Abstract
The relationship between possible sarcopenia and mortality remains ambiguous within Asian populations. To clarify this, we investigated the association in older adults residing in Chinese communities. Utilizing data from the China Health and Retirement Longitudinal Study, this population-based cohort study included individuals aged ≥ 60 years, followed from 2011 to 2012 through 2020. Possible sarcopenia was defined in accordance with the Asian Working Group on Sarcopenia 2019 criteria, and Cox proportional hazards regression was used to analyze its impact on mortality, while exploratory analyses were conducted to investigate the associations of possible sarcopenia with chronic diseases, functional independence, and hospitalization frequency. The study encompassed 5,160 participants (median age: 66 years), nearly half of whom (48.8%) were identified with possible sarcopenia. Over a 9-year follow-up period, there were 1216 recorded deaths. Analysis indicated that individuals with possible sarcopenia faced a significantly elevated mortality risk compared to their counterparts (HR: 1.79, 95% CI: 1.58–2.03; P < 0.001). Further, subgroup analyses confirmed a strong association between possible sarcopenia and all-cause mortality across various subgroups, including those related to sex, obesity status, and living environment. Additionally, exploratory analyses revealed that possible sarcopenia was significantly associated with an increased likelihood of heart disease (OR = 1.18, 95% CI: 1.03–1.34, P = 0.014) and stroke (OR = 1.41, 95% CI: 1.19–1.68, P < 0.001), as well as reduced functional independence (β = -0.17, 95% CI: -0.24–-0.10, P < 0.001). Possible sarcopenia was also associated with a higher frequency of hospitalizations at baseline (Exp(β) = 1.50, 95% CI: 1.25–1.81, P < 0.001), although this association was no longer significant during the follow-up period. In conclusion, in Chinese community-dwelling older adults, possible sarcopenia was associated with an increased risk of all-cause mortality, several chronic diseases, and functional dependence. Thus, alleviating or preventing possible sarcopenia may improve health outcomes and extend the lifespan of these individuals.
Similar content being viewed by others
Introduction
As the global population ages, the prevalence of sarcopenia, which is characterized by a progressive decline in muscle mass and function, is notably increasing, representing a substantial public health concern1. The diagnostic criteria for sarcopenia encompass muscle mass, strength, and physical performance2,3,4,5. Despite extensive research on the link between sarcopenia and heightened mortality risk6,7,8,9,10,11,12, the lack of accessible and reliable equipment for muscle mass assessment in community settings hampers widespread screening efforts4.
In 2019, the European Working Group on Sarcopenia in Older People (EWGSOP2)3 and the Asian Working Group for Sarcopenia (AWGS)4 revised their guidelines for Western and Asian populations, respectively. These revisions introduced the notion of “possible sarcopenia”, identifiable through less complex assessments such as grip strength and chair stand tests, thereby obviating the requirement for muscle mass evaluation. This streamlined and cost-effective methodology facilitates its integration into primary healthcare and preventive services4. Investigating the relationship between “possible sarcopenia” and both disease and mortality is vital, offering significant insights into avenues for disease prevention, treatment modalities, and the improvement of life quality and longevity.
Research has indicated that possible sarcopenia is associated with an increased risk of mortality in Western populations13,14,15,16,17,18,19. However, studies examining this link within Asian populations are scarce and have produced inconsistent results12,20,21,22, underscoring the urgent need for more thorough research in this area. Our study aims to address the following question: Is the association between possible sarcopenia and all-cause mortality among Chinese community-dwelling individuals aged ≥ 60 years similar to that observed in Western populations? We hypothesize that the relationship between possible sarcopenia and mortality in the Asian population mirrors that in Western populations due to shared biological mechanisms23. Our analysis also explored potential variations in the association between possible sarcopenia and mortality across different subgroups, including those related to sex (male or female), residential area (urban or rural), and obesity status (obese or nonobese).
In addition to mortality, sarcopenia has been linked to other adverse health outcomes, such as chronic diseases, functional decline, and increased healthcare utilization1,2. Therefore, our study also explores the relationship between possible sarcopenia and various chronic diseases (e.g., hypertension, diabetes, cancer, chronic lung diseases, heart disease, and stroke), physical function, and hospitalization risk. By including these additional analyses, we aim to provide a more comprehensive understanding of the broader health impact of possible sarcopenia. This is particularly relevant for public health and clinical practice, as understanding the multifaceted effects of possible sarcopenia could lead to more targeted prevention and management strategies.
Results
Study population and baseline characteristics
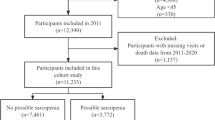
Of 17,705 participants in China Health and Retirement Longitudinal Study (CHARLS) 2011, we excluded 11,916 participants who were aged < 60 years (n = 10,036) or lacked data on possible sarcopenia (n = 1,880). Moreover, we excluded 629 participants for whom data regarding mortality were not available at follow-ups. Consequently, our final analysis included 5,160 individuals aged ≥ 60 years during CHARLS 2011 who completed follow-up until 2020 (Fig. 1). The median age of the study participants was 66 (interquartile range (IQR), 62–72) years, and 2,607 (50.5%) participants were men. The prevalence of possible sarcopenia was 48.8% (2,518 of 5,160 older adults) (Table 1). The Katz ADL score had the highest proportion of missing values at 23.3%, while missing data for other covariates ranged from 0 to 2.0%. These covariates were imputed using multiple imputation and used in subsequent analyses.
