Abstract
Perinatal stem cells have prominent applications in cell therapy and regenerative medicine. Among them, human Wharton’s jelly mesenchymal stem/stromal cells (hWJMSCs) and human amniotic epithelial stem cells (hAESCs) have been widely used. However, the distinction in the therapeutic potential of hWJMSCs and hAESCs is poorly understood. In this study, we reported the phenotypic differences between these two distinct cell types and provided the first systematic comparison of their therapeutic potential in terms of immunomodulation, extracellular matrix (ECM) remodelling, angiogenesis and antioxidative stress using proteomics. The results revealed that the two cell types presented different protein expression profiles and were both promising candidates for cell therapy. Both types of cells demonstrated angiogenic and antifibrotic potential, whereas hAESCs presented superior immunological tolerance and antioxidant properties, which were supported by a series of relevant in vitro assays. Our study provides clues for the selection of appropriate cell types for diverse indications in cell therapy, which contributes to the advancement of their clinical translation and application.
Similar content being viewed by others
Introduction
In recent years, perinatal tissues, including the human amniotic membrane and umbilical cord, have received increasing attention as promising sources for regenerative medicine1,2. Perinatal stem cells are appealing candidates for future clinical applications because of the safety and ethical concerns associated with classical pluripotent stem cells, as well as the disadvantages of adult stem cells, such as their limited natural number, harvesting challenges, and restricted expansion potential3. However, the placenta and its accessory tissues are complex in composition; therefore, a wide range of perinatal stem cell types have been developed, including the most widely utilized mesenchymal cells, including human Wharton’s jelly mesenchymal stem/stromal cells (hWJMSCs), and epithelial cells, represented by human amniotic epithelial stem cells (hAESCs)4. Additionally, although many studies have documented the positive effects of hWJMSCs and hAESCs on the treatment of a variety of diseases, comparative studies on the characteristics of these two cell types and the differences in their protein profiles in terms of therapeutic potential are lacking.
Mesenchymal stem/stromal cells (MSCs) are found in different tissues, such as bone marrow, adipose tissue, Wharton’s jelly, amniotic fluid, and placenta5. The differentiation potential of these cells is prone to mesodermal cell types such as osteoblasts, adipocytes and chondrocytes. They have been investigated for the treatment of various diseases, with promising results in animal models and clinical trials6,7. MSCs derived from various sources may differ in their differentiation potential and patterns of gene expression. Among several types of MSCs, hWJMSCs have recently attracted great interest for allogeneic or autologous applications because of their low cost, easy handling, high yields and high proliferative capacity8. Furthermore, as foetus-derived cells, the low immunogenicity and safety of hWJMSCs have been confirmed by previous studies, demonstrating the significant potential of hWJMSCs for regenerative medicine applications.
Human amniotic epithelial stem cells (hAESCs) are a monolayer of epithelial cells in the innermost part of the amnion. The pluripotency of hAESCs was assumed because they developed from the ectoderm 2 weeks after gastrulation9. Therefore, hAESCs are different from hWJMSCs, which originate from the extraembryonic mesoderm on Day 13 of embryonic development10. Due to their nontumorigenicity, hAESCs can be utilized quite safely. Our previous study reported the safety of hAESCs, laying the foundation for subsequent preclinical trials11. hAESCs also exhibit immune privilege due to their unique expression profile of HLA antigens. In addition, hAESCs perform immunomodulatory functions, showing efficacy in treating autoimmune diseases such as Hashimoto’s thyroiditis, systemic lupus erythematosus and uveitis12,13. Considering the abundant number of cells and relatively easy cell isolation and culture operation, hAESCs are anticipated to serve as foundational cells for large-scale clinical applications in the future.
As neonatal cells, both hWJMSCs and hAESCs present advantages such as easy accessibility, an absence of ethical concerns, low immunogenicity and no DNA damage. Many studies have focused on the possible clinical applications of hWJMSCs and hAESCs. In this study, we compared the molecular characteristics and immunogenicity of hWJMSCs and hAESCs using flow cytometry. Furthermore, we first used proteomics to systematically compare the therapeutic potential of these two cell types in terms of immune regulation, angiogenesis, extracellular matrix (ECM) remodelling and antioxidative stress at the protein level. And a series of relevant in vitro assays were conducted. These differences may have significant implications for the judicious selection of hWJMSCs and hAESCs in future clinical studies.
