Abstract
In recent years, the glymphatic system has received increasing attention due to its possible implications in biological mechanisms associated with neurodegeneration. In the field of human brain mapping, this led to the development of diffusion tensor image analysis along the perivascular space (DTI-ALPS) index. While this index has been repeatedly used to investigate possible differences between neurodegenerative disorders and healthy controls, a comprehensive evaluation of its stability across multiple measurements and different disorders is still missing. In this study, we perform a Bayesian meta-analysis aiming to assess the consistency of the DTI-ALPS results previously reported for 12 studies on Parkinson’s disease and 11 studies on Alzheimer’s disease. We also evaluated if the measured value of the DTI-ALPS index can quantitatively inform the diagnostic process, allowing disambiguation between these two disorders. Our results, expressed in terms of Bayes’ Factor values, confirmed that the DTI-ALPS index is consistent in measuring the different functioning of the glymphatic system between healthy subjects and patients for both Parkinson’s disease (Log10(BF10) = 30) and Alzheimer’s disease (Log10(BF10) = 10). Moreover, we showed that the DTI-ALPS can be used to compare these two disorders directly, therefore providing a first proof of concept supporting the reliability of taking into consideration this neuroimaging measurement in the diagnostic process. Our study underscores the potential of the DTI-ALPS index in advancing our understanding of neurodegenerative pathologies and enhancing clinical diagnostics.
Similar content being viewed by others
Introduction
The glymphatic system is a drainage apparatus of the brain1. In particular, it is thought to contribute to the elimination of soluble proteins and metabolites and to the distribution of glucose, lipids, amino acids, and neuromodulators2. Its mechanism of functioning is based on the movement of cerebrospinal fluid into the perivascular space3.
The activity of the glymphatic system has been classically studied through the administration of tracers, therefore limiting the investigation to animal models. In recent years, the brain imaging community has focused on developing MRI methods to study the glymphatic system in humans. Researchers have specifically adapted diffusion tensor imaging (DTI), originally designed to track water molecules along axons, to study liquid flow in this system. This led to the consolidation of a non-invasive method called diffusion tensor image analysis along the perivascular space (DTI-ALPS)4. This technique produces a quantitative index by measuring diffusion in three directions (i.e., right-left, front-back, and top-bottom) within three spherical regions of interest (ROIs) located at the lateral ventricle body. This mapping distinguishes the contributions of projection, association, and subcortical fibers, which are orthogonal in this anatomical region. The diffusion values in these three directions within the ROIs are then combined arithmetically to quantify diffusion along the direction of the perivascular space, where glymphatic flow is believed to occur. Since its formulation seven years ago4, the DTI-ALPS technique has been used to characterize the glymphatic system in a variety of neurodegenerative conditions, including Alzheimer’s disease (AD), amyotrophic lateral sclerosis, idiopathic normal pressure hydrocephalus, multiple sclerosis, and Parkinson’s disease (PD)4,5,6,7,8,9. Different independent efforts have reported significant between-group differences of the index values computed on clinical subjects and healthy controls10. However, a comprehensive evaluation of the DTI-ALPS index stability across multiple measurements, as well as between different neurological conditions, has not been realized yet.
In this context, the present meta-analysis had two main objectives: first, to evaluate the consistency of the DTI-ALPS values reported across independent studies investigating the same neurodegenerative disorder. Second, to determine whether the DTI-ALPS index alterations are more strongly associated with a specific clinical condition, thereby supporting its potential inclusion in the diagnostic process. The focus of the present work was on PD and AD. These two disorders share a pathophysiological mechanism involving protein deposition, which leads to neurodegeneration11. In the case of PD, protein aggregates may accumulate at the intracellular level, leading to the formation of alpha-synuclein aggregates called Lewy Bodies. In AD, protein sedimentation occurs either at an intracellular level, as with tau neurofibrillary tangles, or at the extracellular level, as with amyloid plaque formation. In both scenarios, the glymphatic system is thought to aid in the removal of these toxic aggregates through its clearance function. This makes studying its role in Alzheimer’s disease (AD) and Parkinson’s disease (PD) particularly important.
