestored control (left) plots without restored or wild staghorn corals, restored (center) plots with restored staghorn, and thicket (right) plots with wild aggregations of staghorn coral. Reef carbonate budget surveys were conducted between October 2021 and December 2022 at all 9 sites in Miami-Dade County. Restoration sites were restored between 2018 and 2020. All three images share the same scale.
Carbonate budgets
Census-based surveys of calcifiers and bioeroders were used to quantify net carbonate production using the ReefBudget methodology29,30 with the following modifications. Benthic composition was recorded by measuring the total linear distance occupied by each taxon along a chain deployed to conform to the reef substrate. The metal chain was deployed along a 10-m linear transect and was chosen over a flexible tape to avoid movement after deployment. Thus, the distances occupied by each taxon were calculated based on a three-dimensional structure of the reef. When surveying branching corals with a complex colony architecture (e.g., staghorn coral), estimates of living tissue coverage were made by recording branch numbers and diameters under the chain transect. The rugosity of the reef was calculated by taking the ratio of the total length of the chain over the 10-m linear distance that the chain occupied29.
Data collected by divers during the surveys was entered into the ReefBudget spreadsheets where coral genera, morphology, size, and literature-reported growth rates were used to calculate carbonate production (G = kg CaCO3 yr− 1) of each coral colony in the survey sites29. CCA cover was also multiplied by a literature-reported calcification rate and integrated into the transect lengths to estimate CCA carbonate production29. The data collected were analyzed as described by Perry & Lange (2019) to yield the percent cover (i.e. proportion of the chain length occupied by each taxon) and gross carbonate production of each transect (Gross Production G)29.
Parrotfish surveys were conducted using the Reef Visual Census (RVC) protocol with data collected from a stationary point with a 5 m radius for five minutes since the plot sizes in this study were only 10 m in diameter compared to the 50 × 5 m transects commonly used for fish surveys as part of the ReefBudget protocol61. These were completed at each plot, recording parrotfish species as well as life phase (juvenile and terminal) and fork length. Parrotfish bioerosion rates were calculated from species and size-specific bite rates (bites hr− 1), bite volume (cm2), and proportion of bites leaving scars29. The data collected were adjusted to the area of the surveys (78.5 m2). Divers swam along each 10 × 1 m transect to record species, abundance, and sizes of bioeroding sponges and urchins and data was entered into the ReefBudget spreadsheets to calculate the rates of bioerosion per transect for these taxa. Sponge surface area was calculated by measuring the approximate two lengths of the encrusting sponge, and urchins were counted when present along the 10 × 1 m transect. Urchin test diameters were estimated using calipers. Rates of macrobioerosion were calculated based on literature-reported rates29. Microbioroerosion was also calculated based on literature-reported rates and the amount of available suitable substrate (all benthic categories minus sand) on the reef for taxa such as cyanobacteria, chlorophytes, and fungi29.
Gross carbonate erosion of each transect was measured by summing together gross bioerosion by microborers, macroborers, parrotfish, and urchins. Gross carbonate production was measured by summing together gross production via stony corals and CCA. Net carbonate production was determined by subtracting gross carbonate erosion from gross carbonate production. Data were averaged for the two transects surveyed per plot to provide a single plot value.
Data analysis
Statistical analyses were performed using R Software (Version 4.4.0) with R studio extension62. All data were tested for normality using the Shapiro-Wilk test and Levene’s test. When normality assumptions were not met, non-parametric Kruskal-Wallis tests were used to test differences between A. cervicornis cover and net carbonate production among the four plot types. Where significant differences were found, the analysis was followed by a Dunn’s test for multiple comparisons with Bonferroni correction to test specific differences between plot types. Where ‘Site’ was considered a potential random factor, mixed-effects models were attempted; however, challenges such as singular fits prompted a reevaluation of including such random effects. Simple linear regression models were used to look at the relationship between net carbonate production and A. cervicornis cover (%) and parrotfish biomass (kg ha− 1) independently. Assumptions of linear regression, including linearity, independence, homoscedasticity of residuals, and normality, were checked using diagnostic plots. A significance level of α = 0.05 was used to determine the presence of statistically significant differences. All figures were created using ggplot2 R package63.