Results
Characteristics of hAESCs and hWJMSCs
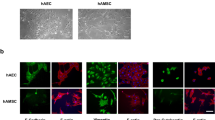
hAESCs and hWJMSCs were obtained from fresh amniotic membranes and the umbilical cord, respectively. As reported previously6,14, hWJMSCs presented a spindle-shaped appearance, whereas hAESCs showed a typical cobblestone-like epithelial appearance (Fig. 1A). Flow cytometry results revealed no expression of the endothelial marker CD31 or the haematopoietic lineage markers CD45 and CD34, indicating that the isolated cells were not contaminated with other cell types (Fig. 1B). As expected, the immunofluorescence and flow cytometry results revealed that hAESCs expressed high levels of the classical epithelial-specific markers pan-cytokeratin and E-cadherin (CD324), with more than 98% of hAESCs expressing CD324 and CD326 (Ep-CAM), whereas hWJMSCs were negative for all epithelial cell markers (Fig. 1C-D). Moreover, hWJMSCs highly expressed the classical mesenchymal markers CD90, CD73 and CD105 (more than 99%). In addition, hAESCs express some mesenchymal stromal cell- and mesenchymal cell-associated antigens15, and flow cytometry results revealed that almost 38% and 99% of the hAESCs in this study expressed CD90 and CD73, respectively, and did not express CD105 (Fig. 1E). A heatmap of the expression of mesenchymal and epithelial markers in the proteomes of hAESCs and hWJMSCs corroborated the distinction between them (Figure S1). The above results suggested that hAESCs and hWJMSCs are two completely different cell types with disparate phenotypic and biological characteristics.
Characteristics of hAESCs and hWJMSCs. (A) Phase-contrast microscopy images showing the morphologies of hAESCs and hWJMSCs. (B) Flow cytometry results for the haematopoietic markers CD34 and CD45 and the endothelial marker CD31. (C-D) Representative images of immunofluorescence staining and flow cytometry results for the epithelial markers pancytokeratin, CD324 (E–cadherin) and CD326 (E–pcam). (E) The MSC markers CD73, CD90, and CD105 were detected by flow cytometry. Scale bars: 100 μm.
The immunogenicity of hAESCs and hWJMSCs
Although hAESCs and hWJMSCs differ in morphology and marker expression, previous reports reported that both types of cells have low immunogenicity because they originate from perinatal tissues16. The expression profile of human leukocyte antigen (HLA) molecules explains the mechanism of low immunogenicity17. Among all HLA antigens, classical HLA-I (HLA-A, -B and -C) and HLA-II (-DR, -DQ, and -DP) molecules contribute to immune recognition and rejection during allografting, whereas nonclassical HLA-I molecules (HLA-G and HLA-E) display limited polymorphisms and create an immune-tolerant environment to improve the survival of allografts after transplantation9. The flow cytometry results revealed high expression levels of HLA class I molecules (HLA-ABC) on both the hAESC and hWJMSC surfaces, which were increased in the presence of interferon-γ (IFN-γ). In response to IFN-γ stimulation, hWJMSCs did not express HLA-II molecules (HLA-DQ), whereas more than half of the hWJMSCs exhibited significantly increased expression of HLA-DR. In addition, the nonclassical MHC class molecule HLA-G was not detected, whereas low HLA-E expression was detected in hWJMSCs after IFN-γ treatment (Fig. 2A, C). In contrast, in hAESCs, HLA-DR and HLA-DQ were not expressed, regardless of IFN-γ treatment. Notably, the HLA-G expression level was very high in hAESCs before and after IFN-γ treatment (percentage over 90%), and the expression of HLA-E was detected in IFN-γ-treated hAESCs (Fig. 2B, D).
