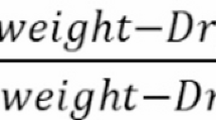Abstract
Cotton (Gossypium hirsutum L.) is a multipurpose crop. Abiotic stresses, especially extreme heat and drought, limit crop growth and thus reduce cotton yield by about 50%. In this study, 30 cotton genotypes were tested against low moisture stress in a pot experiment in triplicates along with control under wire house conditions. At the 3–4 leaf stage, different morpho-physiological and biochemical parameters were measured in order to select the low moisture stress-tolerant genotypes. For the selection of the best performing genotypes, Multi-Trait Genotype-Ideotype Distance Index (MGIDI) was used for the ranking of genotypes on the basis of multiple indices. For biochemical traits, 09 (TPC, TF, TSP, MDA, SOD, POD, CAT, APX, and Proline) out of 24 showed significant genotypic effects and were used for MGIDI. Eight genotypes (N-812 N-1296 N-696 N-377 N-121–896 N-T86, and N-3496) were observed to be best performing than others at 25% selection pressure (SI = 25%). For morpho-physiological traits, 14 out of 15 showed significant genotypic effects and used for MGIDI. Ten genotypes (N-1237 N-812 N-1296 N-696 N-9078 N-377 N-512 N-121 N-375, and N-896) were observed to be best performing at 35% selection pressure (SI = 35%). Six genotypes, i.e. N-812–1296 N-696 N-377 N-121, and N-896 were found common in both MGIDI analysis. In conclusion, three genotypes, i.e. N-696, N-896, and N-T86 proved to be most resilient to low moisture stress. Develop protocols, identified genotypes and markers that can be used for development of climate-smart cotton genotypes.
Similar content being viewed by others
Introduction
Cotton (Gossypium hirsutum L.) is a main agricultural crop that covers the largest textile manufacturing businesses globally, with a substantial influence on the economy of countries about $600 billion worldwide1. Cotton is recognized as a multipurpose crop, and its principal output, cotton lint, is used in the textile sector2. In recent years, cotton production has decreased from 13.960 to 11.935 million bales, a decline of 14%3. Gossypium hirsutum (90%) (features high yield and early system of cultivation4) play critical roles in the production of cotton due to their potential of high yield and cotton textiles manufacturers gained competitive advantage in the market. Cotton is a globally important crop that produces natural fiber. Gossypium hirsutum L. was developed to provide a higher yield of fiber and to endure severe environmental conditions5. Cotton genetic resources are large, scattered geographically over five continents, and include roughly 45 diploid and 5 tetraploid Malvaceae species6,7. Two allotetraploid cotton species, G. hirsutum L. (source of the upland cotton), and G. barbadense L. (source of the Egyptian, Sea Island and Pima cotton), were domesticated about 4000 years ago independently8,9. The remaining three tetraploid species of cotton have not been domesticated and are distributed largely: G. mustelinum Miers ex Watt is found mostly in Brazil10,11. Nowadays, the tetraploid specie, G. hirsutum account for the major production of cotton globally. Evidence suggests that selection by breeders has resulted in significant alterations in cotton cultivars since domestication, including plant habit, lack of developmental responses to light and dark period, early blooming, increased boll size, fiber properties (better fiber strength, length and fineness) and early methods of cultivation12.
Unexpected climate shifts and altering weather extremes have resulted in the high global temperatures and unpredictable patterns of rainfall in recent years, posing serious challenges to cotton production sustainability13. Abiotic stresses, especially extreme heat and drought, limit crop growth and thus reduce cotton yield by about 50%14. Drought stress influences the growth of plants by altering morphological, physiological, biochemical and molecular processes15. Drought stress has developed as a major concern globally that has a considerable impact on production of cotton. Improving the efficiency of water usage and implementing the strategies of water conservation have emerged as critical issues in irrigated agriculture16. The drought had drastic impacts on the photosynthesis of cotton plants. As the drought intensity increased, the gaseous-exchange metrics net photosynthesis, stomatal diffusive resistance, and transpiration showed a declining trend. As the severity of the drought stress increased, both hydrogen peroxide and MDA levels increased, which indicates the oxidative stress17.
At different growth phases, drought stress significantly affects cotton, which influences its physiological processes, yield, and fiber quality18. Drought stress triggers an internal heatwave effect which reduces the photosynthetic activity and stunts growth in plants19. The vulnerability of cotton to low moisture stress varies across different phases of development, with crucial periods during seedling growth, flowering, and boll development. At the seedling stage which is sensitive, drought stress could lead to physiological changes that include increased lipid peroxidation and decreased chlorophyll concentration, indicating oxidative stress20. Drought stress diminishes relative water content (RWC) in cotton seedlings, which is essential for sustaining cell turgor and overall plant survivability. A decrease in RWC leads to a reduction in leaf expansion, root development and negatively impacts plant growth21.
Drought stress frequently results in the gradual increase of the ROS (reactive oxygen species) at the cellular level. Excessive production of ROS causes oxidative stress to the photosynthetic machinery and adversely affects cell activity22. The increased level of ROS considered as a threat to plant cells, but can also act as a secondary messenger in the signal transduction pathway of stress23. The ability of plants to scavenge ROS and reduce their negative impacts may be related to their drought tolerance24. Drought stress affects plants via stress signaling/sensing and the activation of multiple parallel stress responses (including physiological, morphological, molecular, and biochemical processes)25. The indirect effect on performance is connected to an increase in ROS generation, which harms macromolecules during oxidative stress and reduces yield. It is critical to discover the major components and attributes of plant stress (drought) tolerance network in order to generate more drought tolerant cultivars16.
Many researchers have explored numerous factors in the cotton plant, including morphological aspects (leaf, root, and stem growth) and physiological parameters (molecular, histological, and anatomical) as essential selection criteria related with resilience to drought26. Nevertheless, none of these features have been consistently proven to be positively related with drought resistance in cotton27. Based on the duration, timing, and intensity of drought stress, multiple strategies can be used to generate cotton types that are more water efficient and drought tolerant. The first part is to comprehend cotton plant reactions and behavior in response to water scarcity and droughts28. In this regard, scientists revealed that by breeding tolerant cotton plants, we may be able to overcome these critical situations29.
Therefore, in this experiment, numerous physiological, morphological, and biochemical parameters were assessed in normal and low moisture stress conditions in order to screen cotton genotypes at the seedling stage, and their comparison was made to determine which trait is more influenced by low moisture stress. Furthermore, this study presents effective breeding strategies for improving low moisture tolerance in cotton.
Materials and methods
Experimental site
The cotton experiment was conducted in the wirehouse of Marker Assisted Breeding Group (MABG), Plant Breeding and Genetics Division, NIAB Faisalabad (started on March 03, 2023).
Cotton germplasm and growth conditions
In this experiment, thirty diverse cotton genotypes developed at Plant Breeding and Genetics Division, NIAB Faisalabad (Table 1) were grown in CRD design for evaluation of their response to low moisture stress at three to four true leaves and the first square stage. No voucher specimens are involved in this research work. The cotton experiment was carried out with three technical replicates, in which each replication consisted of 15 cotton plants (biological replicates) in 05 bags.
All polythene bags filled with 02 kg of soil were watered at 50% of the field capacity before the seed sowing. The soil used in the study was sandy clay loam composed of 45% sand, 33% silt, and 22% clay sourced from the NIAB experimental field. Seeds were planted (on March 07, 2023), in polythene bags. For sowing, the cotton seeds were submerged in the aerated dehydrated calcium sulphate (CaSO4.2H2O) overnight and the next morning the cotton seeds were sown at approximately 2 cm depth. All plants were watered at field capacity after one week of sowing.
Treatments
At the first true leaf stage (18–20 days after sowing), plants were divided into two groups, i.e., non-stress (control) and low moisture stress. In control plants, water was maintained at field capacity. While low moisture stress was applied by maintaining the water level at 50% field capacity. The water level was maintained by weighing each bag on alternate days for both stressed and control sets. After stress treatment, various physiological parameters were measured and noted between 11 a.m. and 2 p.m. on the day before harvesting. Then, 50 -day-old plants were harvested (27-04-2023) and data for morphological traits was recorded and leaf samples for biochemical analysis were stored at -40 °C till further analysis. Details of the procedures are as follows.
Physiological analysis
Among physiological traits, diffusive resistance (DR), transpiration Rate (TR), and leaf temperature (LT) was recorded with Porometer.
Transpiration rate
The transpiration rate was recorded in a fully expanded fourth leaf of cotton genotypes from the upper surface by using a Li-1600 Steady State Porometer and expressed in unit microgram cm− 2 s− 1.
Stomatal diffusive resistance
The physiological trait stomatal diffusive resistance of cotton leaves was measured by using a Li-1600 Steady State Porometer which has a sensor (lithium chloride). It was calibrated before recording the data30. This data was recorded from a fully expanded leaf of cotton genotypes of every replicate and expressed in unit s cm− 1.
Leaf temperature
The physiological trait leaf temperature was recorded from the upper surface of cotton genotypes of each replicate using a Li-1600 Steady State Porometer. Leaf temperature was expressed in unit ̊C. The measurements of data were taken at 13 ~ 14 h.
Relative water content
Relative Water Content (RWC) of cotton leaf samples was measured and data values were recorded in percentage31. The leaf samples of cotton genotypes were collected for RWC measurement, and leaf fresh, turgid and dry weights were measured by using a sensitive weighing scale. The following formula was used to determine the RWC32.
\({\text{RWC}}=\left( {{\text{fresh weight}} - {\text{dry weight}}} \right)/\left( {{\text{turgid weight}} - {\text{dry weight}}} \right) \times 100\)
SPAD measurements
SPAD (Soil Plant Analysis Development) chlorophyll meter33 was used for the measurement of SPAD values of the fourth fully expanded uppermost leaves. For each leaf sample, three measurements were taken with a SPAD meter on different sections of the leaf.
Morphological analysis
Different morphological traits like fresh leaf weight (FLW), wilted leaf weight (WLW), turgid leaf weight (TLW), dry leaf weight (DLW), dry root weight (DRW), fresh root weight (FRW), dry shoot weight (DSW) and fresh shoot weight (FSW) were recorded in grams. Fresh root length (FRL) and fresh shoot length (FSL) were recorded in cm.
Biochemical analysis
All biochemical analyses were performed at the MAB Lab-1 of the Plant Breeding and Genetics Division, Nuclear Institute of Agriculture and Biology (NIAB), Faisalabad, Pakistan. Details of different methodologies are given below.