Associations of possible sarcopenia with chronic diseases
At the 9-year follow-up, in multivariable-adjusted models, possible sarcopenia was significantly associated with an increased risk of heart disease (odds ratio (OR) = 1.18, 95% confidence interval (CI): 1.03–1.34, P = 0.014) and stroke (OR = 1.41, 95% CI: 1.19–1.68, P < 0.001). These significant associations persisted across all models throughout the 0- to 9-year follow-up period (Table S1 and Table S2). The association between possible sarcopenia and chronic lung disease remained significant from baseline through the 7-year follow-up but gradually weakened and lost statistical significance by the 9-year follow-up (Table S3). In cross-sectional analyses, possible sarcopenia was significantly associated with hypertension; however, in longitudinal analyses, this association became non-significant in the adjusted models (Table S4). No significant associations were observed between possible sarcopenia and either diabetes or cancer (Table S5 and Table S6).
Associations of possible sarcopenia with physical function and hospitalization frequency
In cross-sectional analyses, possible sarcopenia was significantly associated with a lower Katz Index (β = -0.15, 95% CI: -0.19–-0.11, P < 0.001). This association persisted in longitudinal analyses at the 2-year (β = -0.13, 95% CI: -0.18–-0.09, P < 0.001), 4-year (β = -0.21, 95% CI: -0.27–-0.15, P < 0.001), 7-year follow-ups (β = -0.18, 95% CI: -0.24–-0.11, P < 0.001), and 9-year follow-ups (β = -0.17, 95% CI: -0.24–-0.10, P < 0.001) (Table 2), indicating that possible sarcopenia increases the risk of reduced functional independence in both short- and long-term periods.
Using a zero-inflated negative binomial model, after adjusting for potential confounders such as age, sex, comorbidities, and lifestyle factors, possible sarcopenia was significantly associated with an increased hospitalization frequency at baseline (Exp(β) = 1.50, 95% CI: 1.25–1.81, P < 0.001). At the 2-year follow-up, the association was marginally non-significant (Exp(β) = 1.17, 95% CI: 1.00–1.38, P = 0.057). However, at the 4-year, 7-year, and 9-year follow-ups, the association was no longer statistically significant, suggesting that other factors may play a greater role in hospitalization risk over time (Table 3).
Association between possible sarcopenia and all-cause mortality
During the 9-year follow-up period, 1,216 deaths occurred (23.6% of the cohort). In particular, among 2,518 individuals with possible sarcopenia, there were 823 deaths, whereas among 2,642 individuals without possible sarcopenia, there were 393 deaths. Regarding the comparison of the event rate per 1,000 person-years, the rates were 43.0 and 17.7 in participants with and without possible sarcopenia, respectively.
Table 4 shows that after adjusting for age, sex, body mass index (BMI), smoking, alcohol consumption, hypertension, diabetes, cancer, chronic lung diseases, heart disease, and stroke, possible sarcopenia was significantly associated with increased all-cause mortality at 2-year (hazard ratio (HR) = 1.70, 95% CI: 1.23–2.36, P = 0.001), 4-year (HR = 1.69, 95% CI: 1.37–2.08, P < 0.001), 7-year (HR = 1.68, 95% CI: 1.46–1.95, P < 0.001), and 9-year (HR = 1.79, 95% CI: 1.58–2.03, P < 0.001) follow-ups. The sensitivity analyses of our study, involving exclusion of participants with missing covariate values or those who died within 3 months of follow-up, as well as applying multiple imputation for missing muscle strength and physical performance data, yielded almost identical results (Tables S7-S9).
Subgroup analyses indicated a higher prevalence of possible sarcopenia among women (55.2%) than men (42.5%, P < 0.001), and in rural areas (50.8%) compared to urban areas (44.7%, P < 0.001), with no significant difference between obese and non-obese populations (Figure S1). Multivariable-adjusted regression models revealed a significant interaction between sex and the association of possible sarcopenia with all-cause mortality at the 7-year follow-up (interaction P = 0.0496, Figure S1C), indicating that female patients with possible sarcopenia had a higher mortality risk compared to male patients. At the 9-year follow-up, although this trend persisted, the interaction P-value was not statistically significant (interaction P = 0.1047, Figure S1D). Across all follow-up periods, obesity status and residential area did not significantly modify the association between possible sarcopenia and all-cause mortality. At the 2-year follow-up, the association between possible sarcopenia and mortality in obese patients was borderline significant (P = 0.0823, Figure S1A), while possible sarcopenia was associated with increased mortality risk in all other subgroups. At the 4-, 7-, and 9-year follow-ups, the risk of all-cause mortality significantly increased in all subgroups (HR > 1.5, P < 0.05), indicating that possible sarcopenia is associated with an elevated mortality risk in both the short and long term (Fig. 2 and Figure S1).
Associations between possible sarcopenia and risk of all-cause mortality in terms of sex, residence, and obesity. Forest plots display hazard ratios and 95% CIs for mortality during the 2-, 4-, 7-, and 9-year follow-up periods, stratified by sex, residence, and obesity. Risk estimates were adjusted for age, sex (unadjusted in subgroup analysis stratified by sex), body mass index (unadjusted in subgroup analysis stratified by obesity), smoking and alcohol consumption status, hypertension, diabetes, cancer, chronic lung diseases, heart disease, and stroke. CI, confidence interval.
