Abstract
This retrospective study investigated the degree of intraocular lens (IOL) tilt and decentration after cataract surgery in eyes with varying degrees of myopia using the anterior segment optical coherence tomography (AS-OCT). Eyes of 76 patients were analyzed and divided into three groups: mild myopia (emmetropia to − 1.5 diopter [D], Group 1), moderate myopia (− 1.5 D to − 6.0 D, Group 2), and high myopia (over − 6.0 D, Group 3). Postoperative IOL decentration, tilt, and lens diameter were evaluated using swept-source AS-OCT under mesopic conditions without dilation eyedrop. Postoperative parameters revealed no difference in IOL tilt, but IOL decentration was significantly different among the groups, and the degree of decentration was greater in Group 3 (P = 0.007). Univariable regression analysis indicated that age, sex, preoperative uncorrected distant visual acuity, corrected distant visual acuity, intraocular pressure, spherical equivalent, preoperative anterior chamber depth and lens thickness had no influence on postoperative IOL decentration, but axial length (AL) was significantly related to IOL decentration (P = 0.001). This association was confirmed using multivariable regression analysis, establishing a significant correlation between AL and IOL decentration (P = 0.001) on AS-OCT, showing an increase in IOL decentration associated with increasing AL.
Similar content being viewed by others
Introduction
In the past, cataract surgery was used to remove cataracts and securely insert the intraocular lens (IOL) in the bag1. Advances in surgical techniques, development of various intraocular lenses, and improved IOL calculation accuracy have enabled the correction of residual refractive error2,3,4. Despite these efforts to improve accuracy, high-order aberration and residual astigmatism occur due to factors such as the position of the IOL (tilt and decentration)5,6,7.
There have been several factors associated with postoperative IOL tilt and decentration. If the preoperative position of the crystalline lens is tilted, this can affect the position of the IOL postoperatively8,9. In addition, there is a significant increase in IOL decentration in patients with zonular weakness or capsular tension ring insertion compared to those with a healthy zonule10,11. Therefore, pre-existing problems in lens position can contribute to postoperative IOL tilt or decentration10,11,12. Intraoperative factors that can cause IOL decentration include scleral fixation of IOL, large capsulorhexis, and radial tears. Large continuous curvilinear capsulorhexis (CCC) significantly increases the instability of the IOL position5,13. The type of IOL also makes a difference, with studies showing that postoperative decentration was significantly larger for 1-piece IOLs than 3-piece IOLs14,15. Even after uneventful surgery, capsular fibrosis or posterior capsular opacity, and capsulotomy with the Nd: YAG laser can result in IOL subluxation16. The IOL may tilt and decenter due to lower vitreous pressure in patients with pars plana vitrectomy17.
The position of the IOL can be different in eyes with short or long axial length (AL). In eyes with short axial length, it is more common for the IOL to be more prone. However, high myopia with long AL can cause significant inferior decentration and AL was positively correlated with overall IOL decentration17,18. In addition, high myope eyes have higher IOL capsular instability than normal eyes, demonstrating that high myopia was the most prevalent risk factor for late in-the-bag IOL dislocation, accounting for 19.7% of 61 eyes with IOL dislocation19.
In a previous study, Wang et al. used swept-source anterior segment optical coherence tomography (SS-AS-OCT) to investigate the effects of IOL tilt and decenter in patients with high myopia. In the 334 eyes of high myopia patients analyzed, 71 (21.3%) had clinically significant IOL decentration, and 26 (7.78%) had clinically significant IOL tilt. Furthermore, when divided by AL of 30 mm, patients with AL > 30 mm had a highly significant increase in decentration (37.1% vs. 14.0%) and tilt (16.2% vs. 3.90%) compared to patients with AL under 30 mm20. According to Chen et al. the IOL malposition is a common postoperative complication in patients with myopia. It is thought to be caused by the lens capsular bag becoming larger with the elongation of AL. When patients with myopia were divided into high (> 14D), medium (5 to 14D), and low diopter (< 5D), higher diopter was associated with significantly greater IOL inferior decenter and vertical decenter, which was related to higher IOL weight21. The potential for IOL instability, malposition, and increased inferior decentration in patients with myopia have been reported in previous studies, and it was considered important to further investigate the impact of these factors on postoperative outcomes.
