Abstract
Oral lichen planus (OLP) is a chronic inflammatory disorder featured with T lymphocytes infiltration and epithelial basement membrane breakdown. Although matrix metalloproteinase-12 (MMP12) is reported to be involved in the pathogenesis of oral diseases such as oral squamous cell carcinoma and periodontitis, the roles of MMP12 in the context of OLP remain poorly understood. Here, we demonstrate that MMP12 in macrophages derived from mucosal diseased tissues compromises the epithelial barrier integrity in OLP. The increased MMP12 facilitates fibronectin degradation in keratinocytes, leading to oral epithelial barrier disruption. Overexpression of fibronectin in oral keratinocytes prevents epithelial barrier from MMP12-induced damage. In addition, pharmacological inhibition of MMP12 reverses keratinocyte barrier disruption in a cell model. Altogether, our study reveals that MMP12 mediates oral epithelial barrier integrity in the setting of OLP.
Similar content being viewed by others
Introduction
Oral lichen planus (OLP), a common autoimmune disorder, is characterized by numerous T lymphocytes infiltration1,2. The global prevalence of OLP is approximately 1% and the etiology of this disease remains unknown3. The classical feature of OLP is reticular white lesions symmetrically and bilaterally distributed on the oral mucosa, especially the sides of tongue and buccal mucosa2. OLP is identified as a recurrent disease with periods of exacerbations and quiescence which are closely associated with anxiety, trauma, mental stress or exposure to irritants4,5. Particularly, erosive lesions exist in oral mucosa with increasing disease severity2. Erosive tissues present severe symptoms such as pain and sensitivity to strong flavors, owing to the loss of mucosal barrier2. As the activity of disease becomes worse, ulcerative lesions occur in oral mucosa due to the complete loss of epithelium2. The histopathological characteristics of OLP are a dense inflammatory T cell-infiltrated band in the subepithelial area, invasion of lymphocytes into epithelium and basal keratinocytes degeneration6. Moreover, epithelial basement membrane dysfunction including breaks, duplications and branches are common in the lesions of OLP7,8.
The combination of dysregulated immune reactions and overproduction of tissue remodeling enzymes such as matrix metalloproteinases (MMPs) is critical for the onset of inflammatory diseases9. MMPs are released by activated tissue mononuclear myeloid cells to impact tissue integrity, leading to irreversible tissue recession10. The MMP12 or macrophage metalloelastase, which is primarily secreted by macrophages, functions to cleave elastin and epithelial integrity component fibronectin (FN)11,12. MMP12 substrates can be fragmented by the full-length and cropped catalytic domain to act as chemoattractants13. What is more, MMP12 regulates the release of cell membrane-bound cytokines and chemokines to facilitate macrophage migration in inflammatory tissues14,15. The upregulation of MMP12 is closely related to cardiovascular disease, respiratory disease and rheumatoid arthritis16,17,18,19. In addition, the increased levels of MMP12 are also correlated with oral diseases including periodontitis, temporomandibular joint dysfunction (TMD) and oral squamous cell carcinoma (OSCC)20. To date, how MMP12 affects OLP development as well as the oral epithelium remains to be elucidated.
In this study, we reported that MMP12 in macrophages disrupts epithelial barrier integrity in oral lichen planus by degrading keratinocyte-produced fibronectin. Suppression of MMP12 in macrophages or overexpression of fibronectin in keratinocytes could restore the damaged epithelial barrier.
Materials and methods
Human samples
Human oral tissues, blood and saliva samples were collected from healthy individuals and OLP patients at the Stomatological Hospital of Shanxi Medical University. OLP was diagnosed according to the modified World Health Organization diagnostic criteria21. The clinical criteria include the presence of symmetrical or bilateral lesions and the presence of a network of gray-white lines. The bulbous, atrophic, erosive or plaque lesions are only accepted as a subtype in the presence of reticular-type lesions elsewhere. Histopathologic criteria include the occurrence of a band-like zone of cellular infiltration, signs of basal cells’ liquefaction degeneration and the absence of epithelial dysplasia. The inclusion and exclusion criteria were based on previous investigations22. The NEOLP symptom was featured with asymptomatic fine lacelike network of white lines, whereas the EOLP symptom was featured with painful white lines with erosive or ulcerative tissues. Control oral buccal specimens without inflammation were from healthy volunteers who underwent the third molar extractions at the Stomatological Hospital of Shanxi Medical University. Briefly, after local anesthesia, oral tissues were cut and bones were removed prior to tooth removal. The residues of healthy oral buccal mucosal tissues were collected. Sugi® swab was used for saliva collection from each individual according to a protocol23. Briefly, swab was softly pressed onto the surface of the location of interest for 3 min. The pressed swab was then pulled out and centrifuged for 15 min at 1500 g. After centrifugation, 25 µl saliva sample was collected for analysis. 10 ml human blood sample of each participant was collected by venipuncture from the median cubital vein in the arm. The collected peripheral blood samples were stored in tubes for further studies. The protocol for this human study was approved by the Ethical Committee of Shanxi Medical University (#2016LL046). The written informed consent was provided by each participant. All experiments were performed in accordance with relevant guidelines and regulations. All these samples were collected during 2022 and 2023. Consecutive sampling method was used for the enrollment of OLP patients. More details for participants in this study were listed in Table 1. The inclusion and exclusion criteria of human samples were provided in Supplementary Table 1.