Data availability
The datasets generated during and used for the analysis of the current study are available upon request. Please contact the Corresponding Author for requests.
References
Precht, W. F. & Miller, S. L. Ecological shifts along the Florida reef Tract: the past as a key to the future. In (ed Arson, R. B.) Geological Approaches to Coral reef Ecology, 237–312. https://doi.org/10.1007/978-0-387-33537-7_9 (2007).
Manzello, D. P. Rapid recent warming of coral reefs in the Florida Keys. Sci. Rep. 5, 16762 (2015).
Morris, J. T. et al. Low net carbonate accretion characterizes Florida’s coral reef. Sci. Rep. 12 (2022).
Drury, C. & Lirman, D. Genotype by environment interactions in coral bleaching. Proc. R. Soc. B Biol. Sci. 288 (2021).
Alvarez-Filip, L., Dulvy, N. K., Gill, J. A., Côté, I. M. & Watkinson, A. R. Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. R. Soc. B Biol. Sci. 276, 3019–3025 (2009).
Graham, N. A. J. & Nash, K. L. The importance of structural complexity in coral reef ecosystems. Coral Reefs 32, 315–326 (2013).
Carlot, J. et al. Coral reef structural complexity loss exposes coastlines to waves. Sci. Rep. 13, 1–8 (2023).
Toth, L. T. et al. The potential for coral reef restoration to mitigate coastal flooding as sea levels rise. Nat. Commun. 14, 2313. https://doi.org/10.1038/s41467-023-37858-2 (2023).
Ferrario, F. et al. The effectiveness of coral reefs for coastal hazard risk reduction and adaptation. Nat. Commun. 5, 1–9 (2014).
Storlazzi, C. et al. (ed, D.) Rigorously valuing the role of U.S. coral reefs in coastal hazard risk reduction. U.S. Geological Survey Open-File Rep. 2019–1027 42. https://doi.org/10.3133/OFR20191027 (2019).
Lirman, D. & Schopmeyer, S. Ecological solutions to reef degradation: optimizing coral reef restoration in the Caribbean and Western Atlantic. PeerJ 4 (2016).
van Woesik, R. et al. Differential survival of nursery-reared Acropora cervicornis outplants along the Florida reef tract. Restor. Ecol. 29, e13302 (2020).
Ware, M. et al. Survivorship and growth in staghorn coral (Acropora cervicornis) outplanting projects in the Florida Keys National Marine Sanctuary. PLoS One 15, e0231817 (2020).
Schopmeyer, S. A. et al. Regional restoration benchmarks for Acropora cervicornis. Coral Reefs 36, 1047–1057 (2017).
Boström-Einarsson, L. et al. Coral restoration – A systematic review of current methods, successes, failures and future directions. PLoS One 15, e0226631 (2020).
Hein, M. Y., Willis, B. L., Beeden, R. & Birtles, A. The need for broader ecological and socioeconomic tools to evaluate the effectiveness of coral restoration programs. Restor. Ecol. 25, 873–883 (2017).
Calle-Triviño, J. et al. Approach to the functional importance of Acropora cervicornis in outplanting sites in the Dominican Republic. Front. Mar. Sci. 8 (2021).
Rinkevich, B. Rebuilding coral reefs: does active reef restoration lead to sustainable reefs? Curr. Opin. Environ. Sustain. 7, 28–36 (2014).
Ladd, M. C., Miller, M. W., Hunt, J. H., Sharp, W. C. & Burkepile, D. E. Harnessing ecological processes to facilitate coral restoration. Front. Ecol. Environ. 16, 239–247 (2018).
Ridlon, A. D. et al. Culturing for conservation: the need for timely investments in reef aquaculture. Front. Mar. Sci. 10, 1069494 (2023).
Lange, I. D., Perry, C. T. & Alvarez-Filip, L. Carbonate budgets as indicators of functional reef health: a critical review of data underpinning census-based methods and current knowledge gaps. Ecol. Indic. 110, 105857 (2020).
Glynn, P. W. & Manzello, D. P. Bioerosion and coral reef growth: a dynamic balance. Coral Reefs Anthropocene, 67–97. https://doi.org/10.1007/978-94-017-7249-5_4 (2015).