For biochemical analyses and different enzyme estimations, fresh cotton leaves (0.15 g) were crushed in a cold extraction buffer. Specific extraction buffers were used for each enzyme. Samples were centrifuged at 15,000×g for 10 min at 4 °C. The supernatant was used for the determination of various enzyme activities and other biochemical assays. For each treatment, the analysis was performed in duplicate.
Total phenolic content
Total phenolics assay was performed according to the micro colorimetric method, in which the Folin-Ciocalteau (F-C) reagent was used34. First, 0.05 g weighted cotton leaves were homogenized in the dark for 2 days after adding the cold 95% methanol (500 µl). Then samples were centrifuged for 5 min at 14,000 g. The supernatant was used to calculate TPC. The assay procedure was followed as: F-C reagent (150 µl) was added in 100 µl of the sample vortex completely, then added 1.2 ml of Na2CO3 (700 mM). At room temperature, the cotton samples were incubated for 1 h. The phenolic content of cotton samples was determined using a linear regression equation.
Total tannin content
From the TPC experiment, the supernatant after spectrophotometer values were taken. For measurement of total tannin content (TTC) using the method35. 0.1 g polyvinylpolypyrrolidone (PVPP) was added to TPC samples and mixed for 2–3 min. The samples were then centrifuged at 14,000 g. Then, the supernatants of all samples were used to measure their TTC by using UV-Vis (765 nm). Each sample was measured in duplicate. Tannic Acid (0.5 mg/ml) was used for the calibration. Results were calculated based on a standard curve and expressed in units as mg TA/ g plant samples.
Total flavonoid content
Total flavonoid content was determined by using the aluminum chloride colorimetric method36. A 1 ml sample (200 µl + 800 µl H2O) of cotton leaves was added into 50 µl of 2% NH4Cl solution then added 1 M potassium acetate (50 µl). For 40 min, the mixture was incubated at 40℃ with frequent shaking. Then the optical density of the sample mixture was taken at 415 nm using a UV-VIS spectrophotometer against a blank sample without NH4Cl. The resulting data was expressed as mg quercetin equivalents/weight of fresh plant material (g)37.
Ascorbic acid
For analysis of ascorbic acid, the 2,6-dichloroindophenol (DCIP) method was used38. For a brief explanation, vitamin C changed DCIP into DCIPH2. This conversion was observed as a decreasing trend by measuring the absorbance at 520 nm using a spectrophotometer. The ascorbate concentration in the samples was determined using a linear regression equation and standard curve.
Total oxidant status
Total Oxidant Status (TOS) in leaf samples was analyzed by using a novel automated39 formulated method. This approach is based on ferric ions being measured with ferrous ions and xylenol orange being oxidized to ferric ions in an acidic medium by the oxidants of the cotton sample40. The TOS assay solution contained the reagent R1 [xylene orange (stock solution containing 0.38 g in 500 µl of 25 mM H2SO4), 5 ml of glycerol, NaCl (0.4 g) and made volume up to 50 ml with 25 mM H2SO4 made volume up to 50 ml, was included in assay mixture], reagent R2 [contained 0.0196 g of ferrous ammonium sulphate, 0.0317 g of o-dianisidine and added in (10 ml) 25mM H2SO4], and 140 µl sample extract. After 5 min, the absorption of the colour compound produced was taken at 560 nm by using a spectrophotometer (HITACHI U-2800).
Malondialdehyde content
The lipid peroxidation level in the leaf tissue was determined as malondialdehyde (MDA, a lipid peroxidation product) content analyzed by using the thiobarbituric acid (TBA) reaction method41,42. The absorbance was taken at 520 nm and 600 nm using a spectrophotometer and results were measured by an extinction coefficient of 155 mM–1cm –1.
Superoxide dismutase
For the analysis of Superoxide dismutase (SOD) activity, cotton leaves were emulsified in a solution containing 50 mM potassium phosphate buffer (pH 7.8)43. To determine SOD activity, the samples were exposed to white light for 10 min after adding reagents [400 µl water, 250 µl of 0.2 M KH2PO4 buffer, 100 µl L-methane, 100 µl Triton X, 50 µl nitro blue tetrazolium]. The activity of SOD was measured by analyzing its capability of inhibiting the photochemical reduction of nitro blue tetrazolium (NBT) using a previously developed method44. The quantity of enzyme that induced 50% inhibition of NBT photochemical reduction is termed as one unit of SOD activity.
Peroxidase activity
The activity of peroxidase (POD) was analyzed by using the previously described method45. To measure POD activity, cotton leaves were crushed in a solution containing 50 mM KH2PO4 buffer (pH 7.0). The POD activity was measured by using an assay solution (3 ml). The assay solution was made up of distilled water (535 µl), 200 mM potassium phosphate buffer, 100 µl of 200mM guaiacol, 100 µl of 400mM H2O2, and 15 µl extracted leaf sample. The reaction started with the addition of the extract. The optical density at 470 nm was noted for 0 s, 20 s, 40 s, 60 s. The increase in absorbance was observed and a pink color was produced which indicates an enzyme reaction. One unit of POD activity is defined as a 0.01unit min-1 change in absorbance.
Catalase activity
Catalase (CAT) activity was estimated by using the method46 with certain amendments. To estimate the catalase activity, the assay solution was made up of 50 mM KH2PO4 buffer, 59 mM H2O2, and 100 µl sample (enzyme extract). After the addition of the sample, the enzyme reaction was initiated. A decreasing pattern in absorbance at 240 nm was recorded for 0 s, 30 s, and 60 s. One unit of CAT activity was defined as a 0.01unit min-1 change in absorbance. The enzyme activity was expressed on the basis of fresh leaf weight.
Ascorbate peroxidase activity
Ascorbate peroxidase (APX) activity was analyzed by using the previously described method43. To estimate APX activity, cotton leaves were emulsified in a 50 mM potassium KH2PO4 buffer (pH 7.0) solution. To prepare the assay buffer 10 mM ascorbic acid, 0.5 M ethylenediaminetetraacetic acid (EDTA), 0.2 M potassium phosphate buffer (pH 7.0) and 4Mm H2O2 were mixed simultaneously. For the determination of APX, 1 ml of assay buffer and 50 µl sample were added, and the reaction was started when 1 ml of H2O2 was added. The ascorbic acid oxidation rate was calculated by measuring the decreasing trend in absorbance at 290 nm for 0 s, 30 s, and 60 s47.
Total free amino acids
Total free amino acids were analyzed by using the following method48. For the determination of total free amino acids, 50 mM potassium phosphate buffer (pH 7.0) solution. The assay mixture was prepared by 100 µl sample, 10% pyridine (100 µl) and 2% ninhydrin (100 µl). Then, the mixture was warmed in the water bath for 30 min and made a volume up to 5 ml with distilled water. The optical densities of the mixture were recorded at 570 nm using a spectrophotometer.
Total soluble proteins
The estimation of quantitative protein was executed with the previously described method49 by using a spectrophotometer (HITACHI, U2800). To estimate TSP, cotton leaf samples were homogenized in KH2PO4 buffer media. The assay solution contained sample extract (5 µl) and 150 mM NaCl (95 µl) mixed with 1.0 ml Coomassie Brilliant Blue dye (G-250, 100 mg). The solution was kept to develop a protein-dye complex for 5 min. Then, the optical densities were recorded at 595 nm.
Protease activity
For the determination of protease activity, cotton leaves were emulsified in a solution containing 50 Mm KH2PO4 buffer (pH 7.8)50. The required assay solution was prepared by adding reagents [1% casein, 0.01 N NaOH, Tris base, and distilled water] and its pH 7.8 was maintained with H3PO4. Protease activity was analyzed by casein digestion assay as described previously51. In this analytical protocol, one unit is that amount of enzyme, which releases acid soluble fragments equivalent to 0.001 A280 per minute at 37 °C and pH 7.8. Enzyme activity was expressed on a fresh weight basis.
Amylase activity
The amylase activity of cotton leaves was analyzed by using the following method52. For the estimate of α-amylase, two reagents were used. The first solution was the 3,5-dinitro salicylic acid (DNS), which was prepared by dissolving 1 g of DNS in distilled H2O, followed by sodium-potassium tartrate tetrahydride and 2 N NaOH. The second solution was a 1% starch solution. The reaction mixture was incubated for 3 min after adding 200 µl sample extract and 1 ml starch solution, then heated in a water bath for 15 min after adding DNS. Cool down and made volume up to 9 ml with dH2O. Spectroscopically, optical densities were recorded at 540 nm53.
Esterase activity
Esterase activity was analyzed by using the Fast Blue BB method54. The chemical α-naphthyl acetate used as substrate. The reaction mixture contains the assay solution [30mM -naphthyl acetate, 1% acetone, phosphate buffer (0.04 M)] and enzyme extract. The resulting mixture was incubated in the dark for 10 min at 27℃. After 15 min, 1 ml of staining solution (1% Fast blue BB dye and SDS 5% in a 2:5 ratio) was added and incubated in the dark for 20 min at 30℃. The optical densities of the colour compound produced were recorded at 590 nm using a spectrophotometer. Standard curves were prepared by using standards and the enzyme activity (a-naphthyl acetate esterase) was measured in µM min − 1 /g wt.
Total sugar content
The total sugar content in cotton leaf samples were determined using the method and the phenol-sulfuric acid reagent method55. For determination of total sugar level, the assay mixture was prepared by 100 µl sample mixed with 400 µl distilled water, 250 µl 5% phenol, and 1.25 ml H2SO4. Then, we placed for 20 min at 27℃ and the reading was recorded at 490 nm using a spectrophotometer.
Reducing sugars
For the estimation of reducing sugar in cotton leaf samples using the dinitrosalicyclic acid (DNS) method56. To estimate the reducing sugar level of cotton leaves, the 100 µl sample was mixed with 500 µl DNS and 900 µl distilled water. Placed in the water bath for 15 min and cooled at 25℃. Then added 4.5 ml distilled water, and the optical densities of the solution were recorded at 540 nm using a UV-VIS spectrophotometer.
Non-reducing sugar
The non-reducing sugars were then estimated as the difference between the reducing sugars and total soluble sugars.
Pigments analysis
The amount of chlorophyll (a and b) was determined by following the pigment analysis method57 whereas carotenoids were determined following the method58. To extract pigments, 75 mg of cotton leaf samples were emulsified in 80% acetone (80 ml acetone and 20ml H2O) and incubated at room temperature for 1 day in the dark. After 1 day, it was centrifuged for 5 min at 14,000 rpm. Then, the optical densities of the supernatant were measured at 645, 663, 505, 453, and 470 nm.