Cell culture
HOKs (ScienCell, #2610) were grown in oral keratinocyte medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) under 37 °C and 5% CO2 condition. HEK293T cells (ATCC, #CRL-3216) and Raw264.7 mouse macrophages (ATCC, #TIB-71) were both cultured in DMEM with 10% FBS and 1% P/S. HOKs were challenged by recombinant MMP12 for 12 h with distinct doses as indicated. In separate experiments, 20 nM MMP408 (MMP12 inhibitor-1)24, 100 nM RXP470.1 (MMP12 inhibitor-2)25 or 1 µM PF-00356231 hydrochloride (MMP12 inhibitor-3)26 were supplemented into cell culture media for 12 h. Primary human T cells and macrophages were cultured in RPMI 1640 with 10% FBS and 1% P/S. For co-culture systems, all of the cells were cultured with Transwell inserts (0.4 μm pore size; Corning, #3470).
Monocyte-derived macrophages (MDMs) culture
Human monocytes were isolated and differentiated into macrophages as described27. Ficoll-Paque density gradient cell separation was used to purify human blood mononuclear cells prior to monocytes enrichment using Pan Monocyte Isolation Kit (Miltenyi Biotech, # 130-096-537). Monocytes were placed in RPMI 1640 medium including 1% non-essential amino acids, 1% sodium pyruvate, 20 ng/ml human recombinant M-CSF (PeproTech, #300 − 25) and 2 mM glutamine for 7 days to differentiate into macrophages.
Oral epithelial cells, macrophages, and T cells isolation
The fresh buccal tissues were harvested and treated with 0.25% dispase II for 12 h. Oral mucosal epithelial layer was separated by muscle forceps as described28. Both the epithelial layer and the lamina propria tissue were digested into single cells using a Papain Dissociation System (Worthington, #LK003150) in terms of the manufacturer’s protocol. Oral mucosal T cells were enriched using the T Cell Isolation Kit (Miltenyi Biotec, # 130-096-535). Cell sorting of oral mucosal macrophages was performed as reported. In brief, the single cell suspension was incubated with anti-CD16/32 antibody (eBioscience), followed by dead cell detection and cell surface marker staining. Antibodies for human macrophages isolation include anti-CD45-Brilliant Violet 510 (clone 2D1, Biolegend), anti-CD14-PE (clone M5E2, Biolegend), anti-CD11B-Brilliant Violet 711 (clone M1/70, Biolegend) and anti-HLA-DR-Alexa Fluor 700 (clone L243, Biolegend). Cell sorting was performed using the Bigfoot system (Thermo Fisher Scientific).
Lentiviral or plasmid generation
Lentivirus overexpressing human MMP12 or FN1 was constructed by cloning the coding region of human MMP12 [NM_002426.6] or FN1 [NM_212482.4] cDNA into pLV[Exp]-Neo-EF1A lentiviral vector from VectorBuilder. Lentiviral vector harboring cDNAs as well as packaging plasmids were mixed and co-transfected into HEK293T for 48 h. Lentivirus in cell culture medium was harvested and applied to infect cells in the presence of 4 µg/ml polybrene. Primers were listed in Supplementary Table 2.
RT-qPCR
Cells or tissues total RNAs were extracted by TRIzol Reagent (Invitrogen). The first strand cDNA synthesis was performed using the PrimeScript RT Reagent Kit (TaKaRa, #RR037B) according to the manufacturer’s standard protocol. The qPCR was conducted using the SYBR Premix Ex Kit (TaKaRa, #RR420A) in terms of manufacturer’s instruction. GAPDH was served as internal control. The primers regarding qPCR were listed in Supplementary Table 2.