The immune privilege of hAESCs and hWJMSCs. Flow cytometry analysis of HLA-ABC, HLA-DQ, HLA-DR, HLA-G and HLA-E in hWJMSCs and hAESCs cultured in normal culture medium (A, C) or with 10 ng/mL IFN–γ for 72 h (B, D). Representative histograms of HLA antigens are shown in red, and the isotype controls are shown in blue.
These results confirmed the lack of expression of type II HLA markers on both types of cells, as well as the expression of immune-privileged HLA-G on hAESCs. This evidence suggested the potential immunomodulatory capabilities of both types of cells in transplantation, which may allow them to cross major histocompatibility barriers. In particular, hAESCs may be more advantageous in terms of immune tolerance following transplantation.
Characterization of differentially expressed proteins between hAESCs and hWJMSCs
Three biological replicates of hAESCs and hWJMSCs were sequenced with TMT-labelled quantitative proteomics, and their proteomic profiles were analysed to comprehensively understand the differences and similarities between hAESCs and hWJMSCs. Principal component (PCA) analysis was conducted using the expression levels of credible proteins. The results revealed distinct clustering of the hAESC and hWJMSC samples, with significant separation between the two cell types (Fig. 3A). A correlation analysis of the identified proteins between samples revealed good reproducibility across the groups (Fig. 3B). In total, 6693 proteins were identified. Fold changes ≥ 2.0-fold and p values < 0.05 were selected as differential protein screening conditions. The differentially expressed proteins are displayed as normalized heatmap and volcano plots (Fig. 3C, D). A total of 430 significantly differentially expressed proteins were identified, including 205 upregulated proteins (Table S1) and 225 downregulated proteins (Table S2). Figure 3E shows the distribution of multiple changes in the differentially expressed proteins. The majority of the significantly differentially expressed proteins (60%) presented fold changes of 2–3, with 32 proteins having fold changes greater than 6. In terms of the location of the proteins, 33% were from the cytoplasm, 25% were from the nucleus, 36% were from the plasma membrane, and 6% were from the extracellular space (Fig. 3F). These findings indicate that hAESCs and hWJMSCs are completely different cell types with great differences in protein expression profiles.
Characterization of the protein expression profiles of hAESCs and hWJMSCs. (A) Principal component analysis (PCA) of hAESCs and hWJMSCs. (B) Sample correlation analysis of hAESCs and hWJMSCs. (C-D) Heatmaps and volcano plots display differentially expressed proteins between hAESCs and hWJMSCs. (E) Distribution of the fold changes in downregulated genes (green) and upregulated genes (red) in hAESCs. (F) The cellular locations of significantly differentially expressed proteins were characterized.
Next, GO/KEGG enrichment analyses were performed on the differentially expressed proteins to determine their functions. hWJMSCs were significantly enriched in biological process (BP) terms such as “extracellular matrix (ECM) organization”, “collagen fibril organization”, “cell adhesion” and “angiogenesis”. The significantly enriched molecular functions (MFs) included, but were not limited to, “vascular endothelial growth factor receptor 2 binding”, “platelet-derived growth factor binding”, “extracellular matrix structural components that confer tensile strength”, “actin binding” and “collagen binding” (Fig. 4A). The gene set enrichment analysis (GSEA) of hWJMSCs also revealed enrichment in “ossification”, “mesoderm formation”, “collagen fibril organization”, and “chondroitin surface biosynthetic process” (Figure S2). Compared with those in hWJMSCs, the significantly enriched BP terms for differentially expressed genes in hAESCs included “keratinization”, “cell–cell adhesion”, “epidermis development” and “hemidesmosome assembly”. The significantly enriched MFs of the proteins included “calcium binding”, “cadherin binding” and “cadherin binding involved in cell‒cell adhesion” (Fig. 4B). These results were corroborated by the GSEA (Figure S3). These results suggested that hAESCs were significantly enriched in epithelial-related functions and components due to their epithelial properties, whereas hWJMSCs exhibited mesenchymal origin, osteogenic differentiation and collagen-rich properties.