Proline
Cotton leaf samples (0.1 g) were emulsified in 3% sulfosalicylic acid solution (2 ml) in test tubes, vortexed for 10 s, and left for 5 min. Free proline assays were performed on filtered extracts using the procedure59. For the assay, proline extract (1 mL) was treated with 2% ninhydrin solution (1 ml per sample) followed by the addition of glacial acetic acid (1 ml). Then, each sample was vortexed for 10 s and heated for 1 h in the water bath at 95–98 °C. After immediately, cooled in an ice bath to terminate the reaction. Toluene 5 ml was added to each sample and kept for 1–2 min at 27℃. The proline content in the toluene fraction was determined by recording the optical densities at 520 nm using a UV-vis spectrophotometer against a toluene blank. The ultimate findings were expressed as µmol proline/g of frozen or fresh leaf samples60.
Statistical analysis
Microsoft Excel and XLSTAT were used for descriptive analysis and all other statistical analysis was performed in RStudio version1.0.153 including the three-way ANOVA, histogram (by using the “ggplot2” package)61, and correlation62. Principal component analysis (PCA) was performed by using XLSTAT. Best performing genotypes were differentiated using Multi-Trait Genotype-Ideotype Distance Index (MGIDI) as explained in63 with the help of the “metan” package in R64 however selection pressure was kept 50%. PCA (principal component analysis) was performed by using the “factoextra” package in R to minimize the number of dimensions in the data65.
Results
Physiological parameters
Stomatal diffusive resistance
Under the control conditions, stomatal diffusive resistance was found highest in cotton genotype N-868 (0.421 ± 0.03 s cm− 1) while it was lowest in N-9078 (0.298 ± 0.01 s cm− 1). Under low moisture stress, stomatal diffusive resistance was highest in cotton genotypes N-375 and FH-326 (0.346 ± 0.008 s cm− 1), however, it was found lowest in N-4496 (0.272 ± 0.02 s cm− 1). Under low moisture stress conditions, stomatal diffusive resistance was non-significantly increased in N-926, N-123, N-878, FH-326, and FH-490 compared to that of control (Fig. S1a).
Transpiration rate
Under low moisture stress, the transpiration rate was found highest in genotype N-4496 (76.88 ± 4.07 µg cm− 2 s− 1), while it was lowest in N-1296 (46.36 ± 5.6 µg cm− 2 s− 1). Transpiration rate was highest in N-4496 (71.66 ± 1.2 µg cm− 2 s− 1) under the control condition while it was lowest in genotype N-377 (48.85 ± 4 µg cm− 2 s− 1). Transpiration rate under stress treatment was observed retained in all genotypes except for N-377, N-868, N-896, and N-1296 (Fig. S1b). It was significantly increased in N-377 under low moisture stress conditions.
Leaf temperature
Under the control conditions, the leaf temperature was found highest in cotton genotype N-7012 (35.80 ± 0.6 ℃), while it was lowest in N-990 (34.58 ± 0.9 ℃). Leaf temperature was highest in genotype N-878 (36.62 ± 0.09 ℃) under low moisture stress, however, it was lowest in genotype N-213 (35.01 ± 0.3 ℃). Low moisture stress non-significantly affects leaf temperature in comparison to that under control (Fig. S1c).
SPAD measurements
Under the control conditions, the spade value was found highest in genotype N-512 (40.411 ± 0.3), while it was lowest in N-9078 (35.322 ± 0.5). SPAD measurement was highest in N-1286 (40.556 ± 0.7) under low moisture stress while it was lowest in genotype N-153 (31.222 ± 1.6). Non-significant differences in SPAD measurements were found under low moisture stress (Fig. S1d).
Relative water content.
Under low moisture stress conditions, relative water content (RWC) was found highest in cotton genotype N-T86 (86.49 ± 1.2%), while it was lowest in N-912 (81.51 ± 1%). RWC was highest in N-121 (95.87 ± 0.8%) under control conditions and lowest in genotype N-3496 (81.86 ± 1.3%). Overall, there were no significant genotypic differences in RWC among cotton genotypes under low moisture stress (Fig. S1e).
Morphological parameters
Dry and fresh root weight
Under control conditions, fresh root weight was found highest in genotype N-121 (2.133 ± 0.2 g), while it was lowest in N-868 (0.933 ± 0.1 g). Fresh root weight was highest in N-512 (2.66 ± 0.2 g) under low moisture stress while it was lowest in genotype N-T8411 (0.87 ± 0.1 g). Fresh root weight under stress was observed retained in all genotypes except for N-512 and N-T8411, in which it was increased/decreased compared to that under control (Fig. S2a).
Dry root weight was highest in N-512 (1.578 ± 0.1 g) under low moisture stress while it was lowest in genotype N-1496 (0.429 ± 0.1 g). Under control conditions, dry root weight was found highest in genotype N-812 (1.419 ± 0.1 g), while it was lowest in N-868 (0.719 ± 0.08 g). Overall, it was observed that low moisture stress had non-significant effects among the cotton genotypes (Fig. S2b).
Fresh and dry shoot weight
Fresh shoot weight was found highest in N-9078 (18.74 ± 1.2 g) under low moisture stress while it was lowest in genotype N-T8411 (5.11 ± 1.3 g). Under control conditions, fresh shoot weight was found highest in genotype N-812 (24.21 ± 1.3 g), while it was lowest in N-868 (9.30 ± 1.1 g). However, genotypic differences were found non-significantly in all cotton genotypes under non-stress and stress conditions (Fig. S2c).
Under low moisture stress, dry shoot weight was found highest in genotype N-9078 (9.91 ± 0.2 g), while it was lowest in N-T8411 (2.37 ± 0.1 g). Dry shoot weight was highest in N-812 (12.14 ± 0.07 g) under control conditions while it was lowest in genotype N-868 (3.95 ± 0.5 g). Dry shoot weight was observed to non-significantly increase/decrease in all cotton genotypes under low moisture stress (Fig. S2d).
Fresh and dry leaf weight
Under control conditions, fresh leaf weight was found highest in cotton genotype N-T86 (0.673 ± 0.02 g) and it was lowest in FH-490 (0.593 ± 0.01 g). Under low moisture stress, fresh leaf weight was highest in cotton genotype N-T86 (0.682 ± 0.02 g), however, it was found lowest in FH-490 (0.578 ± 0.01 g). Fresh leaf weight (Fig. S3a) and dry leaf weight (Fig. S3b) were observed to be least affected by low moisture stress. However, non-significant genotypic differences were observed in all cotton genotypes.
Root and shoot lengths
Under low moisture stress, root lengths were found highest in genotype N-377 (23.88 ± 1.9 cm), while it was lowest in N-990 (16.58 ± 0.7 cm). Root lengths were highest in N-1296 (27.77 ± 0.7 cm) under control conditions while it was lowest in genotype N-213 (14.33 ± 0.6 cm). Under low moisture stress, an increase in root length was observed for all genotypes with the exception of N-1296, and N-1237 in which it was decreased. Overall, the marked retained root length was observed among the genotypes (Fig. S3c).
Under low moisture stress, shoot length was found highest in genotype N-9078 (34.1 ± 1.2 cm), while it was lowest in FH-490 (20.8 ± 1.2 cm). Shoot length was highest in N-812 (40.1 ± 1.3 cm) under control conditions while it was lowest in genotype FH-490 (23.6 ± 1.08 cm). As compared to control conditions, there were no significant genotypic differences for the shoot lengths under low moisture stress. It was found maintained in N-1286, N-T86, and N-213 under stress and non-stress conditions (Fig. S3d).
Biochemical analysis
Total phenolic content
Under control conditions, total phenolic content (TPC) was found highest in genotype N-1296 (26575 ± 2275 μm/g. f. wt.), while it was lowest in N-374 (14775 ± 275 μm/g. f. wt.). TPC was noted highest in N-4496 (25175 ± 425 μm/g. f. wt.) under low moisture stress, however, it was lowest in genotype N-135 (15100 ± 1450 μm/g. f. wt.). Under low moisture stress treatment, an increase in total phenolic content was noted in N-9078, N-7012, N-T86, N-374, N-375, FH-326 and FH-490 and it was decreased in N-868, N-696, N-1296, N-377 and N-926 (Fig. S4a). Overall, significant decrease in TPC was found in N-1296 out of 30 cotton genotypes.
Total tannins
Under control conditions, total tannins content (TTC) was found highest in genotype N-1296 (3750 ± 250µuM/g. f. wt.), while it was lowest in N-375 (100 ± 25µuM/g. f. wt.). TTC was noted highest in N-T86 (4075 ± 125µuM/g. f. wt.) under low moisture stress, however, it was lowest in genotype N-696 (625 ± 25µuM/g. f. wt.). Under low moisture stress, significantly increased TTC was observed in the 17 cotton genotypes N-868, N-3496, N-990, N-7012, N-1237, N-912, N-T86, N-377, N-135, N-812, N-374, N-375, N-926, N-213, N-878, FH-326 and FH-490 out of 30 (Fig. S4b).
Total flavonoid content
Under low moisture stress, total flavonoid (TF) content was found highest in genotype N-121 (160.9 ± 5.03 µg/ml), while it was lowest in N-512 (115.9 ± 1.3 µg/ml). Under control conditions, TF content was observed highest in N-896 (162.03 ± 2.9 µg/ml), however, it was lowest in genotype N-375 (115.38 ± 0.7 µg/ml). TF contents were maintained in 8 cotton genotypes N-1296, N-1286, N-4496, N-1237, N-7012, and N-213, N-878 and FH-490 out of 30 under non-stress and stress conditions (Fig. S4c). Overall, a significant increase in TF content was found in cotton genotypes N-121 and N-T86.
Ascorbic acid
Under low moisture stress, ascorbic acid (AsA) content was found to highest in genotype N-868 (643 ± 5 µg/g f. wt.), however it was lowest in N-4496 (602.2 ± 3.2 µg/g f. wt.). Under control conditions, AsA content was observed highest in N-1237 (642.5 ± 0.5 µg/g f. wt.), however, it was lowest in genotype FH-490 (604.2 ± 4.7 µg/g f. wt.). Under low moisture stress treatment, significant increase in AsA content was found in N-1296 and FH-490 out of 30 cotton genotypes (Fig. S5a).
Total oxidants status
Under control conditions, total oxidants status (TOS) was found highest in genotype N-512 (18085 ± 715 µM/g. f. wt.) however, it was lowest in N-T8411 (5300 ± 200 µM/g. f. wt.). TOS observed highest in N-153 (15400 ± 100 µM/g. f. wt.) under low moisture stress, however, it was lowest in genotype N-912 (6822.5 ± 177.5 µM/g. f. wt.). TOS was significantly increased in 7 cotton genotypes N-868, N-T8411, N-1496, N-T86, N-153, N-213 and FH-490 under low moisture stress, while it was retained in 2 genotypes N-1296 and N-926 only (Fig. S5b).