Scheimpflug imaging, Purkinje reflection, and ultrasound biomicroscopy have been used to measure IOL tilt and decentration22. Advances in technology have led to the development of AS-OCT, which allows direct confirmation of IOL position9,22. One recent study developing prediction models for estimating IOL decentration and tilt demonstrated that decentration and tilt could be predicted within 0.3 mm/1.5° with 95% confidence intervals through AS-OCT23.
In the present study, we investigated the degree of intraocular lens tilt and decentration in high myopia using AS-OCT compared to mild to moderate myopia after cataract surgery.
Results
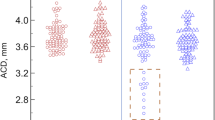
In total, 76 eyes, three groups of mild myopia (Group 1), moderate myopia (Group 2), and high myopia (Group 3) were analyzed. Preoperative baseline characteristics are described in Table 1. The mean age of the patients was 65.93 ± 11.89, and the mean age of Group 3 was 57.0 ± 8.01, significantly lower than that of Groups 1 and 2 (P < 0.001). The preoperative uncorrected distant visual acuity (UDVA) was measured as 0.43 ± 0.77, 0.72 ± 0.72, and 1.30 ± 2.00 in each group, indicating a significant smaller in Group 3 with high myopia (P < 0.001). Preoperative spherical equivalent (SE) was measured as -0.79 ± 0.34, -3.68 ± 1.38, and − 12.02 ± 4.79, respectively, with significant differences among groups (P < 0.001; Bonferroni corrected P-value between Group 1 vs. 2 was < 0.001, Group 1 vs. 3 was < 0.001, and Group 2 vs. 3 was < 0.001). However, corrected distant visual acuity (CDVA) did not differ among groups (P = 0.169). There was no difference in preoperative anterior chamber depth (ACD) and lens thickness (LT) among the groups (P = 0.500 for ACD, and P = 0.290 for LT), but there was a significant difference in AL among groups (P < 0.001; Bonferroni corrected P-value between Group 1 vs. 2 was 0.031, Group 1 vs. 3 was < 0.001, and Group 2 vs. 3 was < 0.001). Preoperatively, lens decentration and tilt obtained from the AS-OCT did not differ among groups (P = 0.280 for decentration, and P = 0.273 for tilt).
Table 2 describes the differences between preoperative and postoperative parameters. Both UDVA and CDVA improved, and the SE decreased from − 4.33 ± 4.76 to -0.41 ± 0.79, all showing significant changes after surgery (all P < 0.001). Comparison of decentration and tilt analyzed by the AS-OCT showed that the former significantly increased after cataract surgery, while the latter was not different before and after surgery (P < 0.001 for decentration, and P = 0.492 for tilt).
Table 3 compares the postoperative parameters among the groups, and there was a significant difference in postoperative intraocular pressure (IOP), with IOP increasingly higher in Group 3 (P = 0.005). Postoperative SE was also significantly different among the groups, and more myopic SE was observed in Group 3 (P = 0.003; Bonferroni corrected P-value between Group 1 vs. 2 was 0.033, Group 1 vs. 3 was 0.002, and Group 2 vs. 3 was 0.043). AS-OCT showed no difference in IOL tilt, but IOL decentration was significantly different among the groups, and the degree of decentration was greater in Group 3 (P = 0.007; Bonferroni corrected P-value between Group 1 vs. 2 was 0.230, Group 1 vs. 3 was 0.003, and Group 2 vs. 3 was 0.018). The axis of the IOL decenter direction was found to be 231.68 ± 79.51 in Group 1, 233.82 ± 63.75 in Group 2, and 216.63 ± 65.28 in Group 3, indicating that inferior decenter occurred in all three groups. There was no significant difference in decenter direction between the groups (P = 0.809).