Western blot
Western blot was performed as described before28. In brief, cells or tissues were lysed using laemmli buffer supplemented with protease inhibitor cocktail. The isolated proteins were separated by SDS–PAGE gels and transferred onto polyvinylidene fluoride (PVDF) membranes, which were then blocked by 5% non-fat milk buffer and incubated by the first and secondary antibodies. The chemiluminescence of ECL Substrate (Thermo Fisher) was used to detect signals. Bands were visualized by an imaging system (Bio-Rad). The details of antibodies were listed in Supplementary Table 3.
Subcellular fractionation
Subcellular fractionation was performed as described29. Briefly, cells or tissues were harvested and dissolved in subcellular fraction buffer (1.5 mM MgCl2, 20 mM HEPES, 250 mM sucrose, 10 mM KCl, 1 mM DTT, 1 mM EDTA and 1 mM EGTA), followed by homogenization with a syringe needle (25-gauge). After 5-min centrifugation at 1000 g, the pellet was saved for the nuclear fraction. The supernatant was then centrifuged at 6000 g for 5 min to separate the mitochondrial fraction. The post-mitochondrial supernatant was centrifuged at 20,000 g to precipitate the membrane fraction, and then the 20,000 g supernatant was saved for the cytosol fraction. All fractions were subjected to western blot assays for protein detection.
Permeability assessment
5000 oral keratinocytes were plated in Transwell inserts (0.4 μm pore size) for 7 days to form the monolayers. Transepithelial electrical resistance (TER) was evaluated by a Millicell-ERS voltohmmeter (Millipore). In another assay, paracellular permeability of monolayer was detected using FITC-conjugated dextran (10 mg/mL; Sigma-Aldrich, #46944) as described previously30. 200 µl of 4000 Da FITC-Dextran was placed into the Transwell inserts for 4-hour incubation, and then 100 µl aliquots of media were collected from the basal chamber. FITC-dextran concentration in the medium was measured under the 490 nm/520 nm (Ex/Em) condition using a microplate reader.
NF-κB activity
NF-κB activity was determined using the commercial NF-κB luciferase reporter kit (BPS Biosci-ence, #60614) following manufacturer’s instruction. Macrophages or keratinocytes were co-transfected with pRL-TK renilla luciferase reporter and NF-κB luciferase reporter. Luciferase activities were monitored using a Dual Luciferase System (Berthold Technologies).Cell viability and proliferation assays. Cell viability was measured using the CellTiter-Glo 2.0 kit (Promega, #G9241) according to the manufacturers’ instructions. Cell proliferation was determined by counting cell number directly on different days. Elisa. MMP12 and fibronectin concentrations were determined using the commercial Human MMP-12 ELISA Kit (Abcam, #ab213811) and Human Fibronectin ELISA Kit (Invitrogen, #BMS2028) according to the manufacturer’s protocols, respectively. The OD values were measured using a microplate reader under 450 nm.
MMP12 activity
MMP12 activity was measured using a commercial kit (Enzo Life Sciences, #BML-AK403-0001) according to the manufacturer’s protocol. Briefly, 20 µl of MMP12 or inhibitor was added into each reaction buffer, followed by 30-min incubation at 37 oC and the addition of 10 µl BML-P277-9090 substrate. MMP12 was used as a positive control. Fluorescence was monitored using a microplate system under 545 nm/576 nm (Ex/Em) condition.
MMP12 or FN1 knockout. The knockout of human MMP12 or FN1 gene was performed using the CRISPR/Cas9 technology. sgRNA sequences targeting the MMP12 gene (5’-ATGATGCACGCACCTCGATG-3’) or FN1 gene (5’-GACCTACCTAGGCAATGCGT-3’) were subcloned into lentiCRISPRv2 vector (Addgene, #52961). LentiCRISPRv2 vector harboring sgRNAs as well as packaging plasmids were mixed and co-transfected into HEK293T for 48 h. Lentivirus in cell culture medium was harvested and applied to infect cells in the presence of 4 µg/ml polybrene.Statistical analysis. Data were shown as means ± standard deviation. GraphPad Prism v8 was used for all statistical analyses. The data we analyzed were normally distributed and had similar variance. The unpaired two-tailed Student’s t test was served for two group’s comparisons. One-way or two-way ANOVA with a Student-Newman-Keuls post hoc test was applied for multiple comparisons, as appropriate. Bonferroni correction was performed for the corrections of multiple comparisons. P < 0.05 were considered to be statistically significant.