Malondialdehyde contents
Under control conditions, malondialdehyde content (MDA) was found highest in genotype N-912 (476.1 ± 13.1 µM/g f. wt.) however, it was lowest in N-153 (285.6 ± 17.8 µM/g f. wt.). Under low moisture stress, MDA was noted highest in N-812 (472.8 ± 17.6 µM/g f. wt.), however, it was lowest in genotype N-696 (192.3 ± 19.7 µM/g f. wt.). Under low moisture stress in N-990 and N-153, a significant increase in MDA was observed. A significant decrease was noted in N-696 followed by N-896, N-8437, and N-374 under low moisture stress conditions (Fig. S5c).
Superoxide dismutase
Under low moisture stress, superoxide dismutase (SOD) activity was found highest in genotype N-812 (337.02 ± 4.3 Units/g f. wt.) however, it was lowest in N-135 (43.96 ± 7.3 Units/g f. wt.). Under control conditions, SOD activity was observed highest in N-375 (228.76 ± 17.6 Units/g f. wt.), however, it was lowest in genotype N-1496 (19.96 ± 3.1 Units/g f. wt.). Low moisture stress caused a significant increase in SOD activity in 12 cotton genotypes N-1096, N-1296, N-1286, N-1496, N-4496, N-990, N-7012, N-1237, N-912, N-512, N-812 and N-153 out of 30. SOD activity was found maintained only in N-896 under non-stress and stress conditions (Fig. S6a).
Peroxidase activity
Under low moisture stress, peroxidase (POD) activity was found highest in genotype N-896 (12287.7 ± 1098.3 Units/g f. wt.) while it was lowest in N-135 (1764.9 ± 99.9 Units/g f. wt.). Under control conditions, POD activity was observed highest in N-896 (9290.7 ± 699.3 Units/g f. wt.), however, it was lowest in genotype N-812 (2564.1 ± 166.5 Units/g f. wt.). Low moisture stress caused a significant increase in POD activity in N-896, N-1496, N-812, N-121, and N-153. However, a significant decrease in plant POD activity was noted in FH-490 (Fig. S6b).
Catalase activity
Under low moisture stress, Catalase activity was found highest in genotype N-512 (510 ± 10 Units/g f. wt.) while it was lowest in N-T8411 (70 ± 10 Units/g f. wt.). Under control conditions, catalase activity was observed highest in N-696 (300 ± 20 Units/g f. wt.), however, it was lowest in genotypes N-1296, N-T8411, N-7012 and n-377 (70.0 ± 10.0 Units/g f. wt.). Catalase activity was maintained under low moisture stress in all cotton genotypes except for N-1296, N-3496, N-9078, N-990, N-7012, N-812, N-512, N-374, N-213 and FH-490 in which increased activity was observed (Fig. S6c). Overall, a significant increase in catalase activity was observed in N-512 and a significant decrease was observed in genotypes N-696 and N-896.
Ascorbate peroxidase
Under control conditions, ascorbate peroxidase (APX) activity was found highest in cotton genotypes N-1296, and N-375 (420 ± 20 Units/g f. wt.), while it was lowest in N-8437 (140 ± 20 Units/g f. wt.). Under low moisture stress, APX activity was observed highest in N-812 (600 ± 40 Units/g f. wt.), however, it was lowest in genotype N-375 (120 ± 0 Units/g f. wt.). Under low moisture stress in genotype N-812, a significant increase in the APX activity was noted and a significant decrease was found in N-1296 and N-375 (Fig. S7a).
Total free amino acids
Under low moisture stress, total free amino acids were found highest in genotype N-3496 (14.74 ± 0.1 µg/ g f. wt.), while it was lowest in N-926 (9.03 ± 0.5 µg/ g f. wt.). Under the control conditions, total free amino acids were observed highest in N-868 (12.57 ± 0.5 µg/ g f. wt.), however, it was lowest in genotype N-374 (7.74 ± 0.1 µg/ g f. wt.). Under low moisture stress, a significant increase was observed in N-3496, N-9078, N-T86, N-153, and FH-490. Total free amino acids were observed to increase/decrease in leftover cotton genotypes, but these differences were non-significant under non-stress and stress conditions (Fig. S7b).
Total soluble proteins
Under the control conditions, total soluble proteins (TSP) were found highest in cotton genotype N-512 (210 ± 3.3 mg/g f. wt.), while it was lowest in N-377 (86.6 ± 5.3 mg/g f. wt.). Under low moisture stress TSP was observed highest in N-812 (220.3 ± 3.6 mg/g f. wt.), however, it was lowest in genotype N-926 (94 ± 2 mg/g f. wt.). Under low moisture stress in genotypes N-377 and N-812, a significant increase in TSP was observed. Significant decrease was found in N-1496, N-7012, N-1237, N-153, N-926, and N-375 (Fig. S7c).
Protease activity
Under control conditions, protease activity was found highest in cotton genotypes N-812 (3230 ± 10 Units/g. f. wt.), while it was lowest in N-1286 (2250 ± 10 Units/g. f. wt.). Under low moisture stress, protease activity was observed highest in N-375 (3160 ± 50 Units/g. f. wt.), however, it was lowest in genotype N-135 (2520 ± 140 Units/g. f. wt.). Under low moisture stress, a significant increase was noted in N-1286 and a significant decrease was found in N-812 (Fig. S8a).
Amylase activity
Under low moisture stress, amylase activity was found highest in genotype N-153 (115.4 ± 4.5 mg/g. f. wt.), while it was lowest in N-868 (9.43 ± 1.8 mg/g. f. wt.). Under the control conditions, amylase activity was observed highest in N-878 (122.2 ± 1.5 mg/g. f. wt.), however, it was lowest in genotype N-512 (11.88 ± 1.6 mg/g. f. wt.). Low moisture stress caused a significant increase in plant amylase activity in N-8437, and N-512 (Fig. S8b).
Esterase activity
Under low moisture stress, genotype N-213 had the maximum esterase activity (116.9 ± 0.3 µM/min/g f. wt.), while genotype N-T86 had the lowest (110.14 ± 0.1 µM/min/g f. wt.). Under control conditions, esterase activity was highest in genotype FH-490 (117.15 ± 0.7 µM/min/g f. wt.), but lowest in genotype N-868 (97.01 ± 6.6 µM/min/g f. wt.). Under low moisture stress, an increase in esterase activity was found in 13 genotypes N-868, N-896, N-696, N-1296, N-1286, N-8437, N-3496, N-9078, N-1237, N-926, N-374, N-213 and N-878. Overall, all cotton genotypes had non-significant results for esterase activity (Fig. S8c).
Total soluble sugars
Under the control conditions, total soluble sugar (TSS) was found highest in cotton genotype N-121 (37.03 ± 0.2 mg/g. f. wt.), while it was lowest in N-1296 (21.85 ± 0.2 mg/g. f. wt.). Under low moisture stress, TSS was observed highest in N-1286 (41.93 ± 1.4 mg/g. f. wt.), however, it was lowest in genotype N-8437 (20.11 ± 0.8 mg/g. f. wt.). Under low moisture stress, in 11 genotypes N-1206, N-1286, N-4496, N-9078, N-1237, N-912, N-512, N-374, N-926, N-812 and FH-326 a significant increase in TSS was observed (Fig. S9a).
Reducing sugars
Under low moisture stress, reducing sugars was found highest in genotype N-121 (16.44 ± 0.4 mg/g. f. wt.), while it was lowest in N-990 (11.31 ± 0.6 mg/g. f. wt.). Under the control conditions, reducing sugar was observed highest in N-213 (15.76 ± 0.1 mg/g. f. wt.), however, it was lowest in genotype N-696 (8.65 ± 0.6 mg/g. f. wt.). Under low moisture stress, a significant increase in reducing sugar was observed in N-696. While, it was maintained in N-896, N-1096, N-1496, N-4496, N-3496, N-9078, N-153 and N-213 (Fig. S9b).
Non-reducing sugars
Under the control conditions, non-reducing sugar was found highest in cotton genotype N-121 (24.38 ± 0.8 mg/g. f. wt.), while it was lowest in N-4496 (7.0 ± 0.1 mg/g. f. wt.). Under low moisture stress, non-reducing sugar was observed highest in N-1286 (26.17 ± 0.5 mg/g. f. wt.), however, it was lowest in genotype N-8437 (3.79 ± 1.2 mg/g. f. wt.). Under low moisture stress in 6 genotypes N-1286, N-4496, N-9078, N-990, N-812, and N-512 out of 30 a significant increase in non-reducing sugar was observed. And a significant decrease was found in N-153 (Fig. S9c).
Lycopene
Under control conditions, lycopene was found highest in cotton genotypes N-374 (12.69 ± 0.2 mg/g f. wt.), while it was lowest in N-375 (9.6 ± 0.1 mg/g f. wt.). Under low moisture stress, lycopene was observed highest in N-135 (13.06 ± 0.3 mg/g f. wt.), however, it was lowest in genotype N-868 (10.97 ± 0.1 mg/g f. wt.). Low moisture stress caused an increase in lycopene content among all genotypes except N-868, N-374, N-926, and FH-326 in which it was reduced. However, lycopene content was maintained in N-912 (Fig. S10a).
Chlorophyll a
Under the control conditions, chlorophyll a content was found highest in cotton genotype N-374 (414.08 ± 7.7 ug/g f. wt.), while it was lowest in N-512 (357.1 ± 1.2 ug/g f. wt.). Under low moisture stress, chlorophyll a was observed highest in N-990 (427.5 ± 1.3 ug/g f. wt.), however, it was lowest in genotype N-135 (282.6 ± 20.09 ug/g f. wt.). Chlorophyll a content was generally less affected by the low moisture stress in most of the genotypes and differences were non-significant under non-stress and stress conditions (Fig. S10b).
Chlorophyll b
Under the control conditions, chlorophyll b content was found highest in cotton genotype N-912 (575.5 ± 15.6 ug/g f. wt.), while it was lowest in N-375 (460.1 ± 20.8 ug/g f. wt.). Under low moisture stress, chlorophyll b was observed highest in N-135 (631.05 ± 19.1 ug/g f. wt.), however, it was lowest in genotype N-868 (527.80 ± 7.07 ug/g f. wt.). The decreased chlorophyll b content was found in N-868, N-926, and FH-326. While it was increased in all leftover cotton genotypes non-significantly under low moisture stress (Fig. S10c).