Table 4 shows the results of a univariable regression analysis to identify preoperative and postoperative factors that can affect postoperative IOL decentration. Age, sex, preoperative UDVA, CDVA, IOP, and SE did not affect postoperative IOL decentration. Preoperative ACD and LT had no influence on postoperative IOL decentration (P = 0.276 for ACD, and P = 0.948 for LT), but AL was significantly related to IOL decentration (P = 0.001). Table 5 shows the results of a multivariable regression analysis to determine preoperative factors that influence postoperative IOL decentration. Age, sex, preoperative IOP, and SE did not affect postoperative IOL decentration. Preoperative ACD and LT had no influence on postoperative IOL decentration (P = 0.489 for ACD, and P = 0.899 for LT), but AL was significantly related to IOL decentration (P = 0.001).
Table 6 shows univariable regression analysis and Table 7 shows the results of a multivariable regression analysis to determine preoperative factors that influence postoperative IOL tilt. Age, sex, UDVA, CDVA, preoperative IOP, and SE did not affect postoperative IOL tilt. Preoperative AL, ACD, and LT had no influence on postoperative IOL tilt also. Only preoperative lens tilt was found to affect postoperative IOL tilt. (P < 0.001 in Table 6, and P < 0.001 in Table 7).
Discussion
In this study, we investigated the degree of IOL tilt and decentration after cataract surgery in eyes with varying degrees of myopia using the AS-OCT. Additionally, we sought to find out whether there is an association between the degree of myopia and the degree of IOL tilt and decentration. The results showed a highly significant correlation between the AL and the degree of IOL decentration obtained from the AS-OCT, revealing an increase in IOL decentration associated with increasing AL. In terms of subgroup analysis, we divided the groups based on the degree of myopia, as there are relatively widespread consensus and references on classifying myopia into mild, moderate, and high categories. However, there has been no clear consensus regarding how to divide AL ranges in highly myopic eyes. Therefore, we initially considered classifying the groups according to the degree of myopia rather than AL.
Among the preoperative parameters that differed between the groups, there was a difference in the age of the patients, and the age at the surgery was low in Group 3 (57.0 ± 8.01years). The Blue Mountains Eye Study showed that posterior subcapsular cataract (PSC) incidence is significantly higher in patients with myopia who suffer from cataract at earlier ages compared to patients without myopia24. High myopia was also associated with early onset of all three types of age-related cataracts, indicating that it is an important risk factor for cataract development25. Therefore, it is postulated that Group 3 consisting of high myopia patients underwent early cataract surgery compared to other groups.
Postoperative IOP, IOL decentration, and SE significantly differed among the groups. Because patients with high myopia are used to seeing near without glasses prior to cataract surgery due to their myopia, cataract surgery targeting emmetropia induces a significant lifestyle change which can be a source of dissatisfaction after cataract surgery. Therefore, many myopic patients often have IOLs with SE closer to − 2.0 D implanted for near vision. This was probably reflected in the results of our study, with significantly more myopic postoperative SE in Group 3.
Regarding ACD, we thought that high myopia patients would have a deeper ACD due to their larger axial length, which would lead to a significant difference, but the results showed no difference between groups. The relationship between ACD and high myopia has been studied in several different studies with varying results. Dong J et al. reported deep ACD, lower corneal refractive power, and greater corneal astigmatism in the high myopia group than in the normal group26. Another large, cross-sectional, population-based study of Chinese individuals reported shallow ACD in persons with myopia, even those with long ALs27. Niu et al. studied patients with AL > 25.0 mm and divided them into two groups, those with ACD ≥ 2.8 mm and those with ACD < 2.8 mm, to determine the effect on ACD. The result showed that the smaller anterior segment, and a thicker and more anteriorly positioned lens, may correlate with shallow ACD in eyes with long axial length28. In this way, the relationship between axial length and ACD in patients with high myopia is not directly correlated, as other variables are involved.
As for the findings of increased IOL decentration with longer AL, previous studies have shown that longer AL is associated with significantly larger capsular bag size. Therefore, IOL may have a greater range of movement and thus decenter more easily in the larger capsular bag in highly myopic eyes29. In addition, AL was significantly associated with IOL decentration ≥ 0.4 mm, and the proportion of participants with IOL decentration in high myopic eyes significantly increased with AL elongation20. Fan et al. reported that the AL of the IOL dislocation cases was significantly longer than that of patients without IOL dislocation. Also, there was a significant association between IOL dislocation and high myopia30. Interestingly, the myopic group presented significantly inferior decentration in the capsular bag compared with the emmetropic group. The overall decentration values were 0.32 ± 0.14 mm in the controls and 0.40 ± 0.18 mm in the myopic group. AL was positively correlated with overall decentration (r = 0.334, P = 0.002)18. The problem of zonular weakness in high myopia is also thought to contribute to IOL decentration. Zonular stress due to axial length elongation in high myopia is more likely to cause zonular weakness compared to normal length eyes19.
