Abstract
The aim of this study was to develop and validate a High-Performance Thin Layer Chromatographic (HPTLC) method for simultaneous determination of ceftriaxone and ceftriaxone e-isomer in powder for injection formulation. Ceftriaxone sodium injection is an antibiotic that used globally. It has Z/E geometrical conformation, in which ceftriaxone sodium and 3 ene-isomer have Z- conformation while (E)-isomer has E- conformation and the potential toxicity of ceftriaxone (E)-isomer has been reported. Thus, to safeguard the public health, a simple and easy to use, rapid and reliable method was developed for qualitative and quantitative determination of ceftriaxone sodium and its (E)–isomer. Samples were applied on HPTLC glass plates precoated with silica gel 60F254 by using Linomat semi-auto sampler. Separation was carried out using acetone, triethyl amine, water, chloroform and ethyl acetate as a mobile phase in different ratios. The Rf values of separated compounds were 0.51 ± 0.01 and 0.62 ± 0.01 for ceftriaxone sodium and ceftriaxone (E)-isomer respectively. The method was validated by studying Specificity, Linearity, Accuracy, Precision, Robustness, Limit of Detection (LOD) and Limit of Quantification (LOQ) and Solution stability. The developed method was successfully, sensitive, simple, precise, accurate, robust and applicable for the simultaneous determination of ceftriaxone sodium and ceftriaxone (E)-isomer in powder for injection formulation.
Similar content being viewed by others
Introduction
A very important stage in the regulatory process of medicines post approval is the quality control (analysis) of medicines1 including isomeric medicines circulating on the market. Isomeric medicines are those medicines composed of active pharmaceutical ingredients (API) having different compounds of the same molecular formula but differ in their overall chemical structure2,3. These mixtures are not drug formulations that containing two or more therapeutic substances, but combination of isomeric substances, with the therapeutic activity residing mainly in one of the molecules4. The separation technique is a challenging experimental task in the separation sciences. The literature survey reveals that different studies have indicated development of different analytical separation techniques for analyzing quality of isomeric medicines depending on the type of medicines5,6. To our knowledge, analytical experience and supported literatures7,8 those methods are expensive and cannot be affordable in resource constraints countries.
Moreover, it was reported that, many National Regulatory Authorities (NRAs) in low- and middle-income countries (LMICs) are under-resourced. These NRAs often lack or have insufficient capacity in terms of separation techniques, infrastructure, expertise and knowledge to assure the quality of medicines circulating in their countries. Due to the above mentioned inadequacies, the public are exposed to the risk of being provided with poor-quality or sub-standard medicines9. It has also been declared that, for the resource-limited countries, NRAs must perform quality control of these chiral medicines by using inexpensive methods10. One study reported that, for the pharmaceutical laboratories, there should be always a need for faster, simpler, cheaper and better performing analytical methods7.
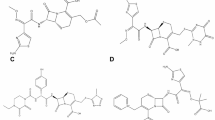
Among other several criteria for quality monitoring of medicines in post approval activities is to monitor highly utilized medicines in order to protect public health. Ceftriaxone sodium is among the widely used medicine globally11 and therefore its quality monitoring is imperative. The medicine is a beta-lactam, third generation cephalosporin used to treat diseases caused by gram-positive and gram-negative bacteria11,12. Its structure has Z/E geometrical conformation, in which ceftriaxone sodium and 3 ene-isomer have Z- conformation while (E)-isomer has E- conformation13 (Fig. 1).
(a–c): Indicating the structure of ceftriaxone disodium, Ceftriaxone (E)-isomer and Ceftriaxone 3-ene isomer respectively. Ceftriaxone Sodium is chemically known as (6R,7R)-7- [[(2Z)- (2-aminothiazol- 4-yl) (methoxyimino) acetyl]amino]-3-[[(2- methyl l-6-oxido-5-oxo-2, 5-dihydro-1,2,4-triazin-3-yl)sulphonyl] methyl] -8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2- carboxylate hemiheptahydrate14.
Ceftriaxone isomers may vary in toxicity13. This has been confirmed in the study which compared the ceftriaxone toxic effects of the (Z)- and (E)- isomers. The study reported that, the ceftriaxone (Z)- LD50 values was higher (24mmol/L) that means low toxic compared to that of the (E)-isomer which was 5.1 mmol/L13. This indicates that (E)-isomer is more toxic compared to (Z)-isomer. The presence of harmful isomers even in any small amount may significantly affect the safety of the product and can harm the patient. Thus, in order to protect the public, there should be a simple, easy to use method for quality analysis of medicines which can determine and separate isomers in the ceftriaxone powder for injection.