In addition to possible sarcopenia, age, smoking, hypertension, diabetes, and chronic lung diseases were significantly associated with an increased risk of mortality over the 9-year follow-up period. In contrast, being female and a higher BMI were significantly associated with a decreased risk of mortality (Figure S2).
Discussion
This study investigated the association between possible sarcopenia, all-cause mortality, and various adverse health outcomes, including chronic diseases, functional decline, and hospitalization frequency, in a representative cohort of 5,160 community-dwelling older adults in China. The association between possible sarcopenia and all-cause mortality persisted across the 2-, 4-, 7 year, and 9-year follow-up periods and remained robust even after adjusting for confounders such as sex, age, BMI, smoking, alcohol consumption, and chronic diseases. Additionally, possible sarcopenia was significantly associated with a higher likelihood of having heart disease and stroke. Individuals with possible sarcopenia also faced a higher likelihood of reduced functional independence. Furthermore, participants with possible sarcopenia exhibited a higher hospitalization frequency at baseline. However, this association was no longer significant during the follow-up period.
While previous studies have demonstrated an association between sarcopenia and increased hospitalization risk within a one-year follow-up period24. Research specifically examining the relationship between possible sarcopenia and hospitalization risk remains limited. Our study addresses this gap by showing a significant association between possible sarcopenia and increased hospitalization frequency at baseline, which diminished over the follow-up period, suggesting that other factors may play a more substantial role in hospitalization risk over time. Moreover, our findings of reduced physical function in individuals with possible sarcopenia are consistent with existing literature that links possible sarcopenia to an increased risk of functional disability25. Previous studies have also reported that possible sarcopenia is associated with a higher risk of chronic diseases, including chronic lung diseases, heart disease, and stroke26,27, which supports our findings of elevated risks for these conditions in individuals with possible sarcopenia. These results highlight the broad impact of possible sarcopenia on functional decline, chronic disease risk, and hospitalization, underscoring the importance of early detection and intervention to mitigate its adverse health outcomes.
In the investigation of the relationship between possible sarcopenia and mortality risk, the association appears more pronounced and consistent in Western populations13,14,15,16,17,18,19. For example, a study from Turkey indicated that possible sarcopenia, defined by both EWGSOP1 and EWGSOP2 standards, correlates with an increased mortality risk, with HR of 4.26 and 2.58, respectively13. This association was further substantiated by research from Norway14 and Sweden15, as well as the United Kingdom17, which, utilizing the EWGSOP2 definition, demonstrated a significant link between possible sarcopenia and higher mortality rates among older adults. Moreover, studies from Brazil have indicated that possible sarcopenia is associated with a heightened risk of mortality in older adults with cancer16 and an increased risk of in-hospital mortality among hospitalized patients18. Similarly, research from Poland has shown that possible sarcopenia is linked to an increased risk of death in older COVID-19 patients19. Several factors inherent to Western lifestyles and healthcare systems may influence these findings. Western countries often exhibit lifestyles that lack sufficient physical activity and include dietary habits high in fat and sugar, which are known to accelerate muscle loss and the onset of possible sarcopenia 28,29. Furthermore, the advanced healthcare resources and comprehensive health management systems in Western countries likely facilitate the more efficient detection and reporting of health issues related to possible sarcopenia30. These combined factors contribute to the more pronounced and consistent association between possible sarcopenia and mortality risk observed in Western populations.
Contrastingly, research on the potential occurrence of sarcopenia and its risk of mortality within Asian populations is less common and yields inconsistent outcomes12,20,21,22. A multicenter cohort study in China, adhering to the AWGS2019 standards, associated possible sarcopenia with an increased risk of mortality in patients with solid tumors, presenting an HR of 1.190 12. Additionally, a study from Thailand identified possible sarcopenia as a risk factor for ten-year all-cause mortality, with an HR of 1.89 20. An 11-year follow-up study also found a significant association between possible sarcopenia and increased mortality risk, although this significance diminished after adjusting for age, sex, and other factors22. However, another study from China observed no significant relationship between possible sarcopenia and mortality among community-dwelling older adults21. Notably, their definition of possible sarcopenia still considered muscle mass, diverging from guidelines21. Our study, strictly following the AWGS 2019 criteria and focusing exclusively on declines in physical performance and muscle strength without evaluating muscle mass, found possible sarcopenia to be a significant risk factor for mortality over nine years among older adults in Chinese communities (HR: 1.79, 95% CI: 1.58–2.03). Therefore, we conclude that, based on our research and prior studies12,20,22, possible sarcopenia, as rigorously defined by the AWGS 2019, consistently correlates with an increased mortality rate.
The confluence of aging and escalating obesity rates has given rise to sarcopenic obesity31. While sarcopenic obesity is linked to higher risks of cardiovascular diseases, diabetes, and physical disabilities compared to sarcopenia alone31, our analysis indicates that the mortality risk remains similar between the two conditions, consistent with previous research31,32. Specifically, our findings show that HR for mortality were comparable between obese and non-obese participants, with HRs consistently slightly higher in the non-obese group during each follow-up. This phenomenon may be explained by the “obesity paradox”, where overweight and obese individuals have a lower mortality risk compared to their normal weight or underweight counterparts33. Our Cox regression results (Figure S2) also confirmed that a higher BMI was significantly associated with a decreased risk of mortality over the 9-year follow-up period. Studies exploring the gender-specific impacts of possible sarcopenia are limited, yet some have identified sex differences in the association between sarcopenia and mortality risk34,35,36. Nonetheless, our research shows an augmented mortality risk associated with possible sarcopenia in both men and women among the older Chinese population, aligning with findings from two Japanese studies that reported a significant link between sarcopenia and increased mortality across both sexes37,38. Additionally, our study revealed that the heightened mortality risk associated with possible sarcopenia impacts elderly individuals living in both urban and rural areas, affirming previous meta-analyses that have flagged sarcopenia as a mortality risk factor, regardless of residential setting11. These findings underscore the urgent necessity for possible sarcopenia assessment in older adults, due to its strong and consistent correlation with increased mortality risk across various demographics and environments. Advancing our understanding of these relationships and devising effective interventions are crucial for subsequent research.