Results
MMP12 levels are increased in the samples from OLP patients
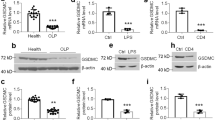
To determine the MMP12 levels in the macrophages of OLP, we collected oral mucosae from healthy people and OLP patients, followed by human macrophages enrichment. As shown, MMP12 levels in macrophages were increased in both the non-erosive OLP (NEOLP) and erosive OLP (EOLP) groups compared to healthy controls, with levels in EOLP being slightly higher than those of NEOLP (Fig. 1a-b). Moreover, our immunohistochemistry data also showed that MMP12 expression was highly increased in the OLP tissues (Supplementary Fig. 1a). Next, we collected serum and saliva samples from healthy donors and OLP individuals for detection. Importantly, MMP12 levels were highly increased in the saliva and serum samples from OLP, especially those from EOLP (Fig. 1c-d).
MMP12 expression in the human samples. (a) Real-time PCR analyses of MMP12 mRNA levels in oral mucosal macrophages from human donors, n = 10. (b) Western blot determinations of MMP12 protein levels in oral mucosal macrophages from human donors (left) and quantitative analyses (right), n = 5. (c-d) Saliva (c) or Serum (d) MMP12 concentrations of healthy or OLP donors measured by Elisa, n = 10. *** P < 0.001 vs. corresponding control group. NEOLP, non-erosive oral lichen planus; EOLP, erosive oral lichen planus. Data were expressed as means ± standard deviation. One-way ANOVA was used for statistical analysis.
Increase of MMP12 compromises epithelial barrier integrity in OLP
We overexpressed MMP12 in monocyte-derived macrophages (MDMs) followed by co-culture with human oral keratinocytes (HOKs) as manifested (Fig. 2a-b). The forced expression of MMP12 increased oral keratinocyte monolayer permeability in the cell co-culture system (Fig. 2c). Consistently, the supplement of recombinant MMP12, which barely influenced HOKs viability and inflammatory responses (Supplementary Fig. 1b-c), enhanced oral keratinocyte monolayer permeability in a dose-dependent way (Fig. 2d). In agreement with these results, the monolayer permeability formed by OLP-derived primary keratinocytes was promoted in relative to controls (Fig. 3a). Next, we isolated primary macrophages from healthy or OLP oral mucosa and co-cultured them with HOKs in transwell plates. In the co-culture model, primary macrophages from either NEOLP or EOLP compromised HOKs monolayer barrier compared to those from healthy donors, especially primary macrophages from EOLP (Fig. 3b). W further isolated primary T cells or oral keratinocytes from OLP participants and healthy volunteers to activate MDMs in a co-culture model, the activated macrophages were then co-cultured with HOKs (Supplementary Fig. 2a). As shown, either primary T cells or keratinocytes from OLP could upregulate MMP12 expression and NF-kB activity in MDMs, and the activated macrophages increased HOKs monolayer permeability (Supplementary Fig. 2b-e). In the following assays, we deleted the MMP12 gene in MDMs (Supplementary Fig. 3a), which were then co-cultured with primary T cells from OLP patients or healthy donors for activation. The T cell-activated macrophages were further co-cultured with HOKs in transwell plates (Fig. 3c). As shown, activated macrophages failed to disrupt HOKs monolayer barrier integrity in the absence of MMP12 (Fig. 3d). Additionally, primary T cells enriched from OLP could increase MMP12 levels in murine macrophages as well (Supplementary Fig. 3b), but failed to damage HOKs monolayer integrity in a T cell-HOK co-culture model (Supplementary Fig. 3c).
Roles of MMP12 overexpression in oral keratinocytes. (a) Schematic showing co-culture of MDMs and HOKs. MDMs were transduced with or without MMP12-lentivirus for 48 h prior to 12-hour co-culture with keratinocytes. (b) Western blot showing MMP12 levels in MDMs with or without MMP12 overexpression. (c) Monolayer permeability detections of HOKs co-cultured with control- or MMP12-lentivirus transduced-MDMs. Permeability was evaluated by detecting TER (left) or FITC-dextran concentrations (right), n = 5. (d) TER (left) or FITC-dextran concentrations (right) of HOKs with recombinant MMP12 treatment at different doses as indicated, n = 5. ** P < 0.01, *** P < 0.001 vs. corresponding control group. Ctrl, control; OE, overexpression. Data were expressed as means ± standard deviation. Student’s t test (c) and One-way ANOVA (d) were used for statistical analysis.