Several methods have been reported in the literatures for separating ceftriaxone sodium from other compounds in combination formulations and some from impurities6,11,12,15,16,17. Among those reported analytical separation methods is the High-Performance Thin Layer Chromatography (HPTLC), however, there is no HTPLC method for the separation of ceftriaxone sodium and its (E)-isomer that has been developed previously.
Therefore, in this study, we aimed at developing a simple, easy to use, rapid and reliable method of separating qualitatively and quantitatively ceftriaxone sodium from its isomers by identifying suitable HPTLC conditions which can allow the separation of the mentioned isomers.
Materials and methods
Chemicals and reagents
The ceftriaxone Sodium 350 mg USP Reference standard of ceftriaxone (Lot R07420) was acquired from Switzerland (USP 12601 Twin brook Pkwy, Rockville). The ceftriaxone sodium E-isomer 25 mg USP reference standard (Lot R131A0) was obtained from Chromachemie Laboratory Pvt., Ltd, VITC Export Bhavan, Plot No 488 B14th Cross, Peenya Industrial Area, Bangalore, Karnataka, India.
All chemicals and reagents used were of analytical grade. These include methanol (Chemi-Lab NV, Zoersel, Belgium), acetone AR (Loba Chemie Pvt, LTD, Mumbai-India), triethylamine AR (Loba Chemie Pvt, LTD, Mumbai-India), ethyl acetate AR (Chem-Lab NV, Zoersel, Belgium), chloroform AR (Surechem products LTD, Suffolk, England), water laboratory grade (TMDA, Tanzania) prepared by reverse osmosis and de-ionization.
Instrumentation
A CAMAG HPTLC system coupled with a CAMAG Linomat semi-automatic applicator, a CAMAG developing tank, and a CAMAG TLC scanner III operated using WinCATS software (version 1.4.3) for data integration, were utilized.
Sample application was performed using a Hamilton syringe with a capacity of 100 µL, all sourced from CAMAG, Muttenz, Switzerland. The pre-coated HPTLC silica plates (60 F254, glass, 20 × 10 cm) were obtained from Merck, Darmstadt, Germany.
Additionally, equipment such as the Evoqua water purification machine, an Ultrasonic bath from Grant Instruments in the United Kingdom, and analytical balances from Shimadzu in Japan were employed.
All glassware employed for measuring volumes was of Class A specifications and made from borosilicate glass. Micropipettes (200 µL and 1000 µL) were obtained from Eppendorf and Isolab, Hamburg, Germany.
Samples collection
Samples of ceftriaxone sodium powder for injection were collected from tertiary hospitals and nearby pharmacies across seven (7) administrative regions of Tanzania namely Dar es salaam, Dodoma, Kilimanjaro, Mwanza, Mbeya, Mtwara and Tabora. The collection process involved two different brands in each region, and each brand was collected in its original packaging as supplied by manufacturer.
Experimental conditions
HPTLC analysis was carried out on silica gel 60F254 HPTLC glass plates (20 × 10 cm) using a Linomat – 5 sample applicators equipped with a 100 µL syringe and operated with settings of band length 3 mm, distance from the plate edge was 10 mm, and distance from the bottom of the plate was 10 mm. The plate was developed for a distance of 70 mm in a chamber previously saturated for 20 min with the mobile phase. For densitometry analysis, the air-dried plate spots were scanned with the CAMAG Scanner III at 281 nm in the reflectance- absorbance mode and controlled by WinCATS software.
Preparation of solutions
Ceftriaxone sodium RS stock solution: About 20 mg of ceftriaxone Sodium RS was weighed and placed into a 10 mL volumetric flask. Seven (7) mL of methanol was then added, and the mixture was thoroughly blended through sonication for 15 min to facilitate dissolution. The volume was adjusted with methanol up to the 10 mL mark, resulting in a stock concentration of 2 mg/mL.
Ceftriaxone (E)-isomer RS stock solution: About 10 mg of Ceftriaxone (E)-isomer RS was weighed and placed into a distinct 100 mL volumetric flask. Subsequently, 50 mL of methanol was added, and the mixture was thoroughly blended through sonication for 15 min to facilitate dissolution. The volume was adjusted with methanol up to the 100 mL mark, resulting in a stock concentration of 0.1 mg/mL.