Functional enrichment analysis of differentially expressed proteins. (A) GO terms significantly enriched with downregulated DEPs. (B) GO terms significantly enriched with upregulated DEPs. (C) KEGG43 enrichment analysis of the top 20 proteins (downregulated). (D) KEGG enrichment analysis of the top 20 proteins (upregulated).
Furthermore, we observed that hWJMSCs were enriched mainly in “protein digestion and absorption”, “leukocyte transendothelial cell migration”, “platelet activation”, “HIF-1 signalling pathway”, “TNF signalling pathway” and other pathways (Fig. 4C). Similar findings were obtained using GSEA, including “cholesterol metabolism” and “cortisol synthesis and secretion” (Figure S4A-C). Compared with hWJMSCs, hAESCs were enriched mainly in “regulation of the actin cytoskeleton”, “tight junction”, “P53 signalling pathway”, and “oestrogen signalling pathway” (Fig. 4D). Interestingly, in the GSEA, hAESCs were enriched in the “neurotrophin signalling pathway” (Figure S4D-F). The above results suggested that hWJMSCs were more enriched in inflammation-related pathways, whereas hAESCs might be more enriched in oestrogen signalling pathways and neurotrophin-related pathways.
Therapeutic potential of hAESCs and hWJMSCs
As perinatal stem cells, hAESCs and hWJMSCs play promising roles in animal models of various diseases. However, the differences in their therapeutic potential remain unclear. Therefore, we conducted a systematic comparison of the differences in protein expression between these two cell types in terms of immunomodulation, ECM remodelling, angiogenesis and oxidation resistance. The results revealed that hWJMSCs highly expressed the chemotactic adhesion-related proteins ICAM1 and CXCL1, as well as the inflammation-related proteins IL6ST, TGFB1, IL17RA and PLAU. hAESCs expressed CD59, MIF, AXL and FSTL1 at high levels, and these proteins play roles in immunosuppression and immune tolerance18,19,20. Interestingly, hAESCs highly expressed IGF2, a foetal growth hormone, which has potent anti-inflammatory properties21. In contrast, the immune tolerance protein CD274 (PDL1) was expressed at comparable levels in both groups (Fig. 5A). The above results indicated that hWJMSCs were more chemotactic, while hAESCs expressed more immunosuppressive proteins. Furthermore, the in vitro analysis demonstrated that co-culture with hAESCs or hWJMSCs effectively downregulated the mRNA expression of proinflammatory factors (IL-1β, IL-6, and TNF-α) in lipopolysaccharide (LPS)-induced RAW264.7 macrophages, especially in the hAESC group (Fig. 6A).
In vitro assays of the therapeutic potential of hAESCs and hWJMSCs. (A) The mRNA expression levels of proinflammatory genes (TNFα, IL-1β and IL-6) in 1 µg/ml LPS-induced RAW264.7 macrophages. (B) The mRNA expression levels of fibrosis-related genes (α-SMA, COL1, CTGF and FN1) in myofibroblasts following 48 h of TGF-β1 treatment, smooth muscle actin (α-SMA), collagen I (COL1), connective tissue growth factor (CTGF), fibronectin 1 (FN1). (C) Representative images of tubes formed on Matrigel, scale bars: 100 μm, quantitative results are shown in (D). (E) Phase-contrast microscopy images of HUVECs showing their migration ability, scale bars: 100 μm, quantitative results are shown in (F). N = 3 independent experiments. Data are mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 vs. control, ns: not significant, one-way ANOVA, Tukey’s multiple comparisons test.
In terms of ECM remodelling-related proteins, hWJMSCs expressed high levels of the matrix metalloproteinases MMP2 and MMP14 and the matrix metalloproteinase inhibitors TIMP1 and TIMP2. Moreover, high levels of extracellular matrix degradation-related proteins, such as histonectin L (CTSL) and CTSZ, were also detected in hWJMSCs. In contrast, MMP9 and TIMP3, and the histone proteases CTSF, CTSD and CTSB were expressed at relatively high levels in hAESCs (Fig. 5B). In addition, we further examined the functional effects of hAESCs and hWJMSCs on ECM remodelling in vitro. Human fibroblasts IMR-90 were stimulated to differentiate into myofibroblasts after treatment with TGF-β1, which were then co-cultured with hAESCs or hWJMSCs. The mRNA expression of most fibrosis-related genes, such as α-SMA, COL1, FN1, and CTGF, was significantly decreased in the treated myofibroblast groups (Fig. 6B). These results suggest that both cell types have potential for ECM remodelling and antifibrotic activity, but the effective molecules responsible for extracellular matrix remodelling and antifibrotic activity may differ between them.