According to Hollyday et al., meaningful IOL decentration is a very small amount, and IOL decentration (≥ 0.4 mm) or tilt (≥ 7°) can lead to suboptimal vision and patient dissatisfaction31. In our study, the degree of IOL decentration was found to be 0.31 ± 0.20 (mm) and tilt was found to be 4.53 ± 1.60 (°). For aspheric IOLs, Weikert et al. found that astigmatism induced by 5 degrees of tilt was 0.08D, 0.11D, and 0.14D for 16.0D, 22.0D, and 28.0D, and 10 degrees of IOL tilt produced 0.33D, 0.44D, and 0.56D of induced astigmatism for 16.0D, 22.0D, and 28.0D32. However, in the case of toric IOLs or multifocal IOLs, it is possible that subtle differences in IOL location could significantly impact astigmatism and postoperative visual acuity. One previous study reported that early postoperative toric IOL rotation is related to large capsular bag size, which shows a correlation with a longer AL33. A greater degree of multifocal IOL (PA154N; Allergan) decentration was associated significantly with worse far and intermediate LogMAR visual acuity (r = 0.460 at 5.0 m). When decentration was 0.9 mm or greater, distance mean visual acuity did not reach 20/32. In contrast, no significant correlation was found between IOL decentration or tilt and visual acuity in the monofocal IOL(MA60BM; Alcon Surgical, Fort Worth, TX) group34. Therefore, when planning cataract surgery in high myopia, it is important to consider that decentration of multifocal or toric IOLs can occur, resulting in decreased postoperative visual quality.
Limitations of this study include its retrospective design, limited sample size, and a short follow-up period of 1 month after surgery. Other causes of postoperative IOL tilt and decentration include posterior capsular opacity (PCO). Uzel et al. found that the PCO was associated with IOL tilt, decentration, and visual impairment. The PCO group had a vertical tilt up to 3.78° and decentration up to 0.55 mm compared to the control group without PCO16. Monitoring IOL tilt and decentration due to PCO and other postoperative causes may be useful with AS-OCT for long-term follow-up. As our study was based on data from the first postoperative month, no cases of PCO were observed, and none of the patients required Nd: YAG capsulotomy within this period. Considering the findings of previous studies on ACD, it is possible that the limited sample size in this study may have hindered the detection of a direct correlation between ACD and IOL tilt or decentration. Therefore, future studies involving a larger cohort of patients with varying ACDs and axial lengths are warranted.
In conclusion, we demonstrated a significant correlation between the AL and the degree of IOL decentration on AS-OCT, which showed an increase in IOL decentration associated with increasing AL. We expect that these results help plan cataract surgery for high myopia patients, choosing the IOL to be used, and following up after surgery.
Methods
This retrospective study analyzed medical records of patients who underwent cataract surgery by a single operator (HL) at a single center (Asan Medical Center, Seoul) from February 2023 to February 2024. This study received approval from the institutional review board of Asan Medical Center, Seoul, South Korea (IRB No. 2024-0767) and conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all subjects.
Overall, 76 eyes of 76 patients were enrolled and divided into three groups: mild myopia (emmetropia to -1.5 Diopter, Group 1), moderate myopia (− 1.5 D to − 6.0 D, Group 2), and high myopia (over − 6.0 D, Group 3). Only a single eye from each patient was included in the analysis. Exclusion criteria included poor dilation, zonular weakness, other ocular diseases such as corneal pathologies, glaucoma, uveitis, strabismus, retinal pathologies, ocular surgical history, ocular trauma history, and complications during cataract surgery. Patients with astigmatism that required a toric IOL implantation were excluded from the analysis. All patients had ophthalmic examinations before the operation. Visual acuities were measured, including UDVA, CDVA by the Snellen chart and converted to logMAR. Slit-lamp examination, auto-refraction, and auto-keratometry (Canon R-50, Canon USA Inc., Huntington, NY, USA), IOP, and measurement of AL, ACD, and LT using the IOLMaster 700 (Carl Zeiss Meditec, Jena, Germany) were performed. Lastly, the fundus examination was completed preoperatively.
