Abstract
Smooth endoplasmic reticulum aggregates (SERa) are a type of dysmorphism in oocytes derived from controlled ovarian stimulation (COS). The effect of SERa on assisted reproductive techniques (ART) outcomes is debatable. Based on some evidence, SERa-positive (SERa+) oocytes cause complications including newborn demise, and compromise the outcome of the unaffected oocytes of the same cycle. While other reports demonstrated equal developmental competence between SERa + and SERa-negative (SERa-) oocytes/cycles. We conducted a prospective cross-sectional study on 315 women candidates for ART and compared the outcome among SERa+ (N = 73) and SERa- cycles (N = 217). Furthermore, for the first time, we investigated the prevalence of SERa + cycles in women with various infertility etiologies. Our results indicated that SERa + patients presented higher levels of Estradiol on the day of ovulation triggering (p = 0.02). Regarding the ART outcome, there were no differences in the number of retrieved oocytes, oocyte maturation and fertilization rates among the groups. However, the quality of the unaffected oocytes (p = 0.03), the rates of day-3 top-quality embryos (p = 0.01, and p = 0.03 for grades A and B, respectively), and clinical pregnancy (p = 0.05) in SERa + group were significantly reduced. Moreover, the prevalence of SERa + cycles gradually increased among endometriosis, POI/POR, PCOS, normal women, tubal factor, and idiopathic groups. Our study suggests that suboptimal situations such as elevated levels of Estradiol can increase the occurrence of SERa + oocytes. This suboptimal phenomenon can negatively influence the outcome of the cycle. Thus, optimization of COS, particularly in vulnerable groups such as women with idiopathic infertility may lower the SERa + cycle occurrence, improving the ART outcome.
Similar content being viewed by others
Introduction
Oocyte plays a fundamental role in embryogenesis1. The quality of the oocyte can influence the embryo development throughout the entire process, from fertilization to live birth2. Several intrinsic (e.g., genetic, epigenetic)3, and extrinsic (e.g., ovarian stimulation, environmental pollution)4,5,6 factors have been uncovered that can affect the oocyte’s developmental competence. Hence, to increase the success rate of assisted reproductive techniques (ART), one of the main approaches is the selection of the oocytes based on the quality7. Currently, the quality of the oocyte is assessed according to the morphological criteria. Morphological analysis includes investigation for any abnormalities in zona pellucida (e.g., dark, irregular), perivitelline space (e.g., enlarged), polar body (e.g., fragmentation), and the oocyte cytoplasm (e.g., refractile body, vacuole, smooth endoplasmic reticulum aggregates (SERa))8. However, it is agreed that oocytes with the non-homogenous cytoplasm might have equal developmental competence to the normal oocytes except for the oocytes with SERa9. SERa can be detected as translucent vacuole-like flat disks in the ooplasm. They are large clusters of tubular SER that are surrounded by dense granules and mitochondria9. Although the exact reason/mechanism behind the SERa is poorly understood, it is suggested that suboptimal ovarian stimulation (e.g., elevated levels of estradiol and progesterone, as well as prolonged stimulation duration) can induce SERa formation9,10.
Due to contradictory reports, there is a dilemma in utilizing SERa-positive (SERa+) oocytes in ART procedures. According to several studies, SERa + oocytes have a higher incidence of meiotic cleavage failure11, lower developmental competence12, elevated rates of early fetal/newborn demise and malformation13,14, as well as obstetric problems12. In this regard, the European Society of Human Reproduction and Embryology (ESHRE) consensus recommended avoiding insemination of the SERa + oocytes in ART cycles in 201115. In addition to the abovementioned complications, there are reports demonstrating that the rates of fertilization, embryo development, and pregnancy outcome of the unaffected oocytes that were retrieved from the SERa + cycles are reduced when compared with those that were from SERa- negative (SERa-) cycles9,16. In contrast, some evidence revealed that SERa do not have any significant influence on the aneuploidy/abnormality rate of the oocyte, and oocytes with SERa can result in healthy babies17,18,19. Hence, more investigations in larger populations are needed to determine the importance of the SERa not only in the affected oocytes but also in the outcome of the whole oocytes driven from SERa + cycles. The exact reason/mechanism behind the SERa aggregates formation is poorly understood. Female infertility etiologies such as polycystic ovarian syndrome (PCOS)20, and endometriosis21 can directly affect oocyte quality. However, there is no study in the literature assessing the prevalence of SERa + in women with various infertility etiologies. This study for the first time, investigated the prevalence of SERa + cycles in women with various infertility etiologies.