Roles of MMP12 deficiency in oral keratinocytes. (a) TER (left) or FITC-dextran concentrations (right) of primary oral mucosal keratinocytes from human donors, n = 5. (b) TER (left) or FITC-dextran concentrations (right) of HOKs co-cultured with primary oral mucosal macrophages from human donors, n = 5. (c-d) Healthy MDMs were transduced with or without sgMMP12-lentivirus for 48 h prior to 12-hour co-culture with primary oral mucosal T cells from control or EOLP participants for activation. The activated macrophages were then co-cultured with HOKs for another 12 h. Schematic exhibiting the macrophage-keratinocyte co-culture system (c); TER (left) or FITC-dextran concentrations (right) of HOKs co-cultured with activated macrophages (d), n = 5. ** P < 0.01, *** P < 0.001 vs. corresponding control group; ###P < 0.001 vs. T cell-OLP sgCtrl group. NEOLP, non-erosive oral lichen planus; EOLP, erosive oral lichen planus; M, macrophage; K, keratinocyte; H, health. Data were expressed as means ± standard deviation. One-way ANOVA was used for statistical analysis.
MMP12 disrupts keratinocyte epithelial barrier via degrading fibronectin
MMP12 is identified to cleave elastin and epithelial integrity component fibronectin11,12. After western blot analysis, we found that fibronectin was expressed in HOKs while elastin was absence (Supplementary Fig. 4a). Therefore, we speculated that fibronectin is the key factor regulated by MMP12 in oral keratinocytes. In HOKs, the mRNA levels of FN1 (encoding fibronectin) were not affected while fibronectin concentrations in the cell culture media were decreased by MMP12 treatment in a dose-dependent fashion (Supplementary Fig. 4b and Fig. 4a). Moreover, recombinant MMP12 decreased fibronectin expression in the cell membrane of HOKs rather than cytosol or nuclear fraction (Fig. 4b). The knockout of fibronectin, which showed slight effects on oral keratinocyte viability, proliferation and inflammatory reaction (Supplementary Fig. 4d-f), significantly weakened the integrity of HOK monolayer barrier (Fig. 4c). Overexpression of fibronectin in HOKs rescued the MMP12-induced disruption of keratinocyte barrier (Supplementary Fig. 4g and Fig. 4d). In agreement with these data regarding HOKs, the levels of fibronectin, which showed negative correlations with MMP12 levels in diseased tissues (Supplementary Fig. 4h-i), were also decreased in oral mucosae and epithelia of OLP (Fig. 4e-g). To further confirm this finding, we treated HOKs with the culture media of primary macrophages derived from health of OLP tissues (Fig. 4h). Elisa data showed that media from OLP-derived primary macrophages suppressed fibronectin levels in HOKs while the depletion of MMP12 by anti-MMP12 antibodies in the media inhibited fibronectin’s downregulation in keratinocytes (Fig. 4i-l).
MMP12 degrades keratinocyte-produced fibronectin. (a) Elisa showing fibronectin concentrations in the culture media of HOKs treated with different doses of recombinant MMP12 for 12 h. (b) Western blot examinations of fibronectin expression in the membrane, cytosol or nuclear fraction of HOKs treated with different doses of recombinant MMP12 for 12 h. (c) TER (left) and FITC-dextran concentrations (right) in HOKs with or without FN1 knockout. (d) TER (left) and FITC-dextran concentrations (right) in FN1-overexpressed HOKs with or without recombinant MMP12 treatment for 12 h. (e-f) Elisa showing fibronectin concentrations in the oral mucosae (left) or epithelia (right) derived from control or EOLP donors. (g) Western blot tests of fibronectin expression in the membrane, cytosol or nuclear fraction of primary keratinocytes from control or EOLP donors. (h-i) Primary oral mucosal macrophages were enriched from healthy or EOLP donors, followed by 12-hour culture. The supernatants of macrophages were then added into HOKs media at a 50% final volumetric concentration for another 12 h. Schematic illustration of HOKs with media from macrophages (h); Elisa assessments of fibronectin concentrations in the supernatants of HOKs (i). (j-k) Primary oral mucosal macrophages were enriched from healthy or EOLP donors, followed by 12-hour culture. The supernatants of macrophage (1 ml) were treated with 20 µg MMP12 antibodies coupled to Pierce Protein A/G Magnetic Beads (ThermoFisher Scientific) for immunodepleting of MMP12, followed by mixture with HOKs media (v/v, 50%/50%) for another 12 h. Schematic illustration of HOKs with media from macrophages (j); western blot showing MMP12 levels in the macrophage media with or without MMP12 antibodies treatment (k); Elisa assessments of fibronectin concentrations in the supernatants of HOKs (l). *** P < 0.001 vs. corresponding control group; ###P < 0.001 vs. MMP or IgG group. NEOLP, non-erosive oral lichen planus; EOLP, erosive oral lichen planus; H, health; Ctrl, control; FN, fibronectin. Data were expressed as means ± standard deviation. All experiments were carried out 5 times. One-way ANOVA (a, d, e, f, l) and Student’s t test (c, i) were used for statistical analysis.