Lens tilt and decentration were measured with CASIA2 AS-OCT (TOMEY, Japan) by referring to the method used in the study by Gu et al. and Kimura et al.8,35 Using the Lens Biometry mode, CASIA2 generated 16 distinct AS-OCT images from 16 different angles of each patient’s crystalline lens and using the IOL Scan mode, 8 distinct AS-OCT images from 8 different angles. The outlines, tilt, and decentration of the crystalline lenses and IOLs were automatically analyzed in three dimensions by the built-in software (Version SS2000), relative to the corneal vertex axis/corneal topographic axis.
Postoperative IOL decentration, tilt, and lens diameter were evaluated using swept-source AS-OCT under mesopic conditions without mydriasis. For the right eye, an axis of 180° indicated the temporal side, whereas an axis of 180° indicated the nasal side for the left eye. To ensure axis orientation consistency between the left and right eyes, we transformed the axis 180° of the right eye into axis 0, and axis 0 of the right eye into axis 180° before analysis. This adjustment was necessary to align the axis orientations between the left and right eyes and achieve uniformity in our statistical evaluations36.
Surgical technique
A clear corneal incision of 2.2 mm was made after CCC and fragmentation using the Femtosecond laser Catalyst FSL platform (Johnson & Johnson Vision Care, Inc). The capsulorhexis center coincided with the lens center and with a size of 5.0 mm. Phacoemulsification was performed using Signature Pro (Johnson & Johnson Vision Care, Inc). Hydrophobic 1-piece IOL (Eyhance non-toric, Johnson & Johnson) was implanted with the lens center aligned with the visual axis.
Postoperative topical medications included levofloxacin hydrate 1.5% (Cravit 1.5%, Santen Pharmaceutical) four times daily, prednisolone acetate 1.0% (Predforte 1.0%, Allergan, Inc.) four times daily, ketorolac tromethamine 0.45% (Acuvail, Allergan, Inc.) two times daily, and cyclosporin 0.1% (Ikervis 0.1%, Santen Pharmaceutical) once daily to manage postoperative dry eye37,38,39.
Statistical analysis
Statistical analysis was performed using SPSS for Windows statistical software (ver. 25.0; SPSS Inc., Chicago, IL, USA). The normality of the data was analyzed using the Shapiro–Wilk test and histograms. The Kruskal-Wallis test was used to compare the three groups, and the Mann-Whitney test was performed. Bonferroni post hoc test was used to analyze the statistical significance of the differences. The Wilcoxon signed-rank test was used to compare preoperative and postoperative parameter differences. Simple linear regression and stepwise multiple linear regression analyses were performed to identify preoperative factors influencing IOL tilt and decentration after 1-month. Statistical significance was set at P < 0.05.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kugelberg, M. & Lundström, M. Factors related to the degree of success in achieving target refraction in cataract surgery: Swedish National Cataract Register study. J. Cataract Refract. Surg. 34, 1935–1939 (2008).
Abdelghany, A. A. & Alio, J. L. Surgical options for correction of refractive error following cataract surgery. Eye Vis. (London England) 1, 2 (2014).
Behndig, A., Montan, P., Stenevi, U., Kugelberg, M. & Lundström, M. One million cataract surgeries: Swedish National Cataract Register 1992–2009. J. Cataract Refract. Surg. 37, 1539–1545 (2011).
Alió, J. L. & Fine, I. H. Minimizing Incisions and Maximizing Outcomes in Cataract Surgery (Springer, 2010).
Oshika, T. et al. Influence of tilt and decentration of scleral-sutured intraocular lens on ocular higher-order wavefront aberration. Br. J. Ophthalmol. 91, 185–188 (2007).