In the current study, we aimed to compare ART outcomes in SERa + and SERa- cycles and determine if the ART outcomes of unaffected oocytes in SERa + cycles differed from those in SERa- cycles. Additionally, for the first time, we investigated the prevalence of SERa + cycles in women with distinct infertility etiologies to find out if the risk of SERa occurrence varies among different female-factor infertility groups.
Materials and methods
Participants recruitment and the study design
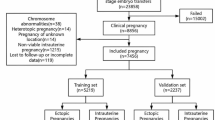
This prospective cross-sectional study was conducted in the infertility clinic of Al-Zahra Hospital, Rasht, Iran from September 2021 to March 2023. The ethics committee of Guilan University of Medical Sciences, Rasht, Iran approved the study (Approval ID: IR.GUMS.REC.1400.539), and signed informed consent was obtained from all patients before enrollment. Regardless of couples with recurrent miscarriage, severe male-factor infertility such as oligoasthenozoospermia and sperm from surgical testicular sperm extraction (such as TESE and microscopic testicular sperm extraction (microTESE)), the number of 315 ICSI candidate women experiencing the first controlled ovarian stimulation (COS) cycle were enrolled in the study. Among the included participants, patients with no oocyte on the day of ovum pick up (OPU) and women who no longer wished to participate in the study were excluded. The sample selection and allocation flowchart are presented in Fig. 1. The patients’ characteristics including age, body mass index (BMI), stimulation protocol, estradiol, and progesterone levels were recorded (Table 1).
.
Controlled ovarian stimulation (COS)
Patients received standard COS protocols, including either Gonadotropin-releasing hormone (GnRH) antagonist or agonist regimes. In GnRH antagonist protocol, ovarian stimulation was performed by injection of 150 IU/day of recombinant follicle-stimulating hormone (rFSH) (Cinal-f, Cinnagen, Iran) from day 2 or 3 of the menstrual cycle. On day 6, to monitor the ovarian response, patients underwent transvaginal ultrasonography. GnRH antagonist (Cetrotid 0.25 mg/day, Merck, Germany) was prescribed when at least one follicle greater than 14 mm was detected. In addition, daily 75 to 150 IU of human menopausal gonadotropin (hMG) (PD homog, Pooyesh Daru, Tehran, Iran) was started. Ultrasonography was performed every 3 days to monitor ovarian stimulation. When at least two follicles greater than 18–20 mm were observed, Triptorelin (Decapeptyle 0.2 mg, Ferring, Switzerland) was administrated as a trigger to prevent Ovarian hyperstimulation syndrome (OHSS). OPU was performed 34–36 h after trigger injection and via transvaginal ultrasound-guided aspiration.
In the GnRH agonist ovarian stimulation regimen, patients received low doses of oral contraceptive pills (Ethinyloestradiol 30 µg + levonorgestrel 150 µg) from day 3 of the menstrual cycle until day 21. Then patients received a subcutaneous injection of 0.5 mg Buserelin Acetate (Cinnafact, CinnaGen, Tehran, Iran) on day 21 of the previous menstrual cycle and reduced to 0.25 mg on day 2 of the next cycle that continued until the day of triggering. From day 2 of the new menstrual cycle, ovarian stimulation was performed using rFSH (Cinnal-f, CinnaGen, Tehran, Iran), and menotropins (PD HOMOG, Pooyesh Darou, Tehran, Iran) were intramuscularly injected. Transvaginal ultrasonography, serum Estradiol, and Progesterone levels were measured to track the ovarian response. After observation of at least 2 follicles with 18–20 mm diameter by transvaginal ultrasonography, 10,000 IU human chorionic gonadotropin (hCG) (PD PREG, Pooyesh darou, Tehran, Iran) were injected intramuscularly. Then OPU was performed 34–36 h after hCG administration.
SERa + oocyte detection, and its prevalence in different groups
Two hours after OPU, cumulus-oocyte complexes (COCs) were denuded in the culture medium (Global for fertilization; LifeGlobal) containing 20 IU/ml hyaluronidase enzyme (LifeGlobal). After removing surrounding cumulus cells, oocytes were assessed under the inverted microscopy (magnification x100) for detection of SERa, and cycles were divided into two groups: SER- cycles when no oocyte with a visible SERa cluster was detected under the inverted microscopy; or SER + cycle which refers to the presence of at least one oocyte containing SERa (Fig. 2).