Materials and methods
Selection of studies
The MEDLINE database was searched to identify all possible experiments performing DTI-ALPS on patients affected by either AD or PD confronting them with healthy controls. The search engine PubMed was used, with the search algorithm consisting of: (DTI-ALPS) AND ((Parkinson) OR (Alzheimer)). Moreover, Google Scholar was searched to find articles that could have been missed during the MEDLINE inspection. At the time of the selection phase (January 2024), 104 full-text articles were initially identified by combining the two databases, and screened for eligibility based on the following criteria: (a) articles had to be published in peer-reviewed English-language journals; (b) articles had to measure DTI-ALPS in patients afflicted by either AD or PD and healthy controls. In particular, with respect to criterion b, articles had to report for each experiment the DTI-ALPS index (measured bilaterally and/or on the left and right sides –Alps L and Alps R, respectively) for the clinical group and the control group, their standard deviations, and the number of clinical and healthy subjects.
Bayes factor in the meta-analytic framework
Most of the past and current clinical research, including meta-analytic approaches, applies the classic hypothesis testing that pertains to frequentist statistics. However, in recent years increasing attention has been gained by the use of alternative solutions taken from Bayesian statistics12,13,14. The main advantage of this approach is the shift from the minimization of the probability of obtaining an erroneous result (i.e., the concept behind p-value) to the quantification of the amount of evidence provided by the data in support of the research hypotheses. This has its technical roots in the Bayes’ theorem:
Where M is the model being evaluated and D are the collected data. The left term of Bayes’ theorem, called “posterior probability”, expresses that the aim is to find the probability of what is hypothesized (M) given that some values had been observed (D). The data-dependent term \(P\left(D|M\right)\)appearing on the right, called “likelihood”, represents the probability of observing those values (D) under the assumption of the validity of what is hypothesized (M). The likelihood is indeed the focal point of the Bayesian comparison between different models (or hypotheses), which can be implemented through the computation of the Bayes’ Factor (BF), which is essentially a ratio between the competing models (i.e., M1 and M2):
As it can be noted, D remains unchanged for both models, taking the role of a normalization constant. In other words, it is the partition function of the inference on the model parameters M once obtained the data D. In the formula above, \({\theta}_{1}\) and \({\theta}_{2}\) allow therefore to parametrize the plausibility of the competing models M1 and M2 considering the observed data D.
This method had been previously used to run secondary analyses of already published literature using their summary statistics rather than the original raw data15,16,17. However, this approach was so far applied to one experiment at a time. In the present work, the aim is instead to analyze multiple research together, in a meta-analytic framework. It could be thought that a way to address this is to take the posterior probabilities of the first experiment as the prior of the second one, and so on, and then simply compute the product of BF. However, this method would not be correct18. To accurately extend the BF to the meta-analytic framework the t-statistics reported as the result in each paper must be thought as the data, while the effect size is modeled as the parameter of interest, δ. In this case, the resulting BF for a single experiment is
and the generalization to M-independent experiments is given by
where Π indicates the product of terms. The important property of this approach is that while it assumes that the true effect size is constant in each experiment, it does not so for a common variance. Therefore, it is applicable to experiments where the unit of measurement varies among them. The computation of the meta-analytic Bayes factor was here completed using the Bayes Factor package for R software (https://cran.r-project.org/package=BayesFactor).
Disorder discrimination
The next step is to consider whether a difference in DTI-ALPS index values between the two diseases, as indicated by the previous analysis, would provide informative results. To address this, a Bayesian analysis was conducted, following the “heuristic of space to move” as proposed by Dienes16. According to this heuristic, if a theory predicts that A is smaller than B, then if B is greater than the case by a quantity r, then A must lie between the case and r, where r represents the space to move.