Angiogenesis is another major therapeutic function of both cell types. We found that platelet-derived growth factor receptor α (PDGFRA), vasorin (VASN), IGFBP4/5/7, EDIL3 (which promotes endothelial cell adhesion22) and TNFRSF12A were highly expressed in hWJMSCs. hAESCs presented increased levels of EFNB2 (which regulates cell adhesion and cell migration23), IGFBP3, DKK3, WNT5A and other angiogenesis-related proteins (Fig. 5C). These results showed that both hAESCs and hWJMSCs have angiogenic potential. Moreover, further investigations were performed using tube formation assays by treating human primary endothelial cells co-cultured with hAESCs or hWJMSCs. The results from the Matrigel tube formation assay revealed that tube length and branches assessed after 6 h were significantly greater in the co-culture treated HUVECs than in the untreated control HUVECs (Fig. 6C-D).
Several studies have shown that hMSCs and hAESCs are highly resistant to oxidative damage24,25,26,27. Our proteomic results revealed that both cell types expressed high levels of antioxidants, including the constitutively expressed antioxidant enzymes SOD, catalase (CAT), glutathione peroxidases (GPXs) and glutathione (GSH). In hWJMSCs, glutathione peroxidases (GPX1, GPX4, and GPX8), CAT, thioredoxin reductase 1 (TXNRD1) and glutathione transferase M2 (GSTM2) were expressed at relatively high levels. Furthermore, hAESCs presented increased levels of the antioxidant enzymes SOD1 and GPX7, prostaglandin E synthase (PTGES) and glutathione S-transferases (GSTP1, GSTK1, and LANCL1). Moreover, thiol-specific peroxidase PRDX5,6, reduced protein metallothionein-1X (MT1X) and thioredoxin (TXN), which have detoxification effects, were expressed at higher levels in hAESCs (Fig. 5D). These results indicated that both cell types have the potential to protect against oxidative stress, while hAESCs are more resistant to oxidative stress and have a greater detoxification capacity. Furthermore, we investigated whether hAESCs and hWJMSCs exerted direct cytoprotective effects. Scratch assays of HUVECs were performed to assess wound healing in vitro. Co-culture with hAESCs or hWJMSCs significantly improved cell viability, particularly in the hAESC group (Fig. 6E-F).
In conclusion, our study suggested that both hAESCs and hWJMSCs are promising candidates for cell therapy. The protein expression profiles of the two cell types are significantly different, especially in terms of immunomodulation, ECM remodelling, angiogenesis and antioxidants associated with therapeutic functions. We found that hAESCs expressed higher levels of nonclassical HLA-I molecules (-E, and -G), indicating their superiority in immune tolerance. In addition, as distinct perinatal stem cell types from different sources, hWJMSCs presented greater mesodermal differentiation and developmental characteristics and stronger angiogenesis-related properties, whereas hAESCs presented greater ectodermal differentiation and developmental properties that were interestingly associated with neural pathways. In terms of the therapeutic potential and direction of these two cell types, hWJMSCs have emerged as superior at chemotaxis, whereas hAESCs have stronger immunosuppressive and antioxidative stress effects. A series of relevant in vitro assays were conducted to further support the proteomic data. In summary, this study provides a comprehensive comparison of the differences and advantages of hWJMSCs and hAESCs in terms of their therapeutic potential from the perspective of protein expression profiles. These insights provide valuable cues for the judicious selection of more appropriate cell types for different indications in future applications.