To compare the incidence of SERa + cycles in women with distinct reasons for infertility, patients were categorized based on the etiology of female-factor infertility, and the incidence of SERa + cycles was calculated according to the number of SERa + cycles in each group/total number of cycles within the group.
ICSI and ART outcome
Following COC denudation, SERa + oocytes were excluded for further analyses and the remaining oocytes were evaluated for the maturity stage and other morphological abnormalities. Regarding the maturation, oocytes were adjusted into germinal vesicle (GV), metaphase I (MI), and metaphase II (MII) groups. Morphological abnormality was assessed according to Rienzi et al.22. MII oocytes underwent ICSI and were incubated at 37oC in 89% N2, 6% CO2, and 5% O2. Followed by 18–20 h, fertilization was confirmed in case of observation of the 2nd polar body and 2 pronuclei. Embryos were monitored on days 1 and 2, and the quality of the day-3 embryos was recorded based on the standard guideline23. Briefly, embryos with grades A (containing 6–8 regular blastomeres, and fragmentation rate ≤ 10%) and B (containing 6–8 regular blastomeres with 10–19% fragmentation rate) were considered top-quality embryos. Day-3 top-quality embryos were transferred (either freshly or in frozen embryo transfer cycles (FET)). Further follow-up was performed in the next two weeks for biochemical pregnancy and four weeks for clinical pregnancy diagnosis by ultrasound detection of the gestational sac. The detailed ART outcome (e.g., top-quality oocyte rate, oocyte maturation rate, top-quality embryo rate) among the SER- and SER + groups was assessed as previously reported24.
Statistical analysis
Data were analyzed using IBM SPSS software version 28.0. Data are expressed as the mean ± standard deviation (SD) for continuous variables, and percentages for categorical variables. Mean values were compared by the Student’s t-test or Mann-Whitney test, based on the Kolmogorov-Smirnov normality test. Percentages were compared through the chi-square test. P < 0.05 was considered statistically significant.
Results
Patients’ characteristics in relation to SERa + oocyte occurrence
A total number of 290 women (age 34.7 ± 5.79) undergoing the first COS were enrolled in the study. Among them, 73 patients (24.3%) presented SERa in at least one oocyte. Age and body mass index (BMI) did not differ significantly between the SERa + and SERa- cycles, concluding these parameters don’t influence SERa + oocyte occurrence. Regarding the hormone levels, only Estradiol level on the day of ovulation triggering was significantly higher in SERa + patients when compared to SERa- women (3407.57 ± 2771.49 pg/ml in SERa + vs. 2656.33 ± 2238.08 pg/ml in SERa- group, P-value = 0.02) (Table 1).
The prevalence of SERa + cycles in different infertility etiologies
Among the included participants, when women were adjusted according to the etiology of the female-factor infertility, 78 patients (26.8%) were diagnosed with PCOS, 13 (4.48%) with endometriosis, 47 (16.2%) with premature ovarian insufficiency/poor ovarian response (POI/POR), 33 (11.37%) with idiopathic infertility, 38 (13.1%) with tubal factor and in 81 cases (27.9%), infertility was due to the male-factor reason and women were considered as normal. Between the mentioned groups, the incidence of SERa + cycle was gradually increased among endometriosis, POI/POR, PCOS, normal women (male-factor infertility), tubal factor, and idiopathic infertility groups (Fig. 3).
ART outcome in SERa + and SERa- cycles
Regarding the ART outcome, there were no significant differences in total number of retrieved oocytes and oocyte maturation rate among SERa + and SERa- groups, while the rate of the top-quality oocytes was significantly lower in SERa + cycles (62.72 ± 34.25 in SER + cycles vs. 71.33 ± 13.63 in SERa- cycles, p = 0.03). Furthermore, there was no significant difference in fertilization rate between the two groups. Although the number of day-3 embryos was significantly higher in SERa + patients (P-value = 0.003), they presented less embryos with grades A and B, when compared with the ratio of day-3 top-quality embryos in SERa- group (P-value = 0.01 and P-value = 0.03 for grades A and B, respectively). Regarding the outcome of pregnancy, the biochemical pregnancy rate was lower in SERa + women but not significantly different (P-value = 0.08), while the clinical pregnancy significantly reduced in SERa + group (2.7% in SERa + vs. 5.5% in SERa- groups, P-value = 0.05) (Table 2).