Taketani, F., Matuura, T., Yukawa, E. & Hara, Y. Influence of intraocular lens tilt and decentration on wavefront aberrations. J. Cataract Refract. Surg. 30, 2158–2162 (2004).
Ashena, Z., Maqsood, S., Ahmed, S. N. & Nanavaty, M. A. Effect of intraocular lens tilt and decentration on visual acuity, dysphotopsia and wavefront aberrations. Vision (Basel Switzerland) 4, 1 (2020).
Kimura, S. et al. Assessment of tilt and decentration of crystalline lens and intraocular lens relative to the corneal topographic axis using anterior segment optical coherence tomography. PLoS ONE 12, e0184066 (2017).
Hirnschall, N. et al. Prediction of postoperative intraocular lens tilt using swept-source optical coherence tomography. J. Cataract Refract. Surg. 43, 732–736 (2017).
Lee, D. H., Shin, S. C. & Joo, C. K. Effect of a capsular tension ring on intraocular lens decentration and tilting after cataract surgery. J. Cataract Refract. Surg. 28, 843–846 (2002).
Takimoto, M., Hayashi, K. & Hayashi, H. Effect of a capsular tension ring on prevention of intraocular lens decentration and tilt and on anterior capsule contraction after cataract surgery. Jpn J. Ophthalmol. 52, 363–367 (2008).
Park, H. J. et al. Effect of co-implantation of a capsular tension ring on clinical outcomes after cataract surgery with monofocal intraocular lens implantation. Yonsei Med. J. 57, 1236–1242 (2016).
Findl, O., Hirnschall, N., Draschl, P. & Wiesinger, J. Effect of manual capsulorhexis size and position on intraocular lens tilt, centration, and axial position. 43, 902–908 (2017).
Sato, T., Shibata, S., Yoshida, M. & Hayashi, K. Short-term dynamics after single- and three-piece acrylic intraocular lens implantation: a swept-source anterior segment optical coherence tomography study. Sci. Rep. 8, 10230 (2018).
Chen, X. Y., Wang, Y. C., Zhao, T. Y., Wang, Z. Z. & Wang, W. Tilt and decentration with various intraocular lenses: a narrative review. World J. Clin. Cases 10, 3639–3646 (2022).
Uzel, M. M., Ozates, S., Koc, M., Taslipinar Uzel, A. G. & Yılmazbaş, P. Decentration and tilt of intraocular lens after posterior capsulotomy. Semin. Ophthalmol. 33, 766–771 (2018).
Chen, X. et al. Characteristics and factors associated with intraocular lens tilt and decentration after cataract surgery. J. Cataract Refract. Surg. 46, 1126–1131 (2020).
Zhu, X. et al. Inferior decentration of multifocal intraocular lenses in myopic eyes. Am. J. Ophthalmol. 188, 1–8 (2018).
Fernández-Buenaga, R. et al. Late in-the-bag intraocular lens dislocation requiring explantation: risk factors and outcomes. Eye 27, 795–802 (2013).
Wang, L. et al. Clinically significant intraocular lens decentration and tilt in highly myopic eyes: a swept-source optical coherence tomography study. Am. J. Ophthalmol. 235, 46–55 (2022).
Chen, Y. et al. Influence of IOL weight on long-term IOL stability in highly myopic eyes. Front. Med. (Lausanne) 9, 835475 (2022).
Wang, X., Dong, J., Wang, X. & Wu, Q. IOL tilt and decentration estimation from 3 dimensional reconstruction of OCT image. PLoS ONE 8, e59109 (2013).
Langenbucher, A., Szentmary, N., Cayless, A., Wendelstein, J. & Hoffmann, P. Prediction of IOL decentration, tilt and axial position using anterior segment OCT data. Graefes Arch. Clin. Exp. Ophthalmol. 262, 835–846 (2024).
Younan, C., Mitchell, P., Cumming, R. G., Rochtchina, E. & Wang, J. J. Myopia and incident cataract and cataract surgery: the blue mountains eye study. Investig. Ophthalmol. Vis. Sci. 43, 3625–3632 (2002).
Lim, R., Mitchell, P. & Cumming, R. G. Refractive associations with cataract: the blue mountains eye study. Investig. Ophthalmol. Vis. Sci. 40, 3021–3026 (1999).