Possible sarcopenia can increase the risk of all-cause mortality in older adults via various mechanisms. Our cross-sectional and longitudinal analyses revealed that individuals with possible sarcopenia were associated with an increased likelihood of chronic conditions (e.g., heart disease and stroke). These underlying health conditions have been reported to significantly contribute to an increased risk of mortality among individuals with possible sarcopenia39. Moreover, reduced muscle strength associated with possible sarcopenia can impair balance and locomotion, rendering older adults more susceptible to accidents that lead to life-threatening consequences, such as falls and fractures40. Our cross-sectional and longitudinal analyses further supported this, showing that individuals with possible sarcopenia were more likely to experience reduced functional independence. Briefly, the impact of possible sarcopenia on mortality encompasses various factors, including those affecting muscle strength and the development or progression of chronic diseases. Therefore, studies focusing on prevention and intervention strategies for possible sarcopenia must be prioritized to improve health outcomes and survival in older adults.
This study has several limitations. First, this was an observational study; thus, there was a possibility of confounding factors influencing the observed relationships. However, we addressed this limitation by employing multiple analytical strategies, including multivariate adjustment and sensitivity analysis, to mitigate the impact of confounding factors and strengthen the robustness of our findings. Second, the estimation of survival time for participants who died during the follow-up periods was dependent on the use of median survival time based on the last two follow-ups. Although this approach was necessary owing to the unavailability of exact death dates, it may have introduced some imprecision. To address these limitations and strengthen our understanding of the relationship between possible sarcopenia and health outcomes, future studies that aim to collect more comprehensive data on death records, employ a longitudinal design with larger sample sizes, and incorporate more detailed assessments of muscle strength and physical performance are warranted.
Conclusions
This study provides evidence for a significant association between possible sarcopenia and increased all-cause mortality in Chinese older adults. Further, our findings indicate possible sarcopenia is significantly associated with higher likelihoods of heart disease, stroke, and reduced functional independence. These findings highlight the importance of recognizing possible sarcopenia as an independent predictor of mortality, as well as physical and functional decline, and underscore the need for further research on prevention and management strategies.
Methods
Data sources and participants
The present analysis was conducted using nationally representative data from the CHARLS. Details about CHARLS have been reported in a previous study41. Notably, CHARLS is a nationally representative ongoing prospective cohort study that aims to collect sociodemographic and health-related data of Chinese individuals aged ≥ 45 years. It uses a multistage-stratified probability-to-scale proportional sampling method for participant selection. The primary objectives of CHARLS are as follows: health status assessment, healthcare utilization, and determination of socioeconomic characteristics of the Chinese population. The CHARLS database contains information about possible sarcopenia and survival/death, which enables to evaluate the relationship between possible sarcopenia and mortality outcomes in the Chinese population. The baseline survey, i.e., CHARLS 2011, was conducted in 2011–2012, and subsequent follow-ups were conducted in 2013, 2015, 2018, and 2020. Data were collected by one-on-one interviews, laboratory tests, and physical measurements to ensure accuracy and reliability.
In the present study, we retrospectively analyzed data of participants aged ≥ 60 years at baseline (2011–2012) and subsequent follow-ups until 2020. The exclusion criteria were as follows: age of < 60 years at baseline, insufficient data to assess possible sarcopenia at baseline, and lack of data on mortality during follow-up.
Assessment of possible sarcopenia
According to AWGS 2019 consensus, possible sarcopenia is defined by low muscle strength or decreased physical performance4. In CHARLS, muscle strength was assessed by grip strength using a mechanical dynamometer (YuejianTM WL-1000, Nantong, China), wherein participants were asked to squeeze the device as hard as they could, and testing for each hand was performed twice. The maximum value among each set of measurements was used in the study. Hand grip strengths of < 28 kg and < 18 kg in men and women were considered as low muscle strengths4. Physical performance was assessed using the 5-time chair stand test. In this test, the participants were requested to sit down and fold their arms in front of their chests. Subsequently, they were asked to stand up and sit down five times continuously as quickly as possible without pausing or moving their arms. During the test, the examiner recorded the time required to complete the test. The participants were considered to have decreased performance if they took ≥ 12 s4 or were unable to complete the test.
Assessment of mortality
In CHARLS, death records were obtained using exit interviews conducted in 2013, 2015, 2018, and 2020. These interviews were primarily answered by family members or close contacts to ensure the accuracy of the information. Although interview dates were available for all three follow-ups, the exact dates of death were only available from the 2013 follow-up and a portion of the 2020 follow-up questionnaires. Due to a large number of unknown or uncertain causes of death, we used all-cause mortality as the study endpoint to ensure the reliability of our results. For surviving participants, the survival time was defined as the duration from the baseline assessment to the most recent follow-up interview. In the case of death, survival time was calculated as the interval between the baseline interview date and the date of participant’s death or, when unavailable, as the median time between the last two surveys42.