Discussion
This study was designed to investigate whether the ART outcome of unaffected oocytes in SERa + cycles are diminished in comparison with oocytes derived from SERa- cycles. Furthermore, the prevalence of SERa + cycles was compared among women with distinct infertility etiologies to assess whether the risk of its prevalence is different in various infertility etiologies. We included 290 women candidates for ICSI. Since suboptimal prolonged ovarian stimulation can increase the frequency of SERa + oocytes9, multiple COS cycles may affect SERa occurrence. Therefore, we only enrolled patients who underwent their first COS to avoid bias in SERa prevalence among different groups. Our results demonstrated that patients’ characteristics such as age and BMI did not have any impacts on the occurrence of SERa + oocyte, as there was no significant difference among the SERa + and SERa- groups. The previous evidence is also in line with no associations between age/BMI and SERa in oocytes25,26,27,28. Regarding the hormones’ effect, SERa + patients in our study presented significantly higher levels of Estradiol on the day of ovulation triggering. SERa abnormalities can be the consequence of COS cycles as they were not observed in the oocytes of unstimulated patients29. Furthermore, suboptimal stimulation can increase the frequency of SERa + oocytes9. It has been reported that patients with SERa + oocytes present elevated levels of serum Estradiol16,30 and Progesterone9 on the day of ovulation triggering. Although we found no significant difference regarding Progesterone levels among the groups, our finding on Estradiol level is in line with the previous reports16,30.
We categorized the participants into different groups according to the type of female-factor infertility to investigate the incidence of SERa + cycles in various etiologies of female infertility. Our results showed that women with idiopathic infertility had the highest SERa + oocytes occurrence. Although the incidence was reduced among tubal factor, normal, and PCOS groups, the prevalence in the mentioned groups stayed much higher than POI/POR and endometriosis patients. Studies in this area are limited. Shebl et al. compared the incidence of SERa + cycles among the endometriosis patients and the control group with the same population and no significant difference was found31. To our knowledge, this is the first report on the investigation of the prevalence of SERa + cycles in women with different infertility etiologies. While it should be noted that our results can be due to an artifact of sample sizes, it seems patients with idiopathic infertility, as well as normal women and patients with tubal factor infertility, are more susceptible to the side effects of (suboptimal) hormonal stimulation regimes. Since SERa are the consequence of COS29, especially suboptimal stimulation9, it seems the abovementioned groups are highly vulnerable to the consequences of exogenous hormone interventions. Thus, COS protocols particularly the dosage and duration of the gonadotropins should be prescribed accurately for these groups. Regarding PCOS patients, due to hormonal imbalances in the pathogenesis of the disorder32, oocytes might have already been influenced by the disrupted endocrine system. Hence, it is reasonable that these patients be susceptible to the COS side effects as well. However, more investigations in a larger population are recommended to confirm the findings of this study.
We also assessed distinct factors in ART outcomes. Based on our results, there were no differences regarding the number of retrieved oocytes and maturation rate between the groups. Previous data on this area is controversial. Some reports demonstrated a higher number of retrieved oocytes9,27, and maturation rate9,27 in SERa + cycles. In contrast, other data presented no differences in the abovementioned factors26,28. Regarding the quality of the retrieved oocytes, the results showed that the quality of the unaffected oocytes in SERa + cycles was lower than oocytes derived from SERa- cycles. To our knowledge, this is the first report analyzing the quality of the unaffected oocytes in SERa + cycles and further research is suggested. To compare the developmental competence of oocytes in SERa + and SERa- cycles, we only included the unaffected oocytes in SERa + cycles. Our results showed that there was no significant difference in fertilization rate between the groups. Although SERa + patients presented a higher number of day-3 embryos, they had fewer top-quality embryos (grades A and B) when compared with SERa- group. Furthermore, the clinical pregnancy in SERa + group was less than SERa- cycles. Altogether, our data shows that SERa appearance in the cycle can be a consequence of suboptimal situations that can impair the quality as well as developmental competence of even unaffected oocytes, particularly in their later stages. Suboptimal COS, especially elevated levels of Estradiol can be an important factor9,12,29. Furthermore, Otsuki et al. reported that in SERa + cycles, even oocytes without any visible SERa under light microscopy can still exhibit small SER clusters, as they could detect the small SERa clusters by transmission electron microscopy in SERa- oocytes according to the light microscopy16. However, it should be noted that controversial evidence exists about the ART outcome of oocytes in SERa + cycles. Although there are several studies in line with the reduced outcome of the SERa + cycle9,16, there are some reports demonstrating that not only the outcome of the unaffected oocytes in SERa + cycles is not impaired26,33, but also the SERa-affected oocytes do have equal developmental competence and birth defect rate25,28.