In the present work, the application of this heuristic is applied as follows: the meta-analysis computes the DTI-ALPS values for the two diseases along with their respective standard deviations. The means and standard deviations are obtained through the samples from the posterior distribution of the DTI-ALPS values obtained in the meta-analysis, using the Bayes Factor package. A new BF is then computed by considering the case in which the DTI-ALPS value of AD is greater than that of PD. The direction of this comparison was determined based on the obtained meta-analytic results, though the reverse would apply if the outcome were the opposite. The difference between DTI-ALPS values for the two diseases is computed, and a semi-Gaussian distribution is used to model the alternative hypothesis H1, since the case of the AD value being greater than the PD value is considered. This distribution is centered on zero, with a standard deviation equal to half the mean DTI-ALPS for AD. This setup implies that the maximum plausible effect size is twice the standard deviation.
Results
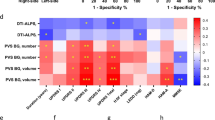
Based on the selection criteria, 23 articles were included in this meta-analysis, amounting to a total of 27 independent experiments: 13 for AD (Table 1) and14 for PD (Table 2), respectively. PRISMA flow chart is reported in Fig. 1. For each selected experiment, a t-value was computed starting from the extracted information. The results of the meta-analytic BF, computed either for the whole-brain, or separately for the left and right hemispheres, are provided in Table 3 for Alzheimer’s disease and in Table 4 for Parkinson’s disease, respectively.
The strength of the evidence, that here was measured in terms of Ban (i.e., the logarithm of the Bayes factor)40 was found to be considerable for AD and strong for PD41. Notably, a marked lateralization emerged for PD, but not for AD. While the supporting evidence was stronger for PD, the median effect size, i.e. the difference between the DTI-ALPS values measured in the healthy or clinical samples, was slightly greater in AD. Finally, the direct comparison between the two disorders returned evidence of \(\text{B}{\text{F}}_{12}=3.86\).
Discussion
Since its development4, the DTI-ALPS has been used to investigate the functionality of the glymphatic system in a variety of neurodegenerative conditions. While independent peer-reviewed studies reported a significant difference between the DTI-ALPS values measured in healthy subjects and patients with PD or AD, this is the first attempt to quantitatively assess the consistency of those multiple results.
Based on the meta-analytic Bayesian analysis of 27 experiments, our results first suggested that there is enough strength of evidence to support the thesis that the DTI-ALPS can indeed capture variations in the glymphatic system associated with AD (compared against healthy controls) or PD (compared against healthy controls). In particular, the results of the BF showed that it is 30 times more likely to observe an altered level of functioning of the glymphatic system in PD patients than in healthy controls. In the domain of Bayesian statistics, a BF value of 30 is regarded as strong evidence. Positive evidence was found for AD as well, although slightly less strong (BF = 10). It should be noted that the observed difference is not due to an imbalanced representation of PD and AD experiments, being indeed 14 and 13, respectively. It is also worth noting that the median effect showed an opposite pattern, indicating that the delta in the DTI-ALPS index measured for healthy subjects and clinical subjects is actually wider for AD than PD. This ability to separate the effect size from the strength of the evidence directly results from using the Bayesian framework. Moreover, unlike frequentist statistics, the Bayesian approach offers the advantage of being unaffected by sample size, providing reliable results even with small datasets14. This analytic method also enabled us to assess whether the DTI-ALPS index can effectively distinguish between the two disorders, even without direct primary comparative experiments. The evidence of glymphatic system impairment was found to differ between AD and PD, suggesting that the DTI-ALPS index may serve as a potential discriminator between the two disorders.
From the pathophysiological point of view, previous studies have demonstrated that glymphatic dysfunction influences the accumulation of pathological protein aggregates both intracellular and extracellular, triggering neuroinflammation42,43. In AD, neurodegeneration is caused by toxic aggregation of extracellular amyloid plaques and intracellular neurofibrillary tangles of hyperphosphorylated tau protein44, while PD neuropathology is characterized by the accumulation of misfolded a-synuclein in intracytoplasmic inclusions called Lewy bodies45. AD pathologies (i.e., beta amyloid and tau) may act synergistically with a-synuclein pathology to confer a worse prognosis in terms of cognitive and executive impairment in patients with PD46. It is also possible to detect a-synuclein in patients with a biological diagnosis of AD, and a-synuclein was also included as a biomarker of non-AD copathology in the latest criteria for diagnosis of AD47.