Subgroup analyses
Both obesity and sarcopenia are associated with adverse health outcomes, and the combination of obesity and sarcopenia, known as sarcopenic obesity, may have a synergistic effect, amplifying their threat to health31. This makes it crucial to examine how obesity status interacts with possible sarcopenia to affect mortality risk. Differences in residential area can affect access to healthcare, nutritional status, and levels of physical activity, all of which are critical factors for muscle health43. Sex differences play a significant role in muscle strength and physical activity levels, affecting the prevalence and impact of sarcopenia44, and thereby influencing mortality risk. Consequently, subgroup analyses were conducted based on sex (male or female), residential area (urban or rural), and obesity status.
Assessment of functional ability and hospitalization
We assessed functional ability using the Modified Katz ADL score, which evaluates participants’ capacity to perform basic daily activities across six domains: feeding, bathing, dressing, toileting, transferring, and continence (Table: Modified Katz Activities of Daily Living (ADL) Scale-MSD Manual Professional Edition (msdmanuals.com))45. For each domain, participants were scored based on their ability to perform the activity independently. If the participant could perform the activity without assistance, it was scored as 1 point. If the participant required help or was unable to perform the activity, it was scored as 0 points. The total score ranges from 0 to 6, with higher scores indicating greater independence in ADL. Hospitalization frequency within the past year can serve as an indicator of healthcare utilization46. Using data from the CHARLS study, the corresponding questions in the questionnaire are: (1) “Have you received inpatient care in the past year?” (2)“How many times have you received inpatient care during the past year?” Modified Katz ADL score and Hospitalization frequency data were collected and analyzed from the baseline, 2013, 2015, 2018, and 2020 CHARLS waves.
Covariates
The covariates adjusted in the statistical analysis, which were previously reported as risk factors for mortality47,48were obtained from baseline questionnaires, laboratory tests, and physical measurements, including age, sex, smoking and alcohol consumption status, BMI, diabetes, hypertension, heart disease, stroke, cancer, and chronic respiratory disease. Additionally, these covariates have also been reported to be associated with the risk of sarcopenia49,50. The participants were considered to have hypertension if (1) their systolic blood pressure was ≥ 140 mmHg or diastolic blood pressure was ≥ 90 mmHg, (2) they were currently receiving antihypertensive medications, or (3) they had previously been diagnosed by a physician. They were considered to have diabetes if (1) their fasting plasma glucose level was ≥ 126 mg/dL, random plasma glucose level was ≥ 200 mg/dL, and HbA1c level was ≥ 6.5%51; (2) they had previously been diagnosed by a physician; or (3) they were currently receiving glucose-lowering treatment. Furthermore, the presence of other comorbidities was determined based on a previous diagnosis or the current use of relevant medications. Heart disease included heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems. Chronic lung disease encompassed chronic bronchitis and emphysema (excluding tumors or cancer). According to the 2022 consensus by the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO), BMI and waist circumference are utilized as initial screening tools for identifying individuals at risk of sarcopenic obesity52. Based on this, in our study, obesity was defined as a BMI of ≥ 28 kg/m2 or waist circumference of ≥ 85 cm and ≥ 90 cm for women and men, respectively53.
Statistical analyses
In the present study, continuous variables were expressed as medians and IQRs if they did not conform to a normal distribution according to the Kolmogorov–Smirnov test. Conversely, categorical variables were expressed as frequencies and percentages. The participants were grouped according to the possible sarcopenia status to describe baseline characteristics. We conducted cross-sectional analyses using baseline data. Logistic regression was employed to analyze the relationship between possible sarcopenia and chronic diseases, including hypertension, diabetes, cancer, chronic lung diseases, heart disease, and stroke. The results are presented as ORs with 95% CIs. The association between possible sarcopenia and ADL scores was analyzed using multivariable linear regression models54,55,56, with results presented as regression coefficients (β) and their 95% CIs. To investigate the associations of possible sarcopenia with hospitalization frequency, a zero-inflated negative binomial model was used57. The results are presented as exponentiated coefficients (Exp(β)) with 95% CIs. The primary focus of our study was longitudinal analyses to investigate the association between possible sarcopenia and mortality at three different time points: 2013 (2-year follow-up), 2015 (4-year follow-up), 2018 (7-year follow-up), and 2020 (9-year follow-up). To investigate the association, we calculated the HRs and corresponding 95%CIs using Cox proportional risk models. Further, subgroup analyses were performed in terms of sex (male or female), obesity status (obese or nonobese), and residential location (urban or rural).
To adjust for potential confounding factors, we used three models in our study. Model 1 was adjusted for age and sex, whereas model 2 included BMI and smoking and alcohol consumption status in addition to the variables adjusted in model (1) Further, model 3 was adjusted for hypertension, diabetes, cancer, chronic lung disease, heart disease, and stroke based on model (2) Multiple imputation methods were applied to impute the missing data58. Moreover, we conducted sensitivity analyses to assess the robustness of the results. First, participants with missing values for covariates were excluded, and a complete case analysis was performed. Second, the participants who died within 3 months of follow-up were excluded to minimize potential reverse causality due to severe disease. Third, we addressed baseline exclusions due to missing muscle strength and physical performance data by applying multiple imputation and reassessing the association between possible sarcopenia and mortality.