All in all, our results revealed that elevated levels of Estradiol can increase SERa + cycle occurrence. Although more investigation is required, according to our data, the SERa + cycle was more prevalent in women with idiopathic infertility. Furthermore, different factors in the ART outcome of unaffected oocytes derived from SERa + cycles (e.g., oocyte quality, day-3 embryo quality, and clinical pregnancy) were reduced when compared with the oocytes of SER- cycles. Hence, it seems that SERa + cycles are due to some suboptimal situations that can impair the outcome of the ART, and COS optimization may improve the outcome.
Conclusions
In conclusion, we demonstrated that suboptimal COS such as elevated levels of Estradiol are involved in SERa observation in MII oocytes in ICSI cycles. This finding highlights the importance of optimal COS in reproductive outcomes in infertility treatment. Furthermore, this study for the first time, investigated the prevalence of SERa + cycles in women with different infertility etiologies and demonstrated that SERa + cycles are more prevalent in women with idiopathic infertility. This finding draws attention to the need for more investigation into the association between infertility etiologies, especially idiopathic infertility, and SERa occurrence. Moreover, the developmental competence of oocytes derived from SERa + cycles, particularly in the later stages such as day-3 embryo quality and clinical pregnancy are diminished in comparison with SERa- cycles. This phenomenon magnifies the importance of personalized COS optimization and can suggest the occurrence of SERa + oocyte as a marker of suboptimal COS.
Data availability
The data of this study is available to share on reasonable request to the corresponding author.
References
Sirard, M. A. et al. Contribution of the oocyte to embryo quality. Theriogenology. 65 (1), 126–136 (2006).
Klimczak, A. M. et al. Role of the sperm, oocyte, and embryo in recurrent pregnancy loss. Fertil. Steril. 115 (3), 533–537 (2021).
Ge, Z. J. et al. Oocyte ageing and epigenetics. Reprod. (Cambridge England). 149 (3), R103 (2015).
Santos, M. A., Kuijk, E. W. & Macklon, N. S. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 139 (1), 23 (2010).
Clark, Z. L. et al. Follicular hyperstimulation dysgenesis: new explanation for adverse effects of excessive FSH in ovarian stimulation. Endocrinology. 163 (9), bqac100 (2022).
Varghese, A. C. et al. Oocyte developmental competence and embryo development: impact of lifestyle and environmental risk factors. Reprod. Biomed. Online. 22 (5), 410–420 (2011).
Ozturk, S. Selection of competent oocytes by morphological criteria for assisted reproductive technologies. Mol. Reprod. Dev. 87 (10), 1021–1036 (2020).
Nikiforov, D. et al. Human oocyte morphology and outcomes of infertility treatment: a systematic review. Reprod. Sci. 29(10), 1–18 (2021).
Massarotti, C. et al. Occurrence of smooth endoplasmic reticulum aggregates in metaphase II oocytes: relationship with stimulation protocols and outcome of ICSI and IVF cycles. Hum. Reprod. 36 (4), 907–917 (2021).
Saito, H. et al. A higher incidence of smooth endoplasmic reticulum clusters with aromatase inhibitors. Reproductive Med. Biology. 18 (4), 384–389 (2019).
Otsuki, J. et al. A higher incidence of cleavage failure in oocytes containing smooth endoplasmic reticulum clusters. J. Assist. Reprod. Genet. 35, 899–905 (2018).
Ebner, T. et al. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod. Biomed. Online. 16 (1), 113–118 (2008).
Akarsu, C. et al. Smooth endoplasmic reticulum aggregations in all retrieved oocytes causing recurrent multiple anomalies: case report. Fertil. Steril. 92 (4), 1496 (2009). e1-1496. e3.
Sá, R. et al. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil. Steril. 96 (1), 143–149 (2011). e7.