Total carotenoids
Total carotenoid content was highest in cotton genotypes N-374 (15.88 ± 0.1 mg/g f. wt.) and lowest in N-512 (10.61 ± 0.4 mg/g f. wt.) under control conditions. Total carotenoids were found highest in genotype N-7012 (16.5 ± 0.2 mg/g f. wt.) and lowest in genotype FH-326 (12.9 ± 0.3 mg/g f. wt.) under low moisture stress. Carotene content was maintained in N-8437, N-3496, and N-374. Total carotene content increased while showing the least significant differences between non-stress and stress conditions (Fig. S11a).
Total chlorophyll
Under low moisture stress, total chlorophyll was found highest in genotype N-990 (1010.3 ± 0.02 ug/g f. wt.), while it was lowest in N-926 (913.1 ± 33.3 ug/g f. wt.). Under control conditions, total chlorophyll was observed highest in N-374 (987.6 ± 16.3 ug/g f. wt.), however, it was lowest in genotype N-375 (847.2 ± 40.2 ug/g f. wt.). Low moisture stress had the least significant difference in total chlorophyll content among the genotypes under the stress condition compared with that in control plants (Fig. S11b).
Proline
Under the control condition, proline was found highest in genotype N-896 (53.504 ± 1.9 ug/g f. wt.), while it was lowest in N-1096 (32.99 ± 0.2 ug/g f. wt.). Proline was observed highest in N-213 (54.07 ± 1.4 ug/g f. wt.) under low moisture stress, however, it was lowest in genotype N-912 (38.68 ± 0.5 ug/g f. wt.). Under low moisture stress, an increase in proline was found in cotton genotypes N-1096, N-1296, N-8437 N-1496, N-4496, N-1237, N-135, N-926, N-213 and N-878. In comparison with non-stress conditions, the genotypic differences for proline in all cotton genotypes under low moisture stress were observed non-significantly (Fig. S11c).
Correlation analysis
Correlation analysis (Pearson test) performed by using the XLSTAT 2011 version 2.06. It was performed for all the parameters with the alpha of 0.05. On the base of the correlation test, parameters that demonstrated significant correlations with one another (indirect effect) and with the genotypes of low moisture stress tolerance (direct effect) were selected, in order to classify the cotton genotypes. Correlation maps of all tested traits under control (Fig. 1) and stress conditions (Fig. 2) were computed.
Cluster analysis
Cluster analysis was performed for grouping of the cotton genotypes under low moisture stress and control conditions by using morpho-physiological (Fig. 3) and biochemical traits (Fig. 4).
Principal component analysis
Principal component analysis performed by using all tested traits measured in controlled and low moisture stress conditions.
For the objective of maintaining all potential diversity among the tested cotton genotypes for the parameters under study, principal component analysis was used to reduce the dimensionality of all datasets in the interpretable approach. For further analysis, an important main component is determined by the eigenvalue. The eigen value > 1 represents total variation of approximately 10%66. Principal component analysis (PCA) was performed on the data. Eigen value greater than 1 was the best indicator of all the attributes in principal components67. Scree plot (Fig. 5) showed that, out of the total 29 principal components, twelve (PC-I to PC-XII) had Eigenvalues greater than 1 and 85.45% of overall cumulative variability was possessed by traits under control conditions. PC-I and PC-II together with a cumulative variability of 29.18%, were the largest contributors to the total cumulative variability in the genetic resource. While under low moisture stress, out of the total 29 principal components, thirteen (PC-I to PC-XIII) had Eigenvalues > 1 and carried 87.04% of the total cumulative variability under control conditions. The greatest contributors to overall cumulative variability in the genetic resource were PC-I and PC-II, with a cumulative variability of 27.46%.
To generate a genotype by trait (G-T) biplot for each genotype and its corresponding trait, the PC-I score (x-axis) was plotted against the PC-II scores (y-axis) under control (Fig. 6) and low moisture stress conditions (Fig. 7). In the genotype by trait (G-T) biplots, the interrelationships between attributes and a visual comparison of multiple traits were effectively demonstrated. The angles formed by the vectors and the distances of the variables from the biplot’s origin were used to extract important data. The vector’s angle to the principal component axis determines its contribution to that PC. The contribution of vectors to a particular PC increases with its parallelism to the principal component axis. In the correlation plot, the vector length represents the representativeness quality of the examined PCA dimensions. If the angle between the two vector traits was < 90, the correlation between that two traits was considered as positive whereas if the angle between the two vector traits was larger than 90, the correlation was regarded as negative. The angle of 90 between the attributes represented that the attributes were not dependent on one another68.
Multi-trait Genotype-Ideotype Distance Index (MGIDI) analysis
For the selection of the best-performing genotypes, MGIDI was used for the ranking of cotton genotypes on the basis of multiple indices as explained earlier69. We estimated the MGIDI index for both biochemical and morpho-physiological indices to identify the best-performing genotypes. For biochemical traits, 09 (TPC, TF, TSP, MDA, SOD, POD, CAT, APX, and Proline) out of 24 indices showed significant genotypic effect and used for MGIDI. Eight genotypes (N-812–1296 N-696 N-377 N-121–896 N-T86, and N-3496) were observed to be best performing than others at 25% selection pressure (SI = 25%) (Fig. 8).
For morpho-physiological traits, 14 (except RWC) out of 15 indices showed a significant genotypic effect and used for MGIDI. Ten genotypes (N-1237 N-812 N-1296 N-696 N-9078 N-377 N-512 N-121 N-375, and N-896) were observed to be best performing than others at 35% selection pressure (SI = 35%) (Fig. 9). Six genotypes, i.e. N-812–1296 N-696 N-377 N-121, and N-896, were common and best performed in both MGIDI based on biochemical and morpho-physiological traits.
The broad-sense heritability (h2) in biochemical traits ranged from 0.3681 (SOD) to 0.7938 (POD) and in morpho-physiological traits ranged between 0.355 (TR) to 0.125 (DSW). Traits including TPC, POD, and TSP showed high value of h2 (h2 > 0.6) that shows that these traits exhibited the good prospect of selection gain. All traits showed desirable gain except TL that showed undesirable gain (Table 2).
Strength and weakness view of variables
A strength and weakness view was provided for all genotypes using biochemical (Figs. 8 and 9), and morpho-physiological traits which comprise total of four factors (FA1, FA2, FA3 & FA4) for each class of traits.
Discussion
Low moisture stress is an adverse environmental condition that has a significant impact on crop yield all over the world. An integrated morphological, physiological, and biochemical approach was used to analyze the low moisture stress responses in cotton, a plant of great economic importance in Pakistan. Water deficit tolerance mechanisms have remained a major concern for plant biologists due to the involvement of multiple parameters70. According to previous research, the development and growth of cotton plants severely affected by drought stress71. Drought resistance is a polygenic trait72 that is correlated with various morphological, physiological, and biochemical parameters.
Screening of tolerant cotton genotypes for low moisture stress was the main objective of this research, as well as identification of best performing genotypes under adverse environmental conditions during post-germination seedling development. According to a previous report, tolerant cotton genotypes accumulated more compatible solutes than sensitive genotypes under drought stress. It could be because of higher levels of suitable solutes such as sugars, amino acids, proline, and polyphenols73. Similar trend was observed for solute levels under low moisture stress in tested cotton genotypes in the present study.
Stomatal diffusive resistance can be utilized to interpret the effects of water stress74. According to reports, the primary method to control transpiration in plants is stomatal conductance regulation75. Stomatal resistance is the ability of mesophyll cells to withstand water evaporation from their outer surfaces, as well as water diffusion across intercellular gaps and the stomatal pore76. The increased resistance of mesophyll cells causes a decrease in transpiration77. In this experiment, stomatal diffusive resistance was maintained in 14 out of 30 cotton genotypes under low moisture stress conditions. Relative water content (RWC) is a helpful feature for identifying drought stress since it is an indicator of water status in plant leaves78. Previous studies reported that physiological factors greatly affect the RWC79 and drought conditions also have an impact on it80. In this study, low moisture stress tolerance was shown among genotypes that retained a high proportion of RWC except for N-121 and N-912 in which it was decreased compared to that under control. Overall, non-significant genotypic differences were observed. A similar pattern of RWC was observed in a previous study3 as cotton genotypes retained a high proportion of RWC.
Genetic variation in transpiration in some crops like sorghum81 and cotton82 in response to drying soil has been reported. In this study, the transpiration rate significantly increased in N-377 under low moisture stress conditions. It was retained in all genotypes except for N-377, N-868, N-896, and N-1296. Genotype N-377 was found to be low moisture stress tolerant based on transpiration rate. As previously reported, tolerant genotypes with a higher fraction of transpirable soil water thresholds can conserve water through decreasing the transpiration rate during the starting days of the drought cycle82.
Cotton cultivars had greater leaf temperatures in dry climates83. In this study, leaf temperature was increased under low moisture stress in cotton genotypes N-696, N-926, N-213, N-1096, and FH-490. Overall, low moisture stress had a non-significant impact on leaf temperature compared to that under control.
In a previous study, the non-significant difference in SPAD values in cotton under water stress was reported84. In this experiment, SPAD values were retained in genotypes N-T86, N-377, and N-121 under low moisture stress. Overall, there was no significant variation in SPAD values between low moisture stress and control in cotton genotypes. Plant water status is an important element influencing crop production, and water potential is increasingly being used to estimate plant water status85. It has been demonstrated in certain research that increased RL improves a plant’s capacity to draw water from the deeper soil86. In this experiment, the marked retained root length was observed among the genotypes. Increasing roots, on the other hand, may have a negative impact on shoot growth while more photosynthates move to the roots87. In the current study, genotypic differences for shoot length under low moisture stress were non-significant. It was found maintained in N-1286, N-T86, and N-213. It was found that the shoots were more responsive to water deficit stress than the roots. The findings of the present study supported prior research88,89. Fresh root weight under stress treatment was retained in all genotypes except for N-512 and N-T8411. Under low moisture stress treatment, dry and fresh shoot weight was observed increased in cotton genotype N-9078 and it was decreased in N-T8411. However, in dry and fresh root weight, dry and fresh shoot weight, and dry and fresh leaf weight genotypic differences were found non-significant in all cotton genotypes. Previous studies have reported the involvement of additive gene action in the measurements of dry weight, density, and root length90.
When there is a water shortage, the leaf functions are greatly harmed, and toxic chemicals, such as ROS, begin to produce at an alarming rate. This happened because of the imbalance between the light capture and utilization of plant photosystems, which results in the formation of singlet oxygen, superoxide anions, hydroxyl radicals, and H2O291. The activity of the cell system is disrupted when ROS affects the cell machinery92. Plants, on the other hand, have developed their metabolic machinery to deal with ROS. The plants ability to withstand oxidative stress depends on activation of antioxidant enzymes like POD, SOD, APX, and CAT93. In this research, APX activity generally increased in cotton genotypes under low moisture stress, with a significant increase only in N-812 and a significant decrease in N-1296 and N-375.