Discussion
Human Wharton’s jelly mesenchymal stem/stromal cells (hWJMSCs) and human amniotic epithelial stem cells (hAESCs) have been investigated for the treatment of various diseases, with many promising results obtained in both animal models and clinical trials28,29. Given the diverse sources of mesenchymal stem cells (MSCs), we specifically chose hWJMSCs, a perinatal tissue source widely used in research, to systematically compare the phenotypic and therapeutic distinctions between hAESCs and hWJMSCs. Our study confirmed the expression of mesenchymal markers, including CD73, CD90 and CD105, in hWJMSCs. In contrast, hAESCs originate from the pluripotent epiblast at approximately 8 days after fertilization and exhibit a typical epithelial cell phenotype, expressing epithelial-specific markers, including pancytokeratin (CK), EpCAM (CD326) and E-cadherin (CD324). The differentiation potential of hAESCs and hWJMSCs allows them to be considered sources for cell replacement therapy. However, despite the promising results in many disease models, little evidence has shown that hAESCs and hWJMSCs are able to differentiate and integrate into host tissues30. Therefore, we focused on comparing the differences between hAESCs and hWJMSCs in terms of immunogenicity, immunomodulation, ECM remodelling, angiogenesis and oxidative stress resistance.
A major challenge in cell therapy is graft rejection. Both hAESCs and hWJMSCs are derived from perinatal tissues, and hAESCs and hWJMSCs exhibit negligible expression of HLA-II molecules, including HLA-DR, DP and DQ31. Other evidence has shown that inflammatory molecules (such as interferon-γ) can induce high expression of HLA-II in mesenchymal stem cells29. Due to their high polymorphism, HLA-II molecules, particularly HLA-DR and HLA-DQ, contribute to immune recognition and rejection during allogeneic transplantation17. On the other hand, nonclassical HLA-G and HLA-E molecules play key roles in maternal immune regulation, potentially establishing an immune-tolerant environment by regulating the immune response of regulatory T cells and NK cells to improve allograft survival after transplantation9. Our results showed that after IFN-γ stimulation, hWJMSCs significantly expressed HLA-DR but not HLA-DQ or immune-privileged molecules (HLA-G and HLA-E). Interestingly, hAESCs did not express HLA-DQ or HLA-DR after IFN-γ stimulation. However, hAESCs presented high expression of HLA-G and modest HLA-E expression, indicating the lower immunogenicity of hAESCs, which may enable them to overcome major histocompatibility barriers. This finding is similar to that of other epithelial cells, which have greater immunomodulatory potential than do MSCs32.
hAESC- and hWJMSC-based therapies have been widely used to treat a variety of inflammatory diseases because of their powerful immunomodulatory functions17,33. Despite differences in morphology and gene expression between hAESCs and hWJMSCs, these cells may have a similar potential to modulate immune responses. Previous studies have identified key regulators of the immunosuppressive effects of both hAESCs and hWJMSCs, including the intracellular enzyme indoleamine 2,3-dioxygenase (IDO), which is expressed in response to inflammatory stimuli9,34. MSCs express a range of chemokines and receptors to sense signals from surrounding tissues, adapting to different pathological conditions. Our results demonstrated that hWJMSCs highly expressed the chemotactic adhesion-related proteins ICAM1 and CXCL1, with ICAM1 significantly promoting the migration of MSCs to inflamed tissues. In contrast, hAESCs expressed high levels of immunosuppressive and immune tolerance-related proteins, such as CD59, MIF and CD276. These results suggested that hWJMSCs were more chemotactic, whereas hAESCs expressed more immunosuppressive proteins.
Among several tissues of origin, the hWJMSC secretome exhibits a more comprehensive angiogenic network with higher concentrations of angiogenesis-related proteins35. In this study, the differential protein enrichment analysis between hAESCs and hWJMSCs revealed that some angiogenic proteins were enriched in hWJMSCs, while some angiogenic proteins were enriched in hAESCs, such as PDGFRB, EFNB2, IGFBP3, DKK3, and WNT5A, suggesting that both cell types possess angiogenic potential.