In this study, all statistical analyses were conducted using R software (version 4.2.1, R Foundation for Statistical Computing). Statistical significance was defined as P < 0.05 for all cases.
Data availability
The datasets utilized in this study are accessible through the open CHARLS databases, which can be found at https://charls.charlsdata.com.
References
Cruz-Jentoft, A. J., Sayer, A. A. & Sarcopenia Lancet (London England) 393, 2636–2646. https://doi.org/10.1016/s0140-6736(19)31138-9 (2019).
Fielding, R. A. et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on Sarcopenia. J. Am. Med. Dir. Assoc. 12, 249–256. https://doi.org/10.1016/j.jamda.2011.01.003 (2011).
Cruz-Jentoft, A. J. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 48, 16–31. https://doi.org/10.1093/ageing/afy169 (2019).
Chen, L. K. et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 21, 300–307e302. https://doi.org/10.1016/j.jamda.2019.12.012 (2020).
Zanker, J. et al. Consensus guidelines for Sarcopenia prevention, diagnosis and management in Australia and New Zealand. J. Cachexia Sarcopenia Muscle. 14, 142–156. https://doi.org/10.1002/jcsm.13115 (2023).
Shu, X. et al. Diagnosis, prevalence, and mortality of Sarcopenia in dialysis patients: a systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle. 13, 145–158. https://doi.org/10.1002/jcsm.12890 (2022).
Tantai, X. et al. Effect of Sarcopenia on survival in patients with cirrhosis: a meta-analysis. J. Hepatol. 76, 588–599. https://doi.org/10.1016/j.jhep.2021.11.006 (2022).
Westbury, L. D. et al. Recent sarcopenia definitions-prevalence, agreement and mortality associations among men: findings from population-based cohorts. J. Cachexia Sarcopenia Muscle. 14, 565–575. https://doi.org/10.1002/jcsm.13160 (2023).
Liu, P. et al. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas. 103, 16–22. https://doi.org/10.1016/j.maturitas.2017.04.007 (2017).
Zhang, X. M. et al. Sarcopenia as a predictor of mortality among the critically ill in an intensive care unit: a systematic review and meta-analysis. BMC Geriatr. 21, 339. https://doi.org/10.1186/s12877-021-02276-w (2021).
Xu, J., Wan, C. S., Ktoris, K., Reijnierse, E. M. & Maier, A. B. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology. 68, 361–376. https://doi.org/10.1159/000517099 (2022).
Yin, L. et al. Association of possible sarcopenia with all-cause mortality in patients with solid cancer: a nationwide multicenter cohort study. J. Nutr. Health Aging. 28, 100023. https://doi.org/10.1016/j.jnha.2023.100023 (2024).
Altinkaynak, M. et al. Associations of EWGSOP1 and EWGSOP2 probable sarcopenia definitions with mortality: a comparative study. Clin. Nutr. 42, 2151–2158. https://doi.org/10.1016/j.clnu.2023.09.019 (2023).
Johansson, J., Grimsgaard, S., Strand, B. H., Sayer, A. A. & Cooper, R. Comparing associations of handgrip strength and chair stand performance with all-cause mortality-implications for defining probable Sarcopenia: the Tromsø Study 2015–2020. BMC Med. 21, 451. https://doi.org/10.1186/s12916-023-03172-3 (2023).
Sobestiansky, S., Michaelsson, K. & Cederholm, T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85–89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr. 19, 318. https://doi.org/10.1186/s12877-019-1338-1 (2019).
Sousa, I. M. & Fayh, A. P. T. Is the ECOG-PS similar to the Sarcopenia status for predicting mortality in older adults with cancer? A prospective cohort study. Support Care Cancer. 31, 370. https://doi.org/10.1007/s00520-023-07845-w (2023).
Spexoto, M. C. B. et al. European Working Group on Sarcopenia in older people 2010 (EWGSOP1) and 2019 (EWGSOP2) criteria or slowness: which is the best predictor of mortality risk in older adults? Age Ageing. 51 https://doi.org/10.1093/ageing/afac164 (2022).
Sousa, I. M., Burgel, C. F., Silva, F. M. & Fayh, A. P. T. Prognostic value of isolated Sarcopenia or Malnutrition-Sarcopenia syndrome for clinical outcomes in hospitalized patients. Nutrients. 14 https://doi.org/10.3390/nu14112207 (2022).
Piotrowicz, K. et al. Factors associated with mortality in hospitalised, non-severe, older COVID-19 patients - the role of Sarcopenia and frailty assessment. BMC Geriatr. 22, 941. https://doi.org/10.1186/s12877-022-03571-w (2022).
Chalermsri, C., Aekplakorn, W. & Srinonprasert, V. Body mass index combined with possible Sarcopenia status is better than BMI or possible Sarcopenia status alone for predicting all-cause mortality among Asian community-dwelling older adults. Front. Nutr. 9, 881121. https://doi.org/10.3389/fnut.2022.881121 (2022).
Xiong, L. et al. The relationship between Sarcopenia and mortality in Chinese community-dwelling adults: a 7-year cohort study with propensity score matching and mendelian randomization. Front. Endocrinol. 14, 1215512. https://doi.org/10.3389/fendo.2023.1215512 (2023).