Previously reported that SOD activity increased throughout the seedling stage in response to drought stress94. In this experiment, low moisture stress caused a significant increase of SOD activity in 12 cotton genotypes N-1096, N-1296, N-1286, N-1496, N-4496, N-990, N-7012, N-1237, N-912, N-512, N-812 and N-153 out of 30. However, it caused significantly decreased SOD activity in N-868, N-696, N-T8411, N-135, N-374, N-926, N-213, N-878 and FH-490. SOD activity was found maintained only in N-896 under non-stress and stress conditions.
Low moisture stress caused a significant increase in peroxidase activity in N-896, N-1496, N-812, N-121, and N-153. While a significant decrease was found in FH-490. Previously, comparable findings have been reported in cotton genotypes under water deficit stress95. Catalase activity was maintained under low moisture stress in all cotton genotypes except for N-1296, N-3496, N-9078, N-990, N-7012, N-812, N-512, N-374, N-213, and FH-490 in which increased activity was observed. Overall, a significant increase in catalase activity was found in N-512. However, in genotypes N-696 and N-896, it was decreased significantly. The same findings were also reported in soybeans96, wheat97, and cotton98. In this research, the enzymes CAT, POD, SOD, and APX increased significantly (p < 0.05) in cotton genotypes compared to that under control.
Previous studies reported that plant cultivars with greater water deficit tolerance had less MDA content under stressed conditions99. In general, MDA is used to identify the lipid peroxidation in crops. MDA is a widely used measure of oxidative lipid damage caused due to abiotic stress. MDA concentration and lipid peroxidation have a positive association, and it may damage cell wall integrity100,101. In the present experiment, Melandialdehyde (MDA) varied significantly in most cotton genotypes. Under low moisture stress in N-990 and N-153, MDA was increased significantly. While, significant decrease was noted in N-696 followed by N-896, N-8437, and N-374. It demonstrates the inability of other genotypes to deal with the high rate of ROS production. Previous research has found the same susceptibility pattern102. The antioxidant activity of plants varies. Scientists hypothesized that the variation in antioxidant activity in genotypes could be caused by a variety of variables, including differences in the degree of stomata closure or other reactions that alter the CO2 fixation degree, or the difference that causes photo-inhibition103.
Phenolic compounds are important secondary metabolites in plants that have redox characteristics and are components of antioxidants (non-enzymatic)104. In response to abiotic stress conditions, plants produce more polyphenols like phenolic acids and flavonoids that help the plant to cope with environmental limitations105. In this study, the genotypic differences of total phenolic contents under low moisture stress which were observed were non-significant. However, total phenolic contents were maintained in N-896, N-1496, N-4496, N-3496, N-990, N-912 and N-812 under non-stress and stress conditions. Overall, a significant decrease in TPC was found in N-1296 out of 30 cotton genotypes.
It is reported that drought stress increased the total tannin (TT) content, total flavonoids (TF), and total phenolics (TP) in the bean leaves106 and cotton107 compared to that under control. In this research experiment, under low moisture stress, a significant increase in the total tannins content was found in 17 cotton genotypes N-868, N-3496, N-990, N-7012, N-1237, N-912, N-T86, N-377, N-135, N-812, N-374, N-375, N-926, N-213, N-878, FH-326 and FH-490 out of 30. However, the significant decrease in TTC was found in 6 genotypes N-696, N-896, N-1296, N-1286, N-8437, and N-4496. Total flavonoid contents were maintained in 8 cotton genotypes N-1296, N-1286, N-4496, N-1237, N-7012 and N-213, N-878 and FH-490 under low moisture stress. Overall, a significant increase in total flavonoid content was observed in cotton genotype N-121. Genotype N-121 was found to be tolerant on the basis of TF contents. Under low moisture stress, ascorbic acid was maintained in many cotton genotypes. Significant increase in ascorbic acid was found in N-1296 and FH-490. These findings were similar to the results of previously reported studies in different plant species106,108,109.
Total oxidants status (TOS) was significantly increased in 7 cotton genotypes N-868, N-T8411, N-1496, N-T86, N-153, N-213 and FH-490 under low moisture stress, while it decreased significantly in N-1096, N-896, N-912, N-512 and FH-326. Significant differences were observed in all cotton genotypes. TOS was retained in 2 genotypes N-1296 and N-926 only. Increased TOS was reported in cotton species under drought stress3. However, significantly increased total free amino acids were observed in N-3496, N-9078, N-T86, N-153, and FH-490. Genotypes N-T86 and FH-490 were found to be tolerant on the basis of total free amino acids and TOS.
In this experiment, compared to non-stress conditions, total soluble proteins varied significantly in many cotton cultivars except N-868, N-3496, and FH-490 in which these were retained. Under low moisture stress in genotypes N-377 and N-812, a significant increase in TSP was found. Significant decrease was found in N-1496, N-7012, N-1237, N-153, N-926, and N-375. These findings were supported by previously reported studies3,110. The same findings trend was also reported in chickpeas that high temperature and low water create a decrease in the production of protein, which could be due to a variety of variables. As a result, plant development and crop output are reduced in susceptible genotypes111.
One of the major plant response mechanisms for achieving tolerance for stress through carbon storage, osmo-protection, and acting on ROS is carbohydrate accumulation under stress conditions112,113. In this study, total soluble sugar varied significantly in most cotton genotypes except N-135 in which it was maintained. Under low moisture stress, in 11 genotypes N-1206, N-1286, N-4496, N-9078, N-1237, N-912, N-512, N-374, N-926, N-812 and FH-326 a significant increase in TSS was noted. Significant decrease was found in N-153. Results revealed that in 6 genotypes N-1286, N-4496, N-9078, N-990, N-812, and N-512, a significant increase in non-reducing sugar was observed. Significant decrease was found in N-153. However, significantly increased reducing sugar was found in N-696. While, it was maintained in N-896, N-1096, N-1496, N-4496, N-3496, N-9078, N-153 and N-213.
Another important biochemical reaction of plants in response to abiotic stimuli (heat, salt, flooding, drought, etc.) is the modulation of various hydrolytic enzymes like esterase and proteases113. In this study, protease activity varied significantly in many cotton genotypes except N-896, N-4496, N-7012, N-912, N-213, and N-878 in which it was maintained. Under low moisture stress, a significant increase was found in N-1286. Significant decrease of protease and esterase activity was observed in N-812. Genotype N-812 was found sensitive to stress on the basis of protease and esterase activity. However, an increase in esterase activity was found in 13 genotypes N-868, N-896, N-696, N-1296, N-1286, N-8437, N-3496, N-9078, N-1237, N-926, N-374, N-213 and N-878. Previously, it was also found that tolerant genotypes of cotton (Gossypium hirsutum) had a significant increase in esterase activity under low moisture conditions95. Low moisture stress caused a significant increase in plant amylase activity observed in N-8437, and N-512. However, a significant decrease in-amylase activity was found in N-868, N-896, N-1096, N-1296, N-1286, N-4496, N-3496, N-9078, N-1237, N-912, N-T86, N-377, N-213, N-878, FH-326 and FH-490. However, an increase in esterase activity was found in 13 genotypes N-868, N-896, N-696, N-1296, N-1286, N-8437, N-3496, N-9078, N-1237, N-926, N-374, N-213 and N-878. In general, all remaining cotton genotypes had non-significant results for amylase, esterase, and protease activity.
According to reports, photosynthesis is the most essential factor influencing crop output during drought conditions114. While photosynthesis depends upon the chlorophyll contents of the plant. In this study, chlorophyll a content under low moisture stress in most cotton genotypes was increased. However, it was decreased in N-868, N-135, N-153, N-374, and FH-326. Low moisture stress caused an increase in chlorophyll b content and the total chlorophyll in all cotton genotypes. While, chlorophyll b content was decreased in genotype N-868, N-926, and FH-326, and total chlorophyll in N-868, N-374, and FH-326. Low moisture stress had the least significant genotypic difference in chlorophyll b, chlorophyll a, and total chlorophyll content in cotton genotypes compared to control plants. Lycopene content was found maintained in N-912.
Increasing proline content may be helpful for both drought stress mitigation and osmotic adjustment. Previous studies found that under the water-deficit conditions, proline content in plants increased115,116. In this study, Proline was observed highest in N-213 under low moisture stress, however, it was lowest in genotype N-912. Under low moisture stress, an increase in proline was found in cotton genotypes N-1096, N-1296, N-8437 N-1496, N-4496, N-1237, N-135, N-926, N-213 and N-878.
Total carotenoids increased in cotton genotype N-7012 under stress treatment but decreased the most in FH-326. Carotenoid content was maintained in N-8437, N-3496, and N-374. The results showed that total carotenoids increased but the differences were non-significant between non-stress and stress conditions. Similar findings were also reported earlier in the cotton cultivars117.
MGIDI, an innovative approach has been illustrated for the selection of best-performing genotypes using multiple attributes64. Several researchers have used this index in different crops including, wheat63,118,119, guar120, barley121, and soybean122. A Monte Carlo simulation research was used to assess the efficacy of the MGIDI index, evaluating its rate of accuracy in determining characteristics with intended outcomes to both conventional and current indices under different circumstances69,123. In this study, results showed that eight genotypes (N-812–1296 N-696 N-377 N-121–896 N-T86, and N-3496) were observed to be best performing by MGIDI for morpho-physiological and biochemical traits. Previously, MGIDI has also been used for similar implications in tomato under control and water deficit stress conditions124. Further, using MGIDI protocol, the best-performing wheat lines under salt stress have also been identified63.
Conclusions
The MGIDI index for both biochemical and morpho-physiological indices was used to identify the best performing genotypes and get insights into involved tolerance mechanisms. Among biochemical traits, TPC, TF, TSP, MDA, SOD, POD, CAT, APX, and Proline proved most efficient indices for selecting cotton genotypes having low moisture stress tolerance. Six genotypes, i.e. N-812–1296 N-696 N-377 N-121, and N-896, were found common and performed best in terms of adjustments in biochemical and morpho-physiological attributes. Three genotypes, i.e. N-696, N-896, and N-T86 proved to be most resilient to low moisture stress and can prove to be a valuable resource for breeding programs aimed to develop climate smart cotton varieties.
Data availability
Data is provided within the manuscript or supplementary information.
References
Jabran, K., Ul-Allah, S., Chauhan, B. S. & Bakhsh, A. An introduction to global production trends and uses, history and evolution, and genetic and biotechnological improvements in cotton. Cotton Prod., 1–22 (2019).