Sample stock solution: Ceftriaxone powder for injection corresponding to 200 mg of ceftriaxone was weighed and placed into a 100 mL Volumetric Flask (VF). Subsequently, 50 mL of methanol was added and thoroughly mixed to facilitate dissolution, aided by sonication for 15 min. The volume was adjusted with methanol up to the 100 mL mark, resulting in a stock concentration of 2 mg/mL.
Working solutions: All stock solutions were prepared by adding respective volumes into 10 mL volumetric flasks, and they were used immediately after preparation due to instability after 12 h. The mobile phase consisting of acetone, triethyl amine, water, chloroform and ethyl acetate in a ratio of 16:5:3.5: 2:0.3 v/v/v/v/v was placed in CAMAG TLC chamber and saturation was performed for 20 min. Final separation was achieved using the above mobile phase.
Method development and optimization
Mobile phase and stationary phase were selected based on literature11,12. Several experiments were performed using different eluent polarities under both normal and reverse phase TLC or HPTLC to attain optimal resolution. Method optimization was performed by identifying major components of the mobile phase that had influence on elution in this case, water and triethylamine.
Analytical method validation
HPTLC method for determination of ceftriaxone sodium and ceftriaxone (E)-isomer was validated in accordance with ICH Q2 R1 guideline13. The following validation performance characteristics were evaluated;
Specificity
Specificity is the ability of the method to accurately measure the analyte in the presence of all potential sample components. This was examined by creating the mobile phase, diluent, and corresponding standard solutions for ceftriaxone sodium and ceftriaxone (E)-isomers. All solutions were administered onto the HPTLC plate, and a visual assessment was conducted to observe co-elution. This process involved separate applications on the plate as well as a mixture of solutions. Additionally, the maximum scanning wavelength for the developed spots was identified.
Linearity
Linearity assesses the capability of the analytical method, within a specified range, to yield a response that is directly proportional to the concentration of the analyte standard. If the method exhibits linearity, the test results are directly proportional to the analyte concentration in samples within a defined range, either directly or through a well-defined mathematical transformation. To evaluate the linearity of the ceftriaxone sodium assay, the ceftriaxone sample matrix was spiked with five standard concentrations (50%, 75%, 100%, 125%, and 150%; corresponding to 100, 150, 200, 250, and 300 µg/mL). For ceftriaxone (E)-isomer, concentrations of 7.5, 15, 30, 45, 60, and 75 µg/mL were used, achieved through serial dilutions from stock solutions of 2 mg/mL and 0.1 mg/mL, respectively. Three spots were generated at each concentration, starting from the lowest concentration to prevent carry-over effects. This process was repeated over three days.
Accuracy
Accuracy signifies the proximity of agreement between the measured value and a recognized true value or an accepted reference value. This is demonstrated as the recovery through the assay of known and added amounts of the analyte. In the preparation of the sample matrix for ceftriaxone sodium, the injectable powder was individually weighed at the nominal concentration, following the procedure outlined in the sample preparation section. The wet spiking approach was employed, involving the addition of a known quantity of standard stock solution (2 mg/mL) of ceftriaxone sodium at levels of 50%, 100%, and 150% (0.75, 1.75, and 2.75 mg/mL) in triplicate. Similarly, ceftriaxone (E)-isomer reference standard was introduced into the same matrix to assess recovery.
Precision
Repeatability assesses the variability encountered by a single analyst using a single instrument. This measure does not differentiate between variations arising from the instrument or system alone and those stemming from the sample preparation process. The assessment involved analyzing multiple replicates of a composite assay sample using the analytical method, with the recovery value being calculated and reported for each instance.
For the preparation of the sample matrix of ceftriaxone sodium, the injectable powder was individually weighed at the nominal concentration, following the procedure outlined in the sample preparation section. The wet spiking approach was employed by adding a known quantity of standard stock solution (2 mg/mL) of ceftriaxone sodium at three assay concentration levels—50%, 100%, and 150%, corresponding to (0.75, 1.5, and 2.25) mg/mL. Additionally, ceftriaxone (E)-isomer reference standard was introduced into the same matrix at LOD, LOQ, and level 3. These steps were repeated over three consecutive days.
Intermediate precision was conducted by a second analyst to assess variations within the laboratory, utilizing the same instrument, as only one was available. For the method to be precise, %RSD should be not more than 2.0% for Ceftriaxone sodium and not more than 15% for Ceftriaxone (E)-isomer.
Limit of detection (LOD) & Limit of quantification (LOQ)
The determination of the Limit of Detection (LOD) and Limit of Quantitation (LOQ) involved utilizing the mean slope of the standard deviation intercept from the calibration curve.