Oxidative stress, which is characterized by cellular damage, inflammation and metabolic dysregulation, is associated with numerous pathologies. Increasing evidence supports the antioxidant properties of hMSCs and hAESCs in animal models of various diseases, potentially explaining their cytoprotective and anti-inflammatory properties36. Cells rely on enzymatic antioxidants, including SOD, CAT, GPx, and small nonenzymatic antioxidants such as GSH to maintain redox homeostasis and prevent excessive production of free radicals24. Our study revealed the expression of multiple antioxidants in both cell types, suggesting their ability to upregulate host antioxidant defences to mitigate oxidative stress. In addition, hAESCs expressed more reducing proteins with detoxification functions, suggesting a potentially stronger antioxidant capacity. Considering that oxidative stress is associated with nearly every disease, these antioxidant properties could explain why hAESCs and hWJMSCs therapies are useful for this seemingly unrelated pathology.
In addition, our study has several limitations that need to be discussed. First, accumulating evidence suggests that both cell types primarily exert their therapeutic effects through paracrine actions33,37,38. Differences in the secretion profiles and protein expression patterns may exist. Subsequent investigations should further analyse the differences in the secretory profiles of the two cell types, comparing them with the proteome, to gain a more comprehensive understanding of their therapeutic potential. Second, previous studies have shown that the properties of hAESCs can be influenced by several sample collection parameters, such as differences between pregnant women, placental quality, the region of the placenta and stage of pregnancy39,40,41. Different samples from different pregnant women, different gestational ages, and different foetal sexes may lead to differences in protein expression profiles among samples of the same cell type. The differences between samples can be reduced by increasing the sample size and controlling for consistent sampling conditions. Finally, our results suggest that both cell types have the potential for immunomodulation, ECM remodelling, angiogenesis and inhibition of oxidative damage, but the effector molecules involved in immunomodulation, ECM remodelling, angiogenesis and inhibition of oxidative damage may differ. For example, hAESCs express high levels of proteins with detoxification effects (PRDX5,6, MT1X and TXN), while these proteins are expressed at low levels in hWJMSCs. These differences may affect their combined therapeutic potential. Moreover, the differences in the therapeutic potential for immunomodulation, angiogenesis and antioxidative stress need to be further compared through cellular and animal experiments. This step is crucial to conclusively determine the therapeutic properties of the two cell types, laying the groundwork for selecting cell therapy types for diverse indications in the future.
Methods
Ethics statement
All methods were performed in accordance with relevant guidelines and regulations. This study complies with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of the International Peace Maternity and Child Health Hospital (No. 201411, Approval date: 27/10/2014). Written consent forms were signed by the participating subjects.
Isolation and culture of hAESCs
After written informed consent was provided by the donors, samples of human amnion membranes were obtained from healthy mothers who were confirmed to be negative for hepatitis A, B, C and D and HIV-I and Treponema pallidum(TPAB) antibodies, as described in our previous study42. Briefly, amniotic membranes were mechanically isolated from the placenta and immersed in PBS buffer to remove the remaining blood clots. Then, the amniotic membranes were digested with 0.25% trypsin at 37 °C for 20 min. After the membranes were removed, hAESCs were collected by centrifugation for 10 min. For cell culture, complete culture medium was prepared with DMEM/F12 containing 10% knockout serum replacement (KSR), 1% antibiotic–antimycotic, 1 mM sodium pyruvate, 1% nonessential amino acids, 2 mM L–glutamine (Thermo Fisher Scientific, Grand Island, NY, USA) and 10 ng/mL human EGF (Peprotech, Rocky Hill, NJ, USA). hAESCs were incubated in an atmosphere of 5.5% CO2 at 37 °C for 3 to 5 days until the cells attached to and filled the dish.