Majeed, S. et al. Heat stress in cotton: A review on predicted and unpredicted growth-yield anomalies and mitigating breeding strategies, Agronomy11(9), 1825 (2021).
Zahid, Z. et al. Dissection of drought tolerance in upland cotton through morpho-physiological and biochemical traits at seedling stage. Front. Plant Sci.12, 627107 (2021).
Campbell, B. et al. Status of the global cotton germplasm resources. Crop Sci.50(4), 1161–1179 (2010).
Hu, Y. et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet.51(4), 739–748 (2019).
Brubaker, C. L., Bourland, F., Wendel, J. F., Smith, C. W. & Cothren, J. T. (1999). Cotton: Origin, history, technology, and production,.
Fryxell, P. A revised taxonomic interpretation of Gossypium L. (Malvaceae), Rheedea, Vol. 2, 108–116 (1992).
Dillehay, T. D., Rossen, J., Andres, T. C. & Williams, D. E. Preceramic adoption of peanut, squash, and cotton in Northern Peru. Science. 316(5833), 1890–1893. https://doi.org/10.1126/science.1141395 (2007).
Wendel, J. F., Schnabel, A. & Seelanan, T. An unusual ribosomal DNA sequence from Gossypium gossypioides reveals ancient, cryptic, intergenomic introgression. Mol. Phylogenet. Evol.4(3), 298–313 (1995).
Wendel, J. F., Rowley, R. & Stewart, J. M. Genetic diversity in and phylogenetic relationships of the Brazilian endemic cotton, Gossypium mustelinum (Malvaceae). Plant Syst. Evol.192, 49–59 (1994).
Barroso, P. A. V. et al. In situ conservation and genetic diversity of three populations of Gossypium Mustelinum Miers ex Watt. Genet. Resour. Crop Evol.57, 343–349 (2010).
Chaudhary, B. et al. Reciprocal silencing, transcriptional bias and functional divergence of homeologs in polyploid cotton (Gossypium). Genetics182(2, 503–517 (2009).
Jameel, S., Hameed, A. & Shah, T. M. Investigation of distinctive morpho-physio and biochemical alterations in desi chickpea at seedling stage under irrigation, heat, and combined stress. Front. Plant Sci.12, 692745 (2021).
Khan, M. K. R. et al. The intervention of multi-omics approaches for developing abiotic stress resistance in cotton crop under climate change. In Sustainable Agriculture in the Era of the OMICs Revolution 37–82 (Springer, 2023).
Rasheed, A. et al. Role of molecular breeding tools in enhancing the breeding of drought-resilient cotton genotypes: An updated review. Water15(7), 1377 (2023).
Zafar, S. et al. Cotton and drought stress: An updated overview for improving stress tolerance. S. Afr. J. Bot.161, 258–268 (2023).
Deeba, F. et al. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem.53, 6–18 (2012).
Babar, M. et al. 12. A comprehensive review on drought stress response in cotton at physiological, biochemical and molecular level, 12(1), 610–622 (2023).
Zafar, M. M. et al. Unraveling heat tolerance in upland cotton (Gossypium hirsutum L.) using univariate and multivariate analysis. 12, 727835 (2022).
Rehman, T., Tabassum, B., Yousaf, S. & Sarwar, G. & U. J. F. i. P. S. Qaisar, Consequences of drought stress encountered during seedling stage on physiology and yield of cultivated cotton. 13, 906444 (2022).
Mahmood, T. et al. Differential seedling growth and tolerance indices reflect drought tolerance in cotton. 22(1), 331 (2022).
Dietz, K. J. & Pfannschmidt, T. Novel regulators in photosynthetic redox control of plant metabolism and gene expression. Plant Physiol.155(4), 1477–1485 (2011).
Foyer, C. H. & Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox. Signal.11(4), 861–905 (2009).
Faize, M. et al. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J. Exp. Bot.62(8), 2599–2613 (2011).
Meshram, J. H., Singh, S. B., Raghavendra, K. & Waghmare, V. Drought stress tolerance in cotton: Progress and perspectives. Climate Change and Crop Stress, 135–169 (2022).
Loka, D. A., Oosterhuis, D. M. & Ritchie, G. L. Water-deficit stress in cotton. Stress Physiol. Cotton. 7, 37–72 (2011).
Sezener, V., Basal, H., Peynircioglu, C., Gurbuz, T. & Kizilkaya, K. Screening of cotton cultivars for drought tolerance under field conditions. Turkish J. Field Crops. 20(2), 223–232 (2015).
Abdelraheem, A., Esmaeili, N., O’Connell, M. & Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod.130, 118–129 (2019).
Singh, K. et al. Genotypic variability among cotton cultivars for heat and drought tolerance using reproductive and physiological traits. Euphytica. 214, 1–22 (2018).
Kanemasu, E. T., Thurtell, G. & Tanner, C. Design calibration and field use of a stomatal diffusion porometer. Plant Physiol.44(6), 881–885 (1969).
Weatherley, P. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytol., 81–97 (1950).
Kumar, P., Pal, M., Joshi, R. & Sairam, R. Yield, growth and physiological responses of mung bean [Vigna radiata (L.) Wilczek] genotypes to waterlogging at vegetative stage. Physiol. Mol. Biol. Plants. 19, 209–220 (2013).
Brown, L. A., Williams, O. & Dash, J. Calibration and characterisation of four chlorophyll meters and transmittance spectroscopy for non-destructive estimation of forest leaf chlorophyll concentration. Agric. For. Meteorol.323, 109059 (2022).
Ainsworth, E. A. & Gillespie, K. M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–ciocalteu reagent. Nat. Protoc.2(4), 875–877 (2007).
Tzekiat, L. & Chiang, L. K. Total phenolics, total tannins and antioxidant activity of Cassia fistula L. extracts of bark, stem, leaf and root under different age classes. Asian J. Pharm. Res. Health Care. 5 (2), 52–57 (2013).
Pothitirat, W., Chomnawang, M. T., Supabphol, R. & Gritsanapan, W. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia80(7), 442–447 (2009).
Vongsak, B. et al. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod.44, 566–571 (2013).
Hameed, A., Iqbal, N., Malik, S. A., Syed, H. & Ahsanul-Haq, M. Age and organ specific accumulation of ascorbate in wheat (Triticum aestivum L.) seedlings grown under etiolation alone and in combination with oxidative stress (2005).
Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem.38(12), 1103–1111 (2005).
Harma, M., Harma, M. & Erel, O. Oxidative stress in women with preeclampsia. Am. J. Obstet. Gynecol.192(2), 656–657 (2005).
Dhindsa, R. S., Plumb-Dhindsa, P. & Thorpe, T. A. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot.32(1), 93–101 (1981).
Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.125(1), 189–198 (1968).
Dixit, V., Pandey, V. & Shyam, R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. Cv. Azad). J. Exp. Bot.52 (358), 1101–1109 (2001).
Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases I. Occurrence in higher plants. Plant Physiol., 59(2), 309–314 (1977).
Chance, B. & Maehly, A. Assay of catalases and peroxidases. Methods Enzymol.2, 764–775 (1955).
Sizer, I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase (1952).
Chen, G. X. & Asada, K. Ascorbate peroxidase in tea leaves: Occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol.30(7), 987–998 (1989).
Hamilton, P. B., Van Slyke, D. D. & Lemish, S. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem.150, 231–250 (1943).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.72(1), 248–254 (1976).
Cupp-Enyard, C. Sigma’s non-specific protease activity assay-casein as a substrate. JoVE (Journal Visualized Experiments). 19, e899 (2008).
Drapeau, G. Protease from Staphylococcus aureus. In Methods in Enzymology Vol. 45 B (ed Lorand, L.) 469 (Academic Press, 1974).
Varavinit, S., Chaokasem, N. & Shobsngob, S. Immobilization of a thermostable alpha-amylase. Science Asia28(3), 247–251 (2002).
Khalid, A. & Hameed, A. Seed biochemical analysis based profiling of Diverse Wheat Genetic Resource from Pakistan. Front. Plant Sci.8, 1276 (2017).
Van Asperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect. Physiol.8(4), 401–416 (1962).
Dubois, M., Gilles, K., Hamilton, J., Rebers, P. & Smith, F. Phenol sulphuric acid method for total carbohydrate. Anal. Chem.26, 350 (1956).
Miller, G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem.31(3), 426–428 (1959).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol.24(1), 1 (1949).
Davies, B. H. T. W. G. Carotenoids, Chemistry and Biochemistry of Plant Pigments 38–165 (Academic Press, 1976).
Bates, L. S., Waldren, R. & Teare, I. Rapid determination of free proline for water-stress studies. Plant. soil.39, 205–207 (1973).
Teklić, T. et al. Assessment of the influence of different sample processing and cold storage duration on plant free proline content analyses. Phytochem. Anal.21(6), 561–565 (2010).
Wickham, H., Chang, W. & Wickham, M. H. Package ‘ggplot2’. In Create Elegant Data Visualisations Using Gramm. Graphics Version. Vol. 2, no. 1, 1–189 (2016).
Epskamp, S., Cramer, A. O., Waldorp, L. J., Schmittmann, V. D. & Borsboom, D. Qgraph: Network visualizations of relationships in psychometric data. J. Stat. Softw.48, 1–18 (2012).
Akram, S. et al. A GBS-based genome-wide association study reveals the genetic basis of salinity tolerance at the seedling stage in bread wheat (Triticum aestivum L). Front. Genet., 13 (2022).
Olivoto, T. & Lúcio, A. D. C. Metan: An R package for multi-environment trial analysis. Methods Ecol. Evol.11(6), 783–789 (2020).
Kassambara, A. & Mundt, F. Package ‘factoextra’. Extract Visualize Results Multivar. Data Analyses76, 2 (2017).
Brejda, J. J., Moorman, T. B., Karlen, D. L. & Dao, T. H. Identification of regional soil quality factors and indicators I. Central and Southern High Plains. Soil Sci. Soc. Am. J.64 (6), 2115–2124 (2000).
Kumar, A. et al. Principal component analysis of agro-morpho-genetic traits in Desi Chickpea (Cicer arietinum L.). SP 362–365 (2019).
Shah, T. M. et al. Selection and screening of drought tolerant high yielding chickpea genotypes based on physio-biochemical indices and multi-environmental yield trials. BMC Plant. Biol.20(1), 1–16 (2020).
Olivoto, T., Nardino, M. & MGIDI: Toward an effective multivariate selection in biological experiments. Bioinformatics37(10), 1383–1389 (2021).
Xiong, L., Schumaker, K. S. & Zhu, J. K. Cell signaling during cold, drought, and salt stress. The plant cell14(suppl_1), S165–S183 (2002).