Lee, W. J., Peng, L. N., Lin, M. H., Loh, C. H. & Chen, L. K. Letter to the editor: disentangling mortality associations: an in-depth comparative study of possible Sarcopenia versus Sarcopenia of AWGS 2019. J. Nutr. Health Aging. 27, 685–686. https://doi.org/10.1007/s12603-023-1953-6 (2023).
Jang, J. Y., Kim, D. & Kim, N. D. Pathogenesis Intervention, and current status of Drug Development for Sarcopenia: a review. Biomedicines. 11 https://doi.org/10.3390/biomedicines11061635 (2023).
Yang, M., Liu, Y., Zuo, Y. & Tang, H. Sarcopenia for predicting falls and hospitalization in community-dwelling older adults: EWGSOP versus EWGSOP2. Sci. Rep. 9, 17636. https://doi.org/10.1038/s41598-019-53522-6 (2019).
Zhou, H., Ding, X. & Luo, M. The association between Sarcopenia and functional disability in older adults. J. Nutr. Health Aging. 28, 100016. https://doi.org/10.1016/j.jnha.2023.100016 (2024).
Wang, H., Qiu, H., Gu, X., Zhang, Y. & Wang, S. The association between Sarcopenia and incident chronic lung disease in the general population: a longitudinal study based on CHARLS data. Exp. Gerontol. 180, 112257. https://doi.org/10.1016/j.exger.2023.112257 (2023).
Gao, K. et al. Association between Sarcopenia and cardiovascular disease among middle-aged and older adults: findings from the China health and retirement longitudinal study. EClinicalMedicine. 44, 101264. https://doi.org/10.1016/j.eclinm.2021.101264 (2022).
Papadopoulou, S. K. et al. Exercise and nutrition impact on osteoporosis and Sarcopenia-the incidence of Osteosarcopenia: a narrative review. Nutrients. 13 https://doi.org/10.3390/nu13124499 (2021).
Clemente-Suárez, V. J., Beltrán-Velasco, A. I., Redondo-Flórez, L., Martín-Rodríguez, A. & Tornero-Aguilera, J. F. Global impacts of western diet and its effects on metabolism and health. Narrative Rev. Nutrients. 15 https://doi.org/10.3390/nu15122749 (2023).
Tagliafico, A. S., Bignotti, B., Torri, L. & Rossi, F. Sarcopenia: how to measure, when and why. Radiol. Med. 127, 228–237. https://doi.org/10.1007/s11547-022-01450-3 (2022).
Wannamethee, S. G. & Atkins, J. L. Sarcopenic obesity and cardiometabolic health and mortality in older adults: a growing health concern in an ageing population. Curr. Diab. Rep. 23, 307–314. https://doi.org/10.1007/s11892-023-01522-2 (2023).
Atmis, V. et al. The relationship between all-cause mortality Sarcopenia and sarcopenic obesity among hospitalized older people. Aging Clin. Exp. Res. 31, 1563–1572. https://doi.org/10.1007/s40520-019-01277-5 (2019).
Dramé, M. & Godaert, L. The obesity paradox and mortality in older adults: a systematic review. Nutrients. 15 https://doi.org/10.3390/nu15071780 (2023).
Yuki, A., Ando, F., Otsuka, R. & Shimokata, H. Sarcopenia based on the Asian Working Group for Sarcopenia criteria and all-cause mortality risk in older Japanese adults. Geriatr. Gerontol. Int. 17, 1642–1647. https://doi.org/10.1111/ggi.12946 (2017).
Kim, J. H. et al. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. J. Gerontol. A. 69, 1244–1252. https://doi.org/10.1093/gerona/glu050 (2014).
Batsis, J. A., Mackenzie, T. A., Barre, L. K., Lopez-Jimenez, F. & Bartels, S. J. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 68, 1001–1007. https://doi.org/10.1038/ejcn.2014.117 (2014).
Kitamura, A. et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J. Cachexia Sarcopenia Muscle. 12, 30–38. https://doi.org/10.1002/jcsm.12651 (2021).
Nakamura, K. et al. Prevalence and mortality of Sarcopenia in a community-dwelling older Japanese Population: the Hisayama Study. J. Epidemiol. 31, 320–327. https://doi.org/10.2188/jea.JE20190289 (2021).
Zhou, M. et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet (London England). 394, 1145–1158. https://doi.org/10.1016/s0140-6736(19)30427-1 (2019).
Luo, C., Liu, R., Shen, X., Zhang, G. & Liu, B. Possible sarcopenia and risk of hip fracture in older adults in China. Arch. Gerontol. Geriatr. 117, 105248. https://doi.org/10.1016/j.archger.2023.105248 (2024).
Zhao, Y., Hu, Y., Smith, J. P., Strauss, J. & Yang, G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 43, 61–68. https://doi.org/10.1093/ije/dys203 (2014).
Hu, W. H. et al. Developing and validating a Chinese multimorbidity-weighted index for middle-aged and older community-dwelling individuals. Age Ageing. 51 https://doi.org/10.1093/ageing/afab274 (2022).
Li, X., Wang, R., Hou, Z. & Sun, Q. Urban-rural differences in the prevalence and associated factors of Sarcopenia: a systematic review and meta-analysis. Arch. Gerontol. Geriatr. 122, 105390. https://doi.org/10.1016/j.archger.2024.105390 (2024).
Lee, D. Y. Sex-specific Sarcopenia prevalence and risk factors in the Korean Population: a cross-sectional epidemiological study. Med. (Kaunas Lithuania). 60 https://doi.org/10.3390/medicina60060899 (2024).