Pharmacological inhibition of MMP12 protects keratinocyte barrier integrity from disruption
To answer whether the inhibition of MMP12 could protect keratinocyte barrier or not, we added MMP12 inhibitors in the macrophage-keratinocyte co-culture system (Fig. 5a). As shown, neither macrophage-produced MMP12 levels nor keratinocyte NF-kB activity were affected by the addition of MMP12 inhibitors (Supplementary Fig. 5a-b). Keratinocyte viability and proliferation were not influenced by MMP12 inhibitors either (Supplementary Fig. 5c-d). However, MMP12 protease activity was suppressed largely after inhibitor treatments (Fig. 5b). Meanwhile, MMP12 inhibitors reversed the breakdown of keratinocyte barrier integrity and inhibited fibronectin decreases in keratinocytes induced by OLP-derived primary macrophages (Fig. 5b-f).
MMP12 inhibitor prevents keratinocyte barrier from destruction. HOKs were co-cultured with primary health- or EOLP-derived oral mucosal macrophages with or without MMP12 inhibitor treatment for 12 h. Three MMP12 inhibitors, MMP408 (inhibitor-1), RXP470.1 (inhibitor-2), PF-00356231 hydrochloride (MMP12 inhibitor-3) were used. (a) Schematic graphic of HOKs and macrophages co-culture. (b) MMP12 activity of primary EOLP-derived oral mucosal macrophages with or without MMP12 inhibitor treatment. Recombinant MMP12 was used for a positive control. (c-f) TER (c) or FITC-dextran concentrations (d) of HOK monolayers, fibronectin concentrations in the supernatants of HOKs (e) and fibronectin expression in the cell membrane fraction of HOKs (f) were tested. *** P < 0.001 vs. corresponding control group; ###P < 0.001 vs. OLP-Ctrl group. Ctrl, control; FN, fibronectin; H, health. Data were expressed as means ± standard deviation. All experiments were carried out 5 times. One-way ANOVA was used for statistical analysis.
Discussion
Although OLP is identified to be a T cell-mediated disease, other immune cells are also involved in the pathogenesis of this disorder2. Activated immune cells are reported to disrupt the epithelial basement membrane to facilitate OLP progression. Mast cells in the lamina propria of OLP produce tryptase and chymase to activate MMPs by proteolytic cleavage31. The increased proteolytic activity accelerates T cells migration and cleaves collagens to cause basement membrane destruction, which allows CD8 + T cells access to the oral keratinocytes2. Here, in the OLP tissues, we confirmed the increases of MMP-12 levels by western blot and the location of MMP-12 by immunohistochemistry. Furthermore, our investigation indicated that activated macrophages secrete MMP12 to destroy oral epithelial barrier, indicating that macrophages may act in concert with mast cells to compromise oral epithelial barrier integrity and facilitate OLP development. In addition, other studies have claimed that keratinocyte barrier dysfunction is a common feature of OLP epithelium6. Although bacteria and infiltration of intra-epithelium T cells are reported to be abundant throughout the epithelium in OLP2,32, the exact underpinning of epithelial barrier destruction is not fully explained. Our current study provided compelling evidence to verify that MMP12, at least in part, is the cause of epithelial barrier dysfunction in the context of OLP. Since the data from both human samples and cell co-culture system are consistent, we think that our human results here are not affected by the unmatched ages between OLP and health groups.
Although MMP12 is closely associated with various oral diseases such as temporomandibular joint dysfunction (TMD), periodontitis and oral squamous cell carcinoma (OSCC)20, the exact pathophysiological functions of MMP12 remain incompletely understood. Therefore, it is essential to explore the roles of MMP12 at cellular and molecular levels since MMP12 could be a potential therapeutic target for the treatment of inflammatory oral diseases. Here we reported that MMP12 is involved in the development of OLP. In addition, we explained that MMP12 compromises epithelial barrier integrity by degrading fibronectin. Fibronectin is revealed to fine-tune human keratinocyte adherence33. Our data showed that fibronectin is dominantly expressed in the cell membrane of oral keratinocyte, which implies its critical roles in cell-cell interactions. We further confirmed that fibronectin is decreased in the oral mucosae and epithelia of OLP. Therefore, oral keratinocyte-produced fibronectin has a critical role in preventing OLP development.