The glymphatic system is a key component of the central nervous system’s solutes clearance and water transport network, and its impairment creates a facilitatory environment for waste product accumulation, with relevant implications in proteinopathies48. Proposed mechanisms for the impairment of brain fluid homeostasis mediated by glymphatic drainage include dilated or increased perivascular spaces and blood-brain barrier disruption. Another key factor is the impairment of aquaporin-4 (AQP-4) water channels, which mediate the flow from periarterial spaces to the brain parenchyma48. The role of AQP4 in neurodegeneration is a potential link between glymphatic disorders and neuroinflammation, triggered by the accumulation of toxic protein aggregates. Studies in an animal model of AD have shown that in APP/PS1 transgenic mice, AQP4 deletion exacerbates cognitive deficits and is associated with an increase in beta-amyloid accumulation49. Another study in an animal model of PD showed that the impairment of the glymphatic system through the deletion of the AQP4 gene reduced the clearance of injected alpha-synuclein from the brain5. It is interesting to note that the glymphatic system appears to interact with various mechanisms involved in neurodegeneration, in general, and not in a disease-specific way. In particular, neuroinflammation via AQP-4 impact renders AQP4 a promising target for further research and a potential avenue for therapeutic interventions in both AD and PD.
An additional aspect worth considering is the speculation that sleep deprivation and circadian rhythm disruptions may be associated with increased accumulation of beta-amyloid, tau and alpha-synuclein levels in humans. Sleep disturbances are widely prevalent both in AD and PD50. In AD, they tend to co-occur with other cognitive and behavioral symptoms from the very early stages of the disease51, and it was observed that shorter sleep duration and poor sleep quality were associated with increased beta-amyloid accumulation52. Among alpha-synucleinopathies, REM-sleep behavior disorder (RBD) is recognized as one of the most promising markers of prodromal PD53. A large prospective multicenter trial found that the overall conversion rate from idiopathic RBD to an overt neurodegenerative syndrome was 6.3% per year, with 73.5% of subjects with idiopathic RBD converting after 12-year follow-up54. Research in mice indicates that interstitial clearance is most effective during REM sleep. Specifically, an increase in water diffusivity during sleep was positively correlated with the REM sleep stage and negatively correlated with the non-REM sleep stage55. It has also been shown that DTI-ALPS values in RBD demonstrated a more severe reduction of glymphatic activity in individuals with phenoconversion to alpha synucleinopathies39. The suggested greater impairment of the glymphatic system in PD could thus be traced back to a time effect, with a prolonged duration of sleep-associated disorder39. However, it should also be noted that PD is characterized by a greater involvement of deep brain regions (i.e., basal ganglia) that are close to the ROI localization used for DTI-ALPS calculation56.
In AD, both beta-amyloid and tau accumulation predominantly affect cortical areas far from the ROIs, with a progressive and hierarchical spreading. Beta-amyloid spreads from the frontotemporal cortices to neocortical areas and medial temporal structures, while tau spreads from the transentorhinal and entorhinal cortices to adjacent medial temporal structures, and subsequently to more distant association and primary cortices57. PD begins as a synucleinopathy in non-dopaminergic structures of the lower brainstem or in the olfactory bulb, with alpha-synuclein deposition progressing rostrally to affect the substantia nigra, then the basal ganglia, the amygdala, the thalamus, and reaching the neocortex only in the latest stages of the disease58.
The sensitivity of the DTI-ALPS method to microstructural changes may be influenced by proximity, particularly regarding neurodegenerative processes in the basal ganglia and thalamus. In a previous study, Chung et al59. showed that a high number of enlarged perivascular spaces (another imaging marker of glymphatic dysfunction) in the basal ganglia was associated with more severely decreased striatal dopamine transporter availability.