Wang, W., Vinocur, B. & Altman, A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance, Planta, vol. 218, pp. 1–14, (2003).
Ahmad, R. T., Malik, T. A., Khan, I. A. & Jaskani, M. J. Genetic analysis of some morpho-physiological traits related to drought stress in cotton (Gossypium hirsutum). Int. J. Agric. Biol.11(4), 235–240 (2009).
Parida, A. K., Dagaonkar, V. S., Phalak, M. S., Umalkar, G. & Aurangabadkar, L. P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant. Biotechnol. Rep.1(1), 37–48 (2007).
Evenson, J. & Rose, C. Seasonal variations in stomatal resistance in cotton. Agric. Meteorol.17(5), 381–386 (1976).
Cochard, H., Coll, L., Le Roux, X. & Améglio, T. Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol.128(1), 282–290 (2002).
Kramer, P. J. Water Relation of Plants (Academic Press, 1983).
Shimshi, D. Effect of soil moisture and phenylmercuric acetate upon stomatal aperture, transpiration, and photosynthesis. Plant Physiol.38(6), 713 (1963).
Sánchez-Blanco, M. J., Rodrıguez, P., Morales, M. A., Ortuño, M. & Torrecillas, A. Comparative growth and water relations of Cistus albidus and Cistus monspeliensis plants during water deficit conditions and recovery. Plant Sci.162(1), 107–113 (2002).
Kramer, P. J. & Boyer, J. S. Water Relations of Plants and Soils (Academic Press, 1995).
Ullah, F., Bano, A. & Nosheen, A. Effects of plant growth regulators on growth and oil quality of canola (Brassica napus L.) under drought stress. Pak. J. Bot.44(6), 1873–1880 (2012).
Gholipoor, M., Sinclair, T. R. & Prasad, P. V. Genotypic variation within sorghum for transpiration response to drying soil. Plant. Soil.357, 35–40 (2012).
Devi, M. J. & Reddy, V. Cotton genotypic variability for transpiration decrease with progressive soil drying. Agronomy10(9), 1290 (2020).
Isoda, A. & Wang, P. Leaf temperature and transpiration of field grown cotton and soybean under arid and humid conditions. Plant. Prod. Sci.5(3), 224–228 (2002).
Isoda, A. Effects of water stress on leaf temperature and chlorophyll fluorescence parameters in cotton and peanut. Plant. Prod. Sci.13(3), 269–278 (2010).
Jones, H. G. Physiological aspects of the control of water status in horticultural crop. HortScience25(1), 19–26 (1990).
Ludlow, M. M. & Muchow, R. C. A critical evaluation of traits for improving crop yields in water-limited environments. Adv. Agron.43, 107–153 (1990).
Saleem, M. F., Sammar Raza, M. A., Ahmad, S., Khan, I. H. & Shahid, A. M. Understanding and mitigating the impacts of drought stress in cotton-a review. Pakistan J. Agricultural Sci.53, 3 (2016).
Govindaraj, M., Shanmugasundaram, P., Sumathi, P. & Muthiah, A. Simple, rapid and cost effective screening method for drought resistant breeding in pearl millet. Electron. J. Plant. Breed.1(4), 590–599 (2010).
Khalid, I., Azhar, F. M. & Khan, I. A. Variability for drought tolerance in cotton (Gossypium hirsutum) and its genetic basis. Int. J. Agric. Biol.13(1), 61–66 (2011).
Devasirvatham, V. & Tan, D. K. Impact of high temperature and drought stresses on chickpea production. Agronomy8(8), 145 (2018).
Munné-Bosch, S. & Penuelas, J. Photo‐and antioxidative protection during summer leaf senescence in Pistacia lentiscus L. grown under mediterranean field conditions. Ann. Botany. 92(3), 385–391 (2003).
Reddy, A. R., Chaitanya, K. V. & Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol.161(11), 1189–1202 (2004).
Ali, Q. & Ashraf, M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defence mechanism. J. Agron. Crop. Sci.197(4), 258–271 (2011).
Ahmadi, A., Emam, Y. & Pessarakli, M. Biochemical changes in maize seedlings exposed to drought stress conditions at different nitrogen levels. J. Plant Nutr.33(4), 541–556 (2010).
Prajapat, P. et al. Effect of water stress on antioxidative enzymes and glycine betaine content in drought tolerant and drought susceptible cotton (Gossypium hirsutum L.) genotypes (2018).
Guler, N. S. & Pehlivan, N. Exogenous low-dose hydrogen peroxide enhances drought tolerance of soybean (Glycine max L.) through inducing antioxidant system. Acta Biol. Hung.67, 169–183 (2016).
Latef, A., Kordrostami, M., Zakir, A., Zaki, H. & Saleh, O. Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants8(9), 303 (2019).
Liu, R. N. et al. Drought-induced increase in catalase activity improves cotton yield when grown under water‐limiting field conditions. J. Agron. Crop. Sci.208(6), 853–867 (2022).
Hameed, A., Bibi, N., Akhter, J. & Iqbal, N. Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol. Biochem.49(2), 178–185 (2011).
Kong, W., Liu, F., Zhang, C., Zhang, J. & Feng, H. Non-destructive determination of Malondialdehyde (MDA) distribution in oilseed rape leaves by laboratory scale NIR hyperspectral imaging. Sci. Rep.6(1), 35393 (2016).
Rani, A. et al. Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. Front. Plant Sci.10, 1759 (2020).
Hafeez, M. et al. Physiological, morphological, biochemical and molecular basis of drought tolerance in cotton. Int. J. Biol. Pharm. Allied Sci.4, 1091–1112 (2015).
Waseem, M. et al. Mechanism of drought tolerance in plant and its management through different methods. Cont. J. Agricultural Sci.5(1), 10–25 (2011).
Giannakoula, A., Therios, I. & Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants10(1), 155 (2021).
Sharma, A. et al. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules24(13), 2452 (2019).
Gaafar, A. A. et al. Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions, Plants9(5), 627 (2020).
Kolahi, M., Faghani, E., Kazemian, M., Goldson-Barnaby, A. & Dodangi, S. Changes in secondary metabolites and fiber quality of cotton (Gossypium hirsutum) seed under consecutive water stress and in silico analysis of cellulose synthase and xyloglucan endotransglucosylase. Physiol. Mol. Biology Plants. 27(8), 1837–1857 (2021).
Ansari, M. A. et al. Comparative transcriptomic analysis and antioxidant defense mechanisms in clusterbean (Cyamopsis tetragonoloba (L.) Taub.) Genotypes with contrasting drought tolerance. Funct. Integr. Genom.22(4), 625–642 (2022).
Öztürk Gökçe, Z. N., Gökçe, A. F., Junaid, M. D. & Chaudhry, U. K. Comparative transcriptomics of drought stress response of taproot meristem region of contrasting purple carrot breeding lines supported by physio-biochemical parameters. Funct. Integr. Genom.22(4), 697–710 (2022).
Abid, M. A. et al. Mode of inheritance for biochemical traits in genetically engineered cotton under water stress. AoB Plants. 8, plw008 (2016).
El-Beltagi, H. S., Mohamed, H. I. & Sofy, M. R. Role of ascorbic acid, glutathione and proline applied as singly or in sequence combination in improving chickpea plant through physiological change and antioxidant defense under different levels of irrigation intervals. Molecules25(7), 1702 (2020).
Kerepesi, I. & Galiba, G. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci.40(2), 482–487 (2000).
Ehsanpour, A. & Amini, F. Effect of salt and drought stress on acid phosphatase activities in alfalfa (Medicago sativa L.) explants under in vitro culture. Afr. J. Biotechnol.2 (5), 133–135 (2003).
Trovato, M., Mattioli, R. & Costantino, P. Multiple roles of proline in plant stress tolerance and development. Rend. Lincei. 19, 325–346 (2008).
Sánchez, F. J., Manzanares, M., de Andres, E. F., Tenorio, J. L. & Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res.59(3), 225–235 (1998).
Alexieva, V., Sergiev, I., Mapelli, S. & Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant. Cell. Environ.24(12), 1337–1344 (2001).
Shah, A. R., Khan, T. M., Sadaqat, H. A. & Chatha, A. A. Alterations in Leaf pigments in Cotton (Gossypium hirsutum) genotypes subjected to Drought stress conditions. Int. J. Agric. Biology, 13, 6, (2011).
Lima, G. W. et al. Genetic diversity in tropical wheat germplasm and selection via multitrait index. Agron. J.114(2), 887–899 (2022).
Farhad, M. et al. Multi-trait selection of bread wheat ideotypes for adaptation to early sown condition. Crop Sci.62(1), 67–82 (2022).
Benakanahalli, N. K. et al. A framework for identification of stable genotypes basedon MTSI and MGDII indexes: An example in guar (Cymopsis tetragonoloba L.), Agronomy, vol. 11, no. 6, p. 1221, (2021).
Pour-Aboughadareh, A. et al. Identification of salt-tolerant barley genotypes using multiple-traits index and yield performance at the early growth and maturity stages. Bull. Natl. Res. Centre. 45(1), 117 (2021).
Maranna, S. et al. Breeding for higher yield, early maturity, wider adaptability and waterlogging tolerance in soybean (Glycine max L.): A case study. Scientific reports11(1), 22853 (2021).
Olivoto, T., Diel, M. I., Schmidt, D. & Lúcio, A. D. J. P. M. MGIDI: a powerful tool to analyze plant multivariate data, vol. 18, no. 1, p. 121, (2022).
Wadood, A., Hameed, A., Akram, S., i., M. J. F. & Ghaffar, P. S. Unraveling the impact of water deficit stress on nutritional quality and defense response of tomato genotypes. 15, 1403895 (2024).
Acknowledgements
The research work is partially supported by ALP funded project entitled “Development of High Yielding Cotton Germplasm for Low Moisture Conditions (Project No: ALP-CS163).
Author information
Authors and Affiliations
Contributions
A.S. executed the experiment as well as the collection of data, analytical work, data organization, presentation of resulting data in the form of graphs, write-up, and revision of the manuscript after the biochemical, physiological and morphological analysis. A.H. planned and designed the experiments, and performed the statistical analysis of data, supervised overall research work and finalized the manuscript. M.H. did the arrangement and provided the seeds of cotton genotypes, contributed to designing and planning of wirehouse experiment, reviewed the finalized manuscript. All authors have equally contributed to the manuscript and approved the final submission of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Safdar, A., Hameed, A. & Hassan, H.M. Biochemical and morpho-physiological insights revealed low moisture stress adaptation mechanisms in cotton (Gossypium hirsutum L.). Sci Rep 14, 25942 (2024). https://doi.org/10.1038/s41598-024-77204-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77204-0