In conclusion, this investigation unveils an unrecognized role of MMP12 in affecting epithelial barrier of OLP. Despite the lack of well-established animal models resembling OLP may limit the strengths of this study, we provided solid data to confirm that either inhibition of MMP12 activity or suppression of MMP12 expression can strengthen the integrity of oral epithelial barrier under inflammatory conditions in multiple cell co-culture systems. Targeting MMP12 is a potential therapeutic approach for OLP treatment. The limitations of this study include the lack of long-term (> 48 h) co-culture experiments, the small human sample size which might result in potential biases and heterogeneity between study groups and the lack of well-established animal models mimicking OLP. To this end, more investigators concerning large human sample size, long-term co-culture system and in vivo studies are required to guarantee that targeting MMP12 is qualified for OLP management.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Cheng, Y. S., Gould, A., Kurago, Z., Fantasia, J. & Muller, S. Diagnosis of oral lichen planus: A position paper of the American Academy of Oral and Maxillofacial Pathology. Oral surgery, oral medicine, oral pathology and oral radiology 122, 332–354. https://doi.org/10.1016/j.oooo.2016.05.004 (2016).
El-Howati, A., Thornhill, M. H., Colley, H. E. & Murdoch, C. Immune mechanisms in oral lichen planus. Oral Dis. 29, 1400–1415. https://doi.org/10.1111/odi.14142 (2023).
Gonzalez-Moles, M. A. et al. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 27, 813–828. https://doi.org/10.1111/odi.13323 (2021).
Kurago, Z. B. Etiology and pathogenesis of oral lichen planus: An overview. Oral surgery, oral medicine, oral pathology and oral radiology 122, 72–80. https://doi.org/10.1016/j.oooo.2016.03.011 (2016).
Carrozzo, M., Porter, S., Mercadante, V. & Fedele, S. Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontology 2000 80, 105–125. https://doi.org/10.1111/prd.12260 (2019).
Sugerman, P. B. et al. The pathogenesis of oral lichen planus. Crit. Reviews oral Biology Medicine: Official Publication Am. Association Oral Biologists 13, 350–365. https://doi.org/10.1177/154411130201300405 (2002).
Jungell, P., Konttinen, Y. T. & Malmstrom, M. Basement membrane changes in oral lichen planus. Proceedings of the Finnish Dental Society. Suomen Hammaslaakariseuran toimituksia 85, 119–124 (1989).
Zhou, X. J., Sugerman, P. B., Savage, N. W. & Walsh, L. J. Matrix metalloproteinases and their inhibitors in oral lichen planus. J. Cutan. Pathol. 28, 72–82. https://doi.org/10.1034/j.1600-0560.2001.280203.x (2001).
Bjornfot Holmstrom, S. et al. Gingival tissue inflammation promotes increased matrix metalloproteinase-12 production by CD200R(low) monocyte-derived cells in periodontitis. J. Immunol. 199, 4023–4035. https://doi.org/10.4049/jimmunol.1700672 (2017).
Minutti, C. M., Knipper, J. A., Allen, J. E. & Zaiss, D. M. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 61, 3–11. https://doi.org/10.1016/j.semcdb.2016.08.006 (2017).
Gronski, T. J. Jr. et al. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J. Biol. Chem. 272, 12189–12194. https://doi.org/10.1074/jbc.272.18.12189 (1997).
Shapiro, S. D., Kobayashi, D. K. & Ley, T. J. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J. Biol. Chem. 268, 23824–23829 (1993).
Lamort, A. S. et al. New insights into the substrate specificity of macrophage elastase MMP-12. Biol. Chem. 397, 469–484. https://doi.org/10.1515/hsz-2015-0254 (2016).
Churg, A. et al. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am. J. Respir. Crit Care Med. 167, 1083–1089. https://doi.org/10.1164/rccm.200212-1396OC (2003).
Shipley, J. M., Wesselschmidt, R. L., Kobayashi, D. K., Ley, T. J. & Shapiro, S. D. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl. Acad. Sci. U S A 93, 3942–3946. https://doi.org/10.1073/pnas.93.9.3942 (1996).
Molet, S et al. Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary disease. Inflamm. Research: Official J. Eur. Histamine Res. Society… et al 54, 31–36. https://doi.org/10.1007/s00011-004-1319-4 (2005).
Amor, M. et al. Identification of matrix metalloproteinase-12 as a candidate molecule for prevention and treatment of cardiometabolic disease. Mol. Med. 22, 487–496. https://doi.org/10.2119/molmed.2016.00068 (2016).