Dong, J. et al. Comparison of anterior segment biometric measurements between Pentacam HR and IOLMaster in normal and high myopic eyes. PLoS ONE 10, e0143110 (2015).
Liu, J. et al. Comparison of the biometric parameters in patients with high myopia and anisometropia. BMC Ophthalmol. 22, 229 (2022).
Niu, L. et al. Anterior segment characteristics of eyes with anterior chamber depth less than 2.8 mm and axial length greater than 25 mm. Ophthalmol. Ther. 12, 1195–1206 (2023).
Tehrani, M. et al. Capsule measuring ring to predict capsular bag diameter and follow its course after foldable intraocular lens implantation. J. Cataract Refract. Surg. 29, 2127–2134 (2003).
Fan, Q. et al. Risk factors of intraocular lens dislocation following routine cataract surgery: a case–control study. Clin. Exp. Optometry. 104, 510–517 (2021).
Holladay, J. T., Piers, P. A., Koranyi, G., van der Mooren, M. & Norrby, N. E. A new intraocular lens design to reduce spherical aberration of pseudophakic eyes. J. Refract. Surg. 18, 683–691 (2002).
Weikert, M. P., Golla, A. & Wang, L. Astigmatism induced by intraocular lens tilt evaluated via ray tracing. J. Cataract Refract. Surg. 44 (2018).
Weinand, F. et al. Rotational stability of a single-piece hydrophobic acrylic intraocular lens: new method for high-precision rotation control. J. Cataract Refract. Surg. 33, 800–803 (2007).
Hayashi, K., Hayashi, H., Nakao, F. & Hayashi, F. Correlation between pupillary size and intraocular lens decentration and visual acuity of a zonal-progressive multifocal lens and a monofocal lens. Ophthalmology 108, 2011–2017 (2001).
Gu, X. et al. Determinants of intraocular lens tilt and decentration after cataract surgery. Ann. Transl. Med. 8, 921 (2020).
Lee, Y. et al. Analysis of intraocular lens decentration and tilt after femtosecond laser-assisted cataract surgery using swept-source anterior optical coherence tomography. Heliyon 10, e29780 (2024).
Hamada, S. et al. Assessment of the effect of cyclosporine-A 0.05% emulsion on the ocular surface and corneal sensation following cataract surgery. Contact Lens Anterior Eye 39, 15–19 (2016).
Ahmadi, H., Tahmasbian, S., Janbazi, M., Amiri, A. & Heidari, Z. Evaluation of cyclosporine 0.05% and artificial tears for the management of dry eye disease following cataract surgery: a randomized controlled trial. Ann. Med. Surg. (Lond.) 86, 1983–1988 (2024).
Chung, Y. W., Oh, T. H. & Chung, S. K. The effect of topical cyclosporine 0.05% on dry eye after cataract surgery. Korean J. Ophthalmol. 27, 167–171 (2013).
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This work was supported by the Korean Fund for Regenerative Medicine, funded by the Ministry of Science and ICT; the Ministry of Health and Welfare (21C0723L1-21, Republic of Korea); by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2024-00438366); and by a grant from the Asan Institute for Life science, Asan Medical Center, Korea (2023IP0069-2).
Author information
Authors and Affiliations
Contributions
Conception and design of study: Y.Y.J., N.P., H.L.; acquisition of data: Y.Y.J., N.P., J.H., H.L.; analysis of data: Y.Y.J., N.P., H.L, K.S.E, J.H., H.S.C, J.Y.K., H.L.; drafting of manuscript: Y.Y.J., N.P., H.L, K.S.E, J.H., H.S.C, J.Y.K., H.L.; revising the manuscript critically for important intellectual content: Y.Y.J., N.P., H.L, K.S.E, J.H., H.S.C, J.Y.K., H.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jeon, Y.Y., Park, N., Lee, H. et al. Analysis of intraocular lens tilt and decentration after cataract surgery in eyes with high myopia using the anterior segment optical coherence tomography. Sci Rep 14, 27987 (2024). https://doi.org/10.1038/s41598-024-78759-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78759-8