Methodological considerations and future directions
The current findings should be interpreted considering several limitations, primarily arising from the meta-level approach used. First, we observe a non-negligible between- and within-experiment heterogeneity of some sociodemographic, clinical, and methodological variables (i.e., age at scan session, sample size, sex distribution, DTI diffusion/encoding directions, and MMSE scores). Inevitably, this issue may contribute to the variability of the published results meta-analyzed and makes it challenging to discriminate possible differences related to specific subpopulations. At the same time, it is important to note that the meta-analytic approach offers significant advantages in this context. In fact, in conjunction with the substantial statistical power provided by the quantitative synthesis60,61, the neuroimaging meta-level approach tends to afford more robust and reliable results in terms of generalization for the population of interest62,63. Second, although systematic search criteria were employed to query the literature, it is possible that some relevant studies may have been missed. Additionally, meta-analytic findings could be influenced by file-drawer problems or publication bias, a primary literature bias relating to null or contra evidence results that are unpublished64. However, it should be noted that Bayesian statistics allows to update results as further literature becomes available, without p-value related issues as in the case of frequentist statistics.
Irrespective of the meta-analytic level, the DTI-ALPS itself has intrinsic limitations. In particular, it aims to represent the state of a whole-brain system through an extremely coarse and circumscribed sampling. Nevertheless, it has repeatedly been shown to correlate with clinical features, and the present work also supports the reliability and informativity of the method. Future research will be instrumental in investigating the relationship between the value measured in the ROIs and the rest of the glymphatic system. The understanding of the human glymphatic system itself is still in its infancy, and much remains to be learned about its role and variability along the lifespan even in healthy conditions. In particular, a better comprehension of the glymphatic-sleep relationship could allow to correctly model a possible relevant co-factor of several disorders. Finally, it is noteworthy that the proposed method can be readily applied to any disorder supposed to affect the brain, offering a further clue of the actual involvement of the glymphatic system across different diseases.
Conclusion
The present research aimed at evaluating the coherence among the increasing corpus of studies reporting an alteration of the human glymphatic system in AD and PD. Results suggest that considerable strong evidence supports the actual capability of the DTI-ALPS technique to quantify the level of functioning of this brain apparatus. The straightforward replicability of the proposed approach could open the way to a systematic investigation of glymphatic impairment across neurological and neurodegenerative disorders.
Data availability
The data that support the findings of this study are available in the tables of the manuscript. Any further request can be addressed to Prof. Tommaso Costa.
References
Hablitz, L. M. & Nedergaard, M. The glymphatic system: A novel component of fundamental neurobiology. J. Neurosci. 41, 7698–7711 (2021).
Bohr, T. et al. The glymphatic system: Current understanding and modeling. iScience 25, 104987 (2022).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl Med. 4, 147ra111 (2012).
Taoka, T. et al. Evaluation of glymphatic system activity with the diffusion MR technique: Diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J. Radiol. 35, 172–178 (2017).
Zhang, Y. et al. Interaction between the glymphatic system and α-Synuclein in Parkinson’s disease. Mol. Neurobiol. 60, 2209–2222 (2023).
Liu, S. et al. Glymphatic dysfunction in patients with early-stage amyotrophic lateral sclerosis. Brain 147, 100–108 (2024).
Bae, Y. J. et al. Altered glymphatic system in idiopathic normal pressure hydrocephalus. Parkinsonism Relat. Disord 82, 56–60 (2021).
Kim, M. et al. Comparative analysis of glymphatic system alterations in multiple sclerosis and neuromyelitis optica spectrum disorder using MRI indices from diffusion tensor imaging. Hum. Brain Mapp. 45, e26680 (2024).
Ruan, X. et al. Diffusion tensor imaging analysis along the perivascular space index in primary Parkinson’s disease patients with and without freezing of Gait. Neuroscience 506, 51–57 (2022).