The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Human reproduction, 26(6): pp. 1270–1283. (2011).
Otsuki, J. et al. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum. Reprod. 19 (7), 1591–1597 (2004).
Mateizel, I. et al. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum. Reprod. 28 (8), 2111–2117 (2013).
Mizobe, Y. et al. Smooth endoplasmic reticulum cluster presence does not affect embryo ploidy. Arch. Gynecol. Obstet. 307 (5), 1607–1612 (2023).
Wang, M. et al. Does smooth endoplasmic reticulum aggregation in oocytes impact the chromosome aneuploidy of the subsequent embryos? A propensity score matching study. J. Ovarian Res. 16 (1), 1–9 (2023).
Palomba, S., Daolio, J. & La Sala, G. B. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol. Metabolism. 28 (3), 186–198 (2017).
Orazov, M. R. et al. Oocyte quality in women with infertility associated endometriosis. Gynecol. Endocrinol. 35 (sup1), 24–26 (2019).
Rienzi, L. et al. The oocyte. Hum. Reprod. 27 (suppl_1), i2–i21 (2012).
Balaban, B. et al. Alpha scientists in reproductive medicine and ESHRE special interest group of embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod, 26(6): pp. 1270–1283. (2011).
Qasemi, M. et al. Cell-free mtDNA level and its biomarker potency for ART outcome are different in follicular fluid of PCOS and non-PCOS women. Mitochondrion. 59, 30–36 (2021).
Fang, T. et al. The impact of oocytes containing smooth endoplasmic reticulum aggregates on assisted reproductive outcomes: a cohort study. BMC Pregnancy Childbirth. 22 (1), 1–9 (2022).
Gurunath, S. et al. Live birth rates in in vitro fertilization cycles with oocytes containing smooth endoplasmic reticulum aggregates and normal oocytes. J. Hum. Reproductive Sci. 12 (2), 156 (2019).
Setti, A. S. et al. Oocytes with smooth endoplasmic reticulum clusters originate blastocysts with impaired implantation potential. Fertil. Steril. 106 (7), 1718–1724 (2016).
Xu, J. et al. Oocytes with smooth endoplasmic reticulum aggregates are not associated with impaired reproductive outcomes: a matched retrospective cohort study. Front. Endocrinol. 12, 688967 (2021).
Nikiforov, D. et al. Clusters of smooth endoplasmic reticulum are absent in oocytes from unstimulated women. Reprod. Biomed. Online. 43 (1), 26–32 (2021).
Ebner, T., Moser, M. & Tews, G. Is oocyte morphology prognostic of embryo developmental potential after ICSI? Reprod. Biomed. Online 12(4), 507–512 (2006).
Shebl, O. et al. Oocyte competence in in vitro fertilization and intracytoplasmic sperm injection patients suffering from endometriosis and its possible association with subsequent treatment outcome: a matched case–control study. Acta Obstet. Gynecol. Scand. 96 (6), 736–744 (2017).
Bergo, I. et al. Hormone imbalance in polycystic ovarian syndrome. Acta Biologica Marisiensis 6(1), 10–20 (2023).
Shaw-Jackson, C. et al. Oocytes affected by smooth endoplasmic reticulum aggregates: to discard or not to discard? Arch. Gynecol. Obstet. 294, 175–184 (2016).
Acknowledgements
The authors would like to thank all participants of this study for their contribution, and the staff of the Reproductive Health Research Center, Guilan University of Medical Sciences for their constant support.
Funding
This study has been supported by Guilan University of Medical Sciences, Rasht, Iran.
Author information
Authors and Affiliations
Contributions
NGG: Conception and study design, statistical analysis, revised the manuscript. SZH: collected clinical data. MQ: drafted the manuscript, data analysis. RK and ZZS: performed patients’ recruitment and treatments. MHB: Data collection. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghanami Gashti, N., Hosseini, S.Z., Qasemi, M. et al. Smooth endoplasmic reticulum aggregates in human oocytes are related to female infertility etiology and diminished reproductive outcomes. Sci Rep 15, 7160 (2025). https://doi.org/10.1038/s41598-024-78366-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78366-7
Keywords
This article is cited by
-
Impact of smooth endoplasmic reticulum aggregates in oocytes on embryo development and clinical outcomes in ICSI cycles: a meta-analysis
Journal of Ovarian Research (2026)