Here, σ represents the standard deviation (SD) of the response, and S denotes slope of the regression line.
Robustness testing
Robustness gauges the capacity of an analytical method to withstand minor yet intentional alterations in method parameters such as pH, mobile phase composition, temperature, and instrument settings. It offers insight into the method’s reliability under typical usage conditions.
To explore this, an investigation was conducted using the analysis system suitability sample solution. The impact on the method was measured by intentionally modifying conditions related to mobile phase composition, TLC/HPTLC plates, slit width, the duration from spotting to development, and the duration from development to scanning. These alterations were made by at least ± 5% for all parameters. Observations were made on the changes in peak area values.
Solution stability
The stability of standards and samples was assessed under typical benchtop conditions (room temperature) and within a refrigerator to ascertain the need for specific storage conditions. Standard solutions and precision sample solutions were analyzed at various time intervals (e.g., 0 and 24 h), and any alterations in the area were compared.
Sample analysis/method application
A total of 27 samples were analysed by using developed and validated HTPLC method.
Results and discussion
Method development and optimization
In this study, the mixture of acetone, triethyl amine, water, chloroform and ethyl acetate was selected as a mobile phase based on previous studies11,18. The selection was concluded based on the trials conducted on several eluent combinations by varying their polarities using normal and reversed phase TLC plates until a good resolution was achieved. The study indicated that, the HPTLC plate showed a good performance in separating ceftriaxone and its (E)-isomer by using the selected mobile phases. The mobile phase composition is shown in Table 1.
Ceftriaxone and its (E)-isomer have structural isomeric similarities thus eluotropic knowledge of solvent polarities was necessary in solvent selection. They also have a log P of around − 1.7 which shows the compound to have more affinity to hydrophilic as compared to lipophilic interactions16. Hence solvents for elution mobile phase had to contain a substantial amount of high polar properties to compete with hydrogen bonding of silica hydroxyl group.
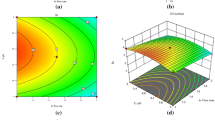
In optimization phase, water and triethylamine were identified as mobile phase components that are critical for elution. Water content, which was 13% was responsible for unlocking bonding between stationary phase and the analyte14 while triethylamine, which was 19% reduced ionization of the analytes hence preventing tailing and fronting21. Other components were used to improve resolution and peak symmetry/shape between ceftriaxone sodium and Ceftriaxone (E)-isomer. The plates were analyzed by densitometry and resolution was shown by densitogram in Fig. 2. Optimized chromatographic conditions are shown in Table 1.
Method validation
Specificity
The method was found to be specific as no peak was observed in the dissolving solvent and there was no co-elution. There was a clear resolution of Ceftriaxone sodium and Ceftriaxone (E)-isomer as shown in Fig. 2 and the maximum analytical wavelength obtained was 281 nm.
Linearity and range
The method was found to be linear in the concentration range of 200–600 ng/spot for ceftriaxone sodium and 15–150 ng/spot for ceftriaxone (E)-isomer. The linear regression coefficient (R2) was 0.997 for ceftriaxone sodium and 0.995 for ceftriaxone (E)-isomer as depicted in Table 2. Linearity curves are presented as Fig. 3 and residual plots showed the distribution of the concentrations around the mean with no tendency which indicated an adequate linear relationship between the peak area and the concentration for each compound.
Accuracy/recovery
The mean recovery for ceftriaxone sodium were found to be 101.02%,102.03% and 99.04% for 50%,100% and 150% levels respectively. Moreover, the accuracy for ceftriaxone (E)-isomer ranged from 87.74 − 103.85% for all six levels used. This suggests that the method has an acceptable recovery of ceftriaxone sodium as well as its (E)-isomer as shown in Table 3.
Precision
Repeatability
The coefficient of variation (%RSD) was below 2% for ceftriaxone sodium at 50%,100% and 150% levels and below 15% for ceftriaxone (E)-isomer at three levels as shown in Tables 4 and 5. The method is therefore repeatable.
Intermediate precision
The coefficient of variation (%RSD) was below 2% for eftriaxone sodium at 50%,100% and 150% levels and below 15% for ceftriaxone (E)-isomer at three levels as shown in Table 5. The method is therefore reproducible and precise.
Limit of detection and Limit of quantitation
From the equations, the LOD was 0.002 mg/mL and 0.005 mg/mL for ceftriaxone sodium and ceftriaxone (E)-isomer respectively and LOQ was 0.0065 mg/mL and 0.015 mg/mL for Ceftriaxone sodium and ceftriaxone (E)-isomer respectively.