White, D. K., Wilson, J. C. & Keysor, J. J. Measures of adult general functional status: SF-36 physical functioning subscale (PF-10), Health Assessment Questionnaire (HAQ), Modified Health Assessment Questionnaire (MHAQ), Katz Index of Independence in activities of daily living, functional independence measure (FIM), and osteoarthritis-function-computer adaptive test (OA-Function-CAT). Arthritis Care Res. (Hoboken). 63 (Suppl 11), 297–307. https://doi.org/10.1002/acr.20638 (2011).
Arueira Chaves, L., de Santos Serio Dos Santos, D.M., Rodrigues Campos, M. & Luiza, V. L. Use of health outcome and health service utilization indicators as an outcome of access to medicines in Brazil: perspectives from a literature review. Public. Health Rev. 40, 5. https://doi.org/10.1186/s40985-019-0115-1 (2019).
World Health Organization. (2009). Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization. https://apps.who.int/iris/handle/10665/44203
World Health Organization. World health statistics 2022: monitoring health for the SDGs, sustainable development goals. https://www.who.int/publications/i/item/9789240051157
Gao, Q. et al. Associated factors of Sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. Nutrients. 13 https://doi.org/10.3390/nu13124291 (2021).
Lavalle, S., Valerio, M. R., Masiello, E., Gebbia, V. & Scandurra, G. Unveiling the intricate dance: how cancer orchestrates muscle wasting and Sarcopenia. vivo (Athens Greece). 38, 1520–1529. https://doi.org/10.21873/invivo.13602 (2024).
2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes care 44, S15-s33. https://doi.org/10.2337/dc21-S002 (2021).
Donini, L. M. et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO Consensus Statement. Obes. Facts. 15, 321–335. https://doi.org/10.1159/000521241 (2022).
[Expert Consensus on Obesity Prevention and Treatment in China]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 43, 609–626. https://doi.org/10.3760/cma.j.cn112338-20220402-00253 (2022).
van Dalen-Kok, A. H. et al. The impact of pain on the course of ADL functioning in patients with dementia. Age Ageing. 50, 906–913. https://doi.org/10.1093/ageing/afaa247 (2021).
Ohtsubo, T., Nozoe, M., Kanai, M., Ueno, K. & Nakayama, M. Association of objectively measured physical activity with physical function in patients with Sarcopenia during Hospitalized Rehabilitation. Nutrients. 14 https://doi.org/10.3390/nu14204439 (2022).
Yagi, T. et al. Sarcopenia affects activities of daily living recovery and hospitalization costs in older adults in convalescent rehabilitation wards. Eur. Geriatr. Med. 12, 1237–1245. https://doi.org/10.1007/s41999-021-00552-x (2021).
Soh, J. G. S., Mukhopadhyay, A., Mohankumar, B., Quek, S. C. & Tai, B. C. Predictors of frequency of 1-year readmission in adult patients with diabetes. Sci. Rep. 13, 22389. https://doi.org/10.1038/s41598-023-47339-7 (2023).
Cummings, P. Missing data and multiple imputation. JAMA Pediatr. 167, 656–661. https://doi.org/10.1001/jamapediatrics.2013.1329 (2013).
Acknowledgements
The data used in this study was obtained from the CHARLS. We would like to express our gratitude to the CHARLS research team, the field team, and all the participants who have dedicated their time and efforts to the CHARLS project.
Funding
This study received support from the Zhejiang Provincial Medical and Health Science and Technology Project [grant number 2023KY1032] and the Natural Science Foundation of Ningbo [grant number 2023J229]. The funders had no role in the study design, data collection, analysis, interpretation, or manuscript preparation and submission.
Author information
Authors and Affiliations
Contributions
BYL and CL conceived the protocol. BYL, RYL, HYJ and CL contributed to the collection, analysis and interpretation of data. BYL and RYL grafted the manuscript. CL, YHJ and YD critically revised the manuscript. All authors take full responsibility for the integrity and accuracy of the work, and have read and approved the final manuscript. The corresponding author had complete access to all the data in the study and made the final decision to submit the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The data collection protocol for CHARLS was approved by the Ethics Review Committee of Peking University (Approval Number: IRB00001052-11015). All study procedures involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committees as well as the 1964 Helsinki Declaration and its subsequent amendments or similar ethical standards. Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, B., Liu, R., Jin, Y. et al. Association between possible sarcopenia, all-cause mortality, and adverse health outcomes in community-dwelling older adults in China. Sci Rep 14, 25913 (2024). https://doi.org/10.1038/s41598-024-77725-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77725-8
Keywords
This article is cited by
-
The quality of life and related factors in older adults with possible sarcopenia and sarcopenia in rural areas of Xinjiang, China: a cross sectional study
BMC Geriatrics (2025)
-
Occurrence of sarcopenia in elderly patients with coronary heart disease and its association with short-term prognosis
BMC Cardiovascular Disorders (2025)
-
Sleep quality and possible sarcopenia in community-dwelling older adults: physical and mental fatigability as mediators
BMC Geriatrics (2025)
-
The relationship between estimated glucose disposal rate and sarcopenia among middle-aged and older adults
Scientific Reports (2025)
-
Food as medicine: white and whole-grain bread consumption in relation to sarcopenia among older adults, insights from the Birjand Longitudinal Aging Study (BLAS)
Aging Clinical and Experimental Research (2025)