Verghese, J. P., Terry, A., de Natale, E. R. & Politis, M. Research evidence of the role of the glymphatic system and its potential pharmacological modulation in neurodegenerative diseases. J. Clin. Med. 11, 6964 (2022).
Xu, B., Fereshtehnejad, S. M., Zeighami, Y. & Editorial Prodromal stage of neurodegenerative proteinopathies: From bench to bedside. Front. Neurosci. 17, 1295344 (2023).
Costa, T. & Cauda, F. A bayesian reanalysis of the Phase III Aducanumab (ADU) trial. J. Alzheimer’s Disease 87, 1009–1012 (2022).
Costa, T., Manuello, J., Cauda, F. & Liloia, D. Retrospective Bayesian evidence of null effect in two decades of Alzheimer’s disease clinical trials. J. Alzheimers Dis. 91, 531–535 (2023).
Costa, T., Liloia, D., Ferraro, M. & Manuello, J. Plausible reasoning in Neuroscience. In Handbook of Abductive Cognition (ed. Magnani, L.) 1581–1618 (Springer, Cham, 2023). https://doi.org/10.1007/978-3-031-10135-9_74.
Gronau, Q. F., Ly, A. & Wagenmakers, E. J. Informed Bayesian t-Tests. Am. Stat. 74, 137–143 (2020).
Dienes, Z. How do I know what my theory predicts? Adv. Methods Practices Psychol. Sci. 2, 364–377 (2019).
Costa, T., Premi, E., Liloia, D., Cauda, F. & Manuello, J. Unleashing the power of bayesian re-analysis: Enhancing insights into Lecanemab (clarity AD) phase III trial through informed t-Test. J. Alzheimers Dis. 95, 1059–1065 (2023).
Rouder, J. N. & Morey, R. D. A Bayes factor meta-analysis of Bem’s ESP claim. Psychon Bull. Rev. 18, 682–689 (2011).
Hsu, J. L. et al. Magnetic resonance images implicate that glymphatic alterations mediate cognitive dysfunction in Alzheimer disease. Ann. Neurol. 93, 164–174 (2023).
Zhang, X. et al. Glymphatic system impairment in Alzheimer’s disease: Associations with perivascular space volume and cognitive function. Eur. Radiol. 34, 1314–1323 (2024).
Ota, M. et al. Relationships between the deposition of Amyloid-β and tau protein and glymphatic system activity in Alzheimer’s disease: Diffusion Tensor Image Study. J. Alzheimer’s Disease 90, 295–303 (2022).
Matsushita, S. et al. The Association of Metabolic Brain MRI, amyloid PET, and clinical factors: A study of Alzheimer’s Disease and normal controls from the Open Access Series of Imaging studies dataset. J. Magn. Reson. Imaging 59, 1341–1348 (2024).
Liang, T. et al. Evaluation of glymphatic system activity by diffusion tensor image analysis along the perivascular space (DTI-ALPS) in dementia patients. Br. J. Radiol. 96, 20220315 (2023).
Steward, C. E. et al. Assessment of the DTI-ALPS parameter along the perivascular space in older adults at risk of Dementia. J. Neuroimaging 31, 569–578 (2021).
Zhong, J., Wang, L., Li, Y. & Jiang, J. A novel diffusion tensor image analysis along the perivascular space method to evaluate glymphatic alterations in Alzheimer’s disease. In Annu Int Conf IEEE Eng Med Biol Soc 1–4, vol. 2023 (2023).
Kamagata, K. et al. Association of MRI indices of glymphatic system with amyloid deposition and cognition in mild cognitive impairment and Alzheimer disease. Neurology 99, e2648–e2660 (2022).
Chang, H. I. et al. Gray matter reserve determines glymphatic system function in young-onset Alzheimer’s disease: Evidenced by DTI-ALPS and compared with age-matched controls. J. Neuropsychiatry Clin. Neurosci. 77, 401–409 (2023).