Perry, C. T. & Hepburn, L. J. Syn-depositional alteration of coral reef framework through bioerosion, encrustation and cementation: taphonomic signatures of reef accretion and reef depositional events. Earth Sci. Rev. 86, 106–144 (2008).
Perry, C. T. et al. Changing dynamics of Caribbean reef carbonate budgets: Emergence of reef bioeroders as critical controls on present and future reef growth potential. Proc. R. Soc. B Biol. Sci. 281, (2014).
Koval, G. et al. Fish predation hinders the success of coral restoration efforts using fragmented massive corals. PeerJ 8, e9978 (2020).
Bruckner, A. W., Bruckner, R. J. & Sollins, P. Parrotfish predation on live coral: ‘Spot biting’ and ‘focused biting’. Coral Reefs 19, 50 (2000).
Enochs, I. C. et al. Ocean acidification enhances the bioerosion of a common coral reef sponge: implications for the persistence of the Florida reef Tract. Bull. Mar. Sci. 91, 271–290 (2015).
Bell, J. J., Bennett, H. M., Rovellini, A. & Webster, N. S. Sponges to be winners under near-future climate scenarios. Bioscience 68, 955–968 (2018).
Perry, C., Lange, I. & ReefBudget Methodology Caribbean Version 2. Retrieved from https://geography.exeter.ac.uk/reefbudget (2019).
Perry, C. T. et al. Estimating rates of biologically driven coral reef framework production and erosion: a new census-based carbonate budget methodology and applications to the reefs of Bonaire. Coral Reefs 31, 853–868 (2012).
Perry, C. T. et al. Caribbean-wide decline in carbonate production threatens coral reef growth. Nat. Commun. 4 (2013).
Toth, L. T., Courtney, T. A., Colella, M. A., Kuffner Johnson, S. A. & Ruzicka, R. R. The past, present, and future of coral reef growth in the Florida Keys. Glob Chang. Biol. 28, 5294–5309 (2022).
Toth, L. T., Courtney, T. A., Colella, M. A. & Ruzicka, R. R. Stony coral tissue loss disease accelerated shifts in coral composition and declines in reef accretion potential in the Florida Keys. Front. Mar. Sci. 10, 1276400 (2023).
Magel, J. M. T., Burns, J. H. R., Gates, R. D. & Baum, J. K. Effects of bleaching-associated mass coral mortality on reef structural complexity across a gradient of local disturbance. Sci. Rep. 9, 1–12 (2019).
Ruzicka, R. R. et al. Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Mar. Ecol. Prog Ser. 489, 125–141 (2013).
National Oceanic and Atmospheric Administration (NOAA). Restoring Seven Iconic Reefs: A Mission to Recover Coral Reefs in the Florida Keys. NOAA Fisheries. https://www.fisheries.noaa.gov/southeast/habitat-conservation/restoring-seven-iconic-reefs-mission-recover-coral-reefs-florida-keys (Accessed 10 April 2024).
Lange, I. D. et al. Coral restoration can drive rapid reef carbonate budget recovery. Curr. Biol. 34 (6), 1341–1348e3 (2024).
Nuñez Lendo, C. I. et al. Carbonate budgets induced by coral restoration of a great barrier reef site following cyclone damage. Front. Mar. Sci. 10, 1298411 (2024).
Muller, E. M., Bartels, E. & Baums, I. B. Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis. eLife 7 (2018).
Tunnicliffe, V. Breakage and propagation of the stony coral Acropora cervicornis. Proc. Natl. Acad. Sci. U.S.A. 78 (4), 2427–2431 (1981).
Montero-Serra, I., Garrabou, J., Doak, D. F., Ledoux, J. B. & Linares, C. Marine protected areas enhance structural complexity but do not buffer the consequences of ocean warming for an overexploited precious coral. J. Appl. Ecol. 56, 1063–1074 (2019).
Loya, Y. et al. Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131 (2001).
Ryan, E. J., Hanmer, K. & Kench, P. S. Massive corals maintain a positive carbonate budget of a Maldivian upper reef platform despite major bleaching event. Sci. Rep. 9, 1–11 (2019).