Robustness
Slight changes in composition of water, ethyl acetate and triethylamine in the mobile phase resulted in minor changes in peak area and demonstrated that the method is robust. Changes in slit dimension resulted in amplification of the densitogram area, hence this was kept constant throughout the study.
Solution stability
Peak broadening was observed after solutions were stored for 24 h at room temperature and in the refrigerator. Ceftriaxone (E)-isomer stored at room temperature showed degradation products close to the main peak. Storage in refrigerator resulted in higher stability even though the overall change in area after storage was more than ± 2%. There was generally a decrease in concentration of the analytes with time, with a higher rate following storage at room temperature. This observation suggested that prepared solutions should be used immediately and not more than 12 h after preparation.
Sample analysis/method application
The results for 27 marketed analysed samples for Ceftriaxone sodium was found to be between 91.37 and 105.56% (99.1 ± 3.72%). This was within the recommended USP assay limit of 90–115%22. Though the method did not detect ceftriaxone (E)-isomer in the samples of ceftriaxone injections, it successfully separated the drug from its impurity with remarkable resolution. The validation results were found within the acceptable limits, specific and sensitive to detect ceftriaxone (E)-isomer in finished ceftriaxone injection formulation.
Conclusion
The HPTLC method for qualitative and quantitative determination of Ceftriaxone sodium and Ceftriaxone (E)-isomer in powder for injection formulations was successfully developed and validated as per ICH guidelines. This simple, reliable, selective, precise and accurate method is ideal for routine analysis in pharmaceutical companies, medicines regulatory agency and research laboratories.
Data availability
The datasets generated and analyzed during the study are available at the TMDA office. All data are available with permission from the TMDA.
References
Hajjou, M. et al. Monitoring the quality of medicines: Results from Africa, Asia, and South America. Am. J. Trop. Med. Hyg. 92, 68–74 (2015).
Chhabra, N., Aseri, M. L. & Padmanabhan, D. A review of drug isomerism and its significance. Int. J. Appl. Basic. Med. Res. 3, 16–18 (2013).
Phillips, S. T., Dodds, J. N., May, J. C. & McLean, J. A. Isomeric and Conformational Analysis of small drug and drug-like molecules by ion mobility-mass spectrometry (IM-MS). In Bioinformatics and Drug Discovery (eds. Larson, R. S. & Oprea, T. I.) 161–178, vol. 1939 (Springer, 2019).
Mitra, S. & Chopra, P. Chirality and anaesthetic drugs: A review and an update. Indian J. Anaesth. 55, 556–562 (2011).
Heydari, R. & Shamsipur, M. Enantiomeric separation and quantitation of tenofovir disoproxil fumarate using amylose-based chiral stationary phases by high-performance liquid chromatography. Acta Chromatogr. 27, 583–595 (2015).
Sajewicz, M., Piętka, R. & Kowalska, T. Chiral separations of Ibuprofen and Propranolol by TLC. A study of the mechanism and thermodynamics of Retention. J. Liq Chromatogr. Relat. Technol. 28, 2499–2513 (2005).
Sreeman Narayana, P., Shiva Reddy, A., Ramesh, B. & Sita Devi, P. Simultaneous determination and method validation of fluconazole and its impurities by high performance thin layer chromatography using reflectance scanning densitometry. Eurasian J. Anal. Chem. 81 39–49 (8), 39–49 (2013).
Rashmin, P., Mrunali, P., Nitin, D., Nidhi, D., & Bharat, P. HPTLC method development and validation: Strategy to minimize methodological failures. 藥物食品分析 20 (2012).
Ravinetto, R. et al. Fighting poor-quality medicines in low- and middle-income countries: The importance of advocacy and pedagogy. J. Pharm. Policy Pract. 9 (36), s40545–s40016 (2016).
Shewiyo, D. H. et al. Development and validation of a normal-phase high-performance thin layer chromatographic method for the analysis of sulfamethoxazole and trimethoprim in co-trimoxazole tablets. J. Chromatogr. A. 1216, 7102–7107 (2009).
Eric-Jovanovic, S., Agbaba, D., Zivanov-Stakic, D. & Vladimirov, S. HPTLC determination of ceftriaxone, cefixime and cefotaxime in dosage forms. J. Pharm. Biomed. Anal. 18, 893–898 (1998).