Saito, Y. et al. Multisite harmonization of diffusion tensor image analysis along the perivascular space using the COMBined Association Test. Jpn J. Radiol. 41, 1072–1083 (2023).
Bae, Y. J. et al. Glymphatic function assessment in Parkinson’s disease using diffusion tensor image analysis along the perivascular space. Parkinsonism Relat. Disord. 114, 1–7 (2023).
Saito, Y. et al. Glymphatic system impairment in corticobasal syndrome: Diffusion tensor image analysis along the perivascular space (DTI-ALPS). Jpn J. Radiol. 41, 1226–1235 (2023).
Qin, Y. et al. Neuroimaging uncovers distinct relationships of glymphatic dysfunction and motor symptoms in Parkinson’s disease. J. Neurol. 270, 2649–2658 (2023).
Gu, L. et al. Noninvasive neuroimaging provides evidence for deterioration of the glymphatic system in Parkinson’s disease relative to essential tremor. Parkinsonism Relat. Disord. 107, 1–9 (2023).
Cai, X. et al. Diffusion along perivascular spaces provides evidence interlinking compromised glymphatic function with aging in Parkinson’s disease. CNS Neurosci. Ther. 29, 111–121 (2023).
Shen, T. et al. Diffusion along perivascular spaces as marker for impairment of glymphatic system in Parkinson’s disease. npj Parkinsons Dis. 8, 1–10 (2022).
Si, X. et al. Neuroimaging evidence of glymphatic system dysfunction in possible REM sleep behavior disorder and Parkinson’s disease. npj Parkinsons Dis. 8, 1–9 (2022).
Ma, X. et al. Diffusion Tensor Imaging along the perivascular space index in different stages of Parkinson’s disease. Front. Aging Neurosci. 13, 1–7 (2021).
Chen, H. L. et al. Associations among cognitive functions, plasma DNA, and diffusion tensor image along the perivascular space (DTI-ALPS) in patients with Parkinson’s disease. Oxid Med Cell Longev 2021, 4034509 (2021).
Meng, J. C. et al. Correlation of glymphatic system abnormalities with Parkinson’s disease progression: A clinical study based on non-invasive fMRI. J. Neurol. 271, 457–471 (2024).
Bae, Y. J. et al. Altered brain glymphatic flow at diffusion-tensor MRI in rapid eye movement sleep behavior disorder. Radiology 307, e221848 (2023).
Good, I. J. Weight of evidence, corroboration, explanatory power, information and the utility of experiments. J. Roy. Stat. Soc.: Ser. B (Methodol.) 22, 319–331 (1960).
Kass, R. E. & Raftery, A. E. Bayes factors. J. Am. Stat. Assoc. 90, 773–795 (1995).
Szlufik, S., Kopeć, K., Szleszkowski, S. & Koziorowski, D. Glymphatic system pathology and neuroinflammation as two risk factors of neurodegeneration. Cells 13, 286 (2024).
Yamada, K. & Iwatsubo, T. Involvement of the glymphatic/meningeal lymphatic system in Alzheimer’s disease: Insights into proteostasis and future directions. Cell. Mol. Life Sci. 81, 192 (2024).
Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 12, 383–388 (1991).
Balestrino, R. & Schapira, A. H. V. Parkinson disease. Eur. J. Neurol. 27, 27–42 (2020).
Compta, Y. et al. Combined dementia-risk biomarkers in Parkinson’s disease: A prospective longitudinal study. Parkinsonism Relat. Disord 19, 717–724 (2013).
Jack, C. R. et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 20, 5143–5169 (2024).
Buccellato, F. R., D’Anca, M., Serpente, M., Arighi, A. & Galimberti, D. The role of glymphatic system in Alzheimer’s and Parkinson’s disease pathogenesis. Biomedicines 10, 2261 (2022).
Xu, Z. et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener 10, 58 (2015).
Matsumoto, S. & Tsunematsu, T. Association between sleep, Alzheimer’s, and Parkinson’s disease. Biology (Basel) 10, 1127 (2021).