Coury, F. et al. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat. Med. 14, 81–87. https://doi.org/10.1038/nm1694 (2008).
Di Sabatino, A. et al. Stromelysin-1 and macrophage metalloelastase expression in the intestinal mucosa of Crohn’s disease patients treated with infliximab. Eur. J. Gastroenterol. Hepatol. 21, 1049–1055. https://doi.org/10.1097/MEG.0b013e3283293d0f (2009).
Lin, B. et al. The emerging role of MMP12 in the oral environment. Int. J. Mol. Sci. 24 https://doi.org/10.3390/ijms24054648 (2023).
van der Meij, E. H. & van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. oral Pathol. Medicine: Official Publication Int. Association Oral Pathologists Am. Acad. Oral Pathol. 32, 507–512. https://doi.org/10.1034/j.1600-0714.2003.00125.x (2003).
Cheng, Y. S. et al. Salivary endothelin-1 potential for detecting oral cancer in patients with oral lichen planus or oral cancer in remission. Oral Oncol. 47, 1122–1126. https://doi.org/10.1016/j.oraloncology.2011.07.032 (2011).
Ciurli, A. et al. Spatially resolved sampling of the human oral cavity for metabolic profiling. STAR. Protocols 2, 101002. https://doi.org/10.1016/j.xpro.2021.101002 (2021).
Yi, C. et al. Macrophage elastase (MMP12) critically contributes to the development of subretinal fibrosis. J. Neuroinflamm. 19 https://doi.org/10.1186/s12974-022-02433-x (2022).
Johnson, J. L. et al. A selective matrix metalloproteinase-12 inhibitor retards atherosclerotic plaque development in apolipoprotein E-knockout mice. Arterioscler. Thromb. Vasc. Biol. 31, 528–535. https://doi.org/10.1161/ATVBAHA.110.219147 (2011).
Jiang, Y. et al. ADAM-10 regulates MMP-12 during Lipopolysaccharide-Induced Inflammatory Response in macrophages. J. Immunol. Res. 3012218. https://doi.org/10.1155/2022/3012218 (2022).
Wang, X. et al. N(6)-methyladenosine of Spi2a attenuates inflammation and sepsis-associated myocardial dysfunction in mice. Nat. Commun. 14, 1185. https://doi.org/10.1038/s41467-023-36865-7 (2023).
Zhao, B. et al. Vitamin D/VDR signaling suppresses microRNA-802-induced apoptosis of keratinocytes in oral lichen planus. FASEB Journal: Official Publication Federation Am. Soc. Experimental Biology 33, 1042–1050. https://doi.org/10.1096/fj.201801020RRR (2019).
Zhang, M. et al. A STAT3 palmitoylation cycle promotes T(H)17 differentiation and colitis. Nature 586, 434–439. https://doi.org/10.1038/s41586-020-2799-2 (2020).
Cheadle, G. A. et al. Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PloS One 8, e69042. https://doi.org/10.1371/journal.pone.0069042 (2013).
Lees, M., Taylor, D. J. & Woolley, D. E. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases a and B. Eur. J. Biochem. 223, 171–177. https://doi.org/10.1111/j.1432-1033.1994.tb18980.x (1994).
Choi, Y. S. et al. The presence of bacteria within tissue provides insights into the pathogenesis of oral lichen planus. Sci. Rep. 6, 29186. https://doi.org/10.1038/srep29186 (2016).
Clark, R. A., Folkvord, J. M. & Wertz, R. L. Fibronectin, as well as other extracellular matrix proteins, mediate human keratinocyte adherence. J. Invest. Dermatol. 84, 378–383. https://doi.org/10.1111/1523-1747.ep12265466 (1985).
Acknowledgements
We thank Dr. Fang Zhang (Shanxi Medical University) and Dr. Yongfeng Cheng (Shanxi Medical University) for the diagnosis of OLP and healthy samples collection.
Funding
This work was supported by the Natural Science Foundation of Shanxi (202303021211127, 202103021224231, 202302020101009).
Author information
Authors and Affiliations
Contributions
X.G. and X.R. conceived and designed the research. Z.X. and X.W. performed the experiments. Z.X. collected human samples. X.W. analyzed data. X.G. supervised the patient research. X.G. wrote the manuscript. X.G. and X.W. acquired funding. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, Z., Wang, X., Ren, X. et al. MMP12 disrupts epithelial barrier integrity in oral lichen planus by degrading fibronectin. Sci Rep 14, 27511 (2024). https://doi.org/10.1038/s41598-024-78930-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78930-1