De Aléssio, P. V., Kogawa, A. C. & Salgado, H. R. N. Quality of ceftriaxone sodium in lyophilized powder for injection evaluated by clean, fast, and efficient spectrophotometric method. J. Anal. Methods Chem. 7530242 (2017).
Zhang, J., Qian, J., Tong, J., Zhang, D. & Hu, C. Toxic effects of cephalosporins with specific functional groups as indicated by zebrafish embryo toxicity testing. Chem. Res. Toxicol. 26, 1168–1181 (2013).
Malgundkar, S. S., & Mulla, S. Validated HPTLC method for simulteneous determination of ceftriaxone sodium and sulbactam sodium in combined dosage form. IOSR J. Pharm. Biol. Sci. 9, 1–5 (2014).
Phattanawasin, P., Sotanaphun, U., Sriphong, L. & Kanchanaphibool, I. Stability-indicating TLC-image analysis method for quantification of ceftriaxone sodium in pharmaceutical dosage forms. J. Planar Chromatogr. – Mod. TLC. 24, 30–34 (2011).
Da Trindade, M. T. & Salgado, H. R. N. A critical review of analytical methods for determination of ceftriaxone sodium. Crit. Rev. Anal. Chem. 48, 95–101 (2018).
Hancu, G., Simon, B., Rusu, A., Mircia, E. & Gyéresi, Á. principles of micellar electrokinetic capillary chromatography applied in pharmaceutical analysis. Adv. Pharm. Bull. EISSN 2251–7308. https://doi.org/10.5681/APB.2013.001 (2013).
Prajapati, L. M., Patel, A., Patel, J. R., Joshi, A. K. & Kharodiya, M. High performance thin layer chromatography method for simultaneous estimation of cefepime hydrochloride and sulbactam sodium. Asian J. Pharm. Anal. 6, 207 (2016).
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Validation of analytical procedures: Text and methodology Q2(R1). (2005).
Krzemińska, K., Bocian, S., Pluskota, R. & Buszewski, B. Surface properties of stationary phases with embedded polar group based on secondary interaction, zeta potential measurement and linear solvatation energy relationship studies. J. Chromatogr. A. 1637, 461853 (2021).
Bian, M., Zhang, Z. & Yin, H. Effects and mechanism characterization of ionic liquids as mobile phase additives for the separation of matrine-type alkaloids by liquid chromatography. J. Pharm. Biomed. Anal. 58, 163–167 (2012).
United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary (USP 41-NF 38. ). https://online.uspnf.com/uspnf/document/1_GUID-0111DA94-EAAF-4FAE-905E-0C25EE2DDB16_3_en-US?source=Search%20Results&highlight=Ceftriaxone (2023).
Acknowledgements
The authors would like to acknowledge the European and Developing Countries Clinical Trials Partnership (EDCTP2 Program), which funded the PhD studies. The authors are also thankful to the management of the Tanzania Medicines and Medical Devices Authority (TMDA) for allowing the laboratory analysis to be conducted in its TMDA – WHO pre-qualified laboratory located at Dar es Salaam, Eastern zone.
Funding
The study was part of the EDCTP2 Program supported by the European Union partly under the ASCEND project (CSA2019ERC- 2683) and partly under TMDA. The funder had no role in study design, analysis, or manuscript writing. However, the report of the study and progress of what has been done was of interest to the funder to ensure that the study results are useful in protecting public health.
Author information
Authors and Affiliations
Contributions
K.W.M contributed to conception, design, funding acquisition, project administration, laboratory method development analysis, optimization, validation, writing, and approval of the manuscript. B.N, M.A and G.S contributed to the method development, optimization, validation, method utilization, writing, and approval of the manuscript. G.G.N and E.Y contributed to data analysis, writing and approval of the manuscript for publication. A.M.F, V.M and W.S contributed to review and approve the manuscript for publication. D.H.S, M.C.S, A.D.S, and E.A.K supervised, reviewed, and approved the manuscript for publication. All authors commented on the previous versions of the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical clearance was granted by the National Institute for Medical Research, Tanzania (certificate number NIMR/HQ/R.8 a/vol. IX/3086) and the Institutional Review Board of Muhimbili University of Health and Allied Sciences (MUHAS) (certificate number DA.282/298/01.C).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mwamwitwa, K.W., Nyamweru, B., Iwodyah, M.A. et al. A high-performance thin-layer chromatography densitometric method for the separation of isomeric ceftriaxone in powder for injection formulation. Sci Rep 14, 26308 (2024). https://doi.org/10.1038/s41598-024-77334-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77334-5