Tortolero-Langarica, J. J. A., Rodríguez-Troncoso, A. P., Alvarez-Filip, L., Cupul-Magaña, A. L. & Carricart-Ganivet, J. P. The contribution of assisted coral restoration to calcium carbonate production in Eastern Pacific reefs. Rev. Biol. Trop. 71, e54849–e54849 (2023).
Lessios, H. A. Diadema antillarum 10 years after mass mortality: Still rare, despite help from a competitor. Proc. R. Soc. B Biol. Sci. 259, 331–337 (1995).
Lessios, H. A. The great Diadema antillarum die-off: 30 years later. Ann. Rev. Mar. Sci. 8, 267–283 (2016).
Hylkema, A. et al. The 2022 Diadema antillarum die-off event: comparisons with the 1983–1984 mass mortality. Front. Mar. Sci. 9 (2023).
Kuffner, I. B. et al. Improving estimates of coral reef construction and erosion with in-situ measurements. Limnol. Oceanogr. 64, 2283–2294 (2019).
Hoey, A. S. & Bellwood, D. R. Cross-shelf variation in the role of parrotfishes on the great barrier reef. Coral Reefs 27, 37–47 (2008).
Burkepile, D. E. & Hay, M. E. Impact of herbivore identity on algal succession and coral growth on a Caribbean reef. PLoS One 5 (2010).
Yates, K. K., Zawada, D. G., Smiley, N. A. & Tiling-Range, G. Divergence of seafloor elevation and sea level rise in coral reef ecosystems. Biogeosciences 14, 1739–1772 (2017).
Mccauley, D. J. et al. Positive and negative effects of a threatened parrotfish on reef ecosystems. Conserv. Biol. 28, 1312–1321 (2014).
Mumby, P. J., Steneck, R. S., Roff, G. & Paul, V. J. Marine reserves, fisheries ban, and 20 years of positive change in a coral reef ecosystem. Conserv. Biol. 35, 1473–1483 (2021).
Mumby, P. J. et al. Trophic cascade facilitates coral recruitment in a marine reserve. Proc. Natl. Acad. Sci. U. S. A. 104, 8362–8367 (2007).
Bellwood, D. R. & Choat, J. H. A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. Environ. Biol. Fishes. 28, 189–214 (1990).
Harrell, C. & Lirman, D. Dictyota defense: developing effective chemical protection against intense fish predation for outplanted massive corals. PeerJ 11, e14995 (2023).
Hughes, T. P. et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science (1979) 359, 80–83 (2018).
McLeod, I. M. et al. Coral restoration and adaptation in Australia: the first five years. PLoS One 17 (11), e0273325 (2022).
van Oppen, M. J. H. et al. Shifting paradigms in restoration of the world’s coral reefs. Glob. Change Biol. 23, 3437–3448 (2017).
Lirman, D. et al. Propagation of the threatened staghorn coral Acropora cervicornis: methods to minimize the impacts of fragment collection and maximize production. Coral Reefs 29, 729–735 (2010).
Bohnsack, J. A. & Bannerot, S. P. A stationary visual census technique for quantitatively assessing community structure of coral reef fishes. NOAA Tech. Rep. NMFS 41, 1–15 (1986).
RStudio Team. RStudio: Integrated Development for R (2020).
Wickham, H. ggplot2: elegant graphics for data analysis (Springer-Verlag New York, 2016).
Acknowledgements
We are grateful for the assistance of University of Miami’s Coral Restoration and Benthic Ecology Lab in the data collection and thank the Florida Department of Environmental Protection and the National Fish and Wildlife Foundation for their funding support.
Author information
Authors and Affiliations
Contributions
E.E.* led the data collection, analysis, and drafted the original manuscript. D.L. and J.M. supervised the study and, along with I.E. and N.B., contributed to the analysis. D.L., I.E., J.M., and N.B. were involved in writing, revision, and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Esplandiu, E., Morris, J., Enochs, I. et al. Enhancing reef carbonate budgets through coral restoration. Sci Rep 14, 27599 (2024). https://doi.org/10.1038/s41598-024-76799-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76799-8
Keywords
This article is cited by
-
Coral restoration can drive rapid increases in reef accretion potential
Scientific Reports (2025)
-
Reduced Atlantic reef growth past 2 °C warming amplifies sea-level impacts
Nature (2025)