Wang, C. & Holtzman, D. M. Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology 45, 104–120 (2020).
Spira, A. P. et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 70, 1537–1543 (2013).
Hu, M. T. REM sleep behavior disorder (RBD). Neurobiol. Dis. 143, 104996 (2020).
Postuma, R. B. et al. Risk and predictors of dementia and Parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 142, 744–759 (2019).
Tuura, R. O., Volk, C., Callaghan, F., Jaramillo, V. & Huber, R. Sleep-related and diurnal effects on brain diffusivity and cerebrospinal fluid flow. Neuroimage 241, 118420 (2021).
McGregor, M. M. & Nelson, A. B. Circuit mechanisms of Parkinson’s disease. Neuron 101, 1042–1056 (2019).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Morris, H. R., Spillantini, M. G., Sue, C. M. & Williams-Gray, C. H. The pathogenesis of Parkinson’s disease. Lancet 403, 293–304 (2024).
Chung, S. J. et al. Perivascular spaces in the basal ganglia and long-term motor prognosis in newly diagnosed Parkinson disease. Neurology 96, e2121–e2131 (2021).
Fox, P. T., Lancaster, J. L., Laird, A. R. & Eickhoff, S. B. Meta-analysis in human neuroimaging: Computational modeling of large-scale databases. Annu. Rev. Neurosci. 37, 409–434 (2014).
Kruschke, J. K. & Liddell, T. M. The Bayesian new statistics: Hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychon Bull. Rev. 25, 178–206 (2018).
Manuello, J., Costa, T., Cauda, F. & Liloia, D. Six actions to improve detection of critical features for neuroimaging coordinate-based meta-analysis preparation. Neurosci. Biobehavioral Reviews 137, 104659 (2022).
Müller, V. I. et al. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav Rev. 84, 151–161 (2018).
Samartsidis, P. et al. Estimating the prevalence of missing experiments in a neuroimaging meta-analysis. Res. Synth. Methods 11, 866–883 (2020).
Acknowledgements
None.
Funding
This research was supported by the PRIN 2022 Grant (Prot. 202223XMAF; 3B4D - Blood-Based Biomarkers for Dementia: Using blood-based biomarkers to improve diagnostic discrimination between Alzheimer’s disease and Frontotemporal Lobar Degeneration through Bayesian statistics) Prof. Tommaso Costa P.I.
Author information
Authors and Affiliations
Contributions
TC: Conceptualization, Methodology, Investigation, Formal Analysis, Software, Resources, Data curation, Writing - original draft, Writing - review & editing, Project administration, Funding acquisition. JM: Methodology, Investigation, Writing - original draft, Writing - review & editing. EP: Methodology, Writing - original draft, Writing - review & editing. IM: Writing - original draft, Writing - review & editing; LL: Data curation, Writing - original draft, Writing - review & editing. CBL: Data curation, Writing - original draft, Writing - review & editing. FC: Writing - review & editing. SD: Writing - review & editing; DL: Methodology, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Costa, T., Manuello, J., Premi, E. et al. Evaluating the robustness of DTI-ALPS in clinical context: a meta-analytic parallel on Alzheimer’s and Parkinson’s diseases. Sci Rep 14, 26381 (2024). https://doi.org/10.1038/s41598-024-78132-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78132-9
Keywords
This article is cited by
-
Evaluation of glymphatic system function in patients with thalassemia: a study based on DTI-ALPS technology
Neuroradiology (2025)
-
Tracking glymphatic dysfunction in isolated rapid eye movement sleep behavior disorder: a longitudinal neuroimaging study
Sleep and Breathing (2025)
-
Clearance mechanisms of the glymphatic/lymphatic system in the brain: new therapeutic perspectives for cognitive impairment
Cognitive Neurodynamics (2025)
-
Similar DTI-ALPS metrics in Parkinson’s disease and essential tremor: a cross-sectional comparative analysis
Neuroradiology (2025)