Isolation and culture of hWJMSCs
Human umbilical cords were obtained after full-term birth, and written informed consent was provided by the donors. The procedure was approved by the Institutional Patient and Ethics Committee of the International Peace Maternal and Child Health Hospital, Shanghai Jiao Tong University. The umbilical cords were washed to remove blood and clots and cut into 1.5 cm long pieces to isolate cells from Wharton’s jelly (WJ). Each piece was then cut longitudinally to remove the umbilical artery, vein and umbilical cord epithelium to obtain WJ. The remaining gelatinous tissue around the vessels was collected and crushed into small pieces (1 mm2). After the tissue was allowed to adhere to the culture plate, standard proliferation medium was gently added. After the first cellular outgrowth was detected, the tissue blocks were removed, and the cells were harvested for subsequent passaging. The hWJMSCs were isolated and expanded in an incubator at 37 °C with 5% CO2in MEMα supplemented with 10% FBS, and a 1% antibiotic–antimycotic mixture (Thermo Fisher Scientific, Grand Island, NY, USA), as described in a previous study35.
Flow cytometry
Multiple cell markers (CD31, Invitrogen, Cat# 11-0319-42; CD34, Invitrogen, Cat# 12-0349-41; CD45, Invitrogen, Cat# 11-0459-41; CD324, BioLegend, Cat# 324105; CD326, BioLegend, Cat# 324206; CD90, Invitrogen, Cat# 11-0909-42; CD73, Invitrogen, Cat# 11-0739-41; and CD105, BioLegend, Cat# 800503) were detected via flow cytometry to determine the basic characteristics of hWJMSCs and hAESCs. hWJMSCs and hAESCs were collected after an incubation with 10 ng/mL IFN-γ (Peprotech, Cat# 300-02-100) for 72 h to determine immunogenicity. Then, they were stained with anti-HLA-ABC (Invitrogen, Cat# 11-9983-41), anti-HLA-DR (BioLegend, Cat# 307604), anti-HLA-DQ (BioLegend, Cat# 318104), anti-HLA-G (BioLegend, Cat# 335909), anti-HLA-E (BioLegend, Cat# 342603) and relevant isotype control antibodies according to the manufacturer’s instructions and analysed by flow cytometry (FACS Calibur; BD Biosciences, Franklin Lakes, NJ). Analyses were performed using three biological replicates.
Immunostaining
After fixation with 4% paraformaldehyde in PBS for 15 min, the cells were permeabilized with 0.25% Triton X-100 in PBS for 5–10 min and blocked for 60 min with 5% goat serum. The cells were subsequently incubated for 60 min at room temperature with the following primary antibodies: anti-pancytokeratin antibody (Abcam, ab7753) and anti-E-cadherin antibody (Abcam, ab40772). The cells were then incubated for 2 h at room temperature with the corresponding secondary antibodies: Alexa Fluor 594-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch, Cat# 715-586-150) and Alexa Fluor 488-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch, Cat# 711-546-152). Fluorescence images were acquired with a confocal microscope (Zeiss LSM 800, Carl Zeiss).
Liquid chromatography and tandem mass spectrometry (LC–MS/MS)
The cell samples were divided into two groups, hWJMSCs and hAESCs, with three biological replicates (n = 3). All the cells were seeded in culture dishes overnight and cultured for another 48 h before harvest. The culture media were discarded, and the dishes were placed on absorbent paper to drain the medium. The cell surface was subsequently washed with precooled PBS solution 2–3 times to wash away the culture medium. PBS that had been precooled at 4 °C was added to the Petri dish. The dish was gently shaken, and the cells were scraped off with a clean cell scraper (quickly). A pipette was used to transfer the cells into a precooled centrifuge tube. After centrifugation at 300 × g for 5 min, cell pellet was obtained, and the supernatant was removed. Total protein was subsequently extracted in radioimmunoprecipitation assay (RIPA) buffer (Solarbio Life Science, China) supplemented with a protease inhibitor on ice-cold plates. Specifically, 300 L of RIPA working mixture was added, mixed thoroughly, steel balls were added, the samples were ground at a low temperature of 45 Hz for 4 min and the samples were centrifuged at 4 °C for 10 min at 12000 rpm; the samples were ultrasonicated in an ice water bath with a cell crusher for 20 min to fully crack the samples. The mixture was centrifuged at 12000 rpm for 10 min at 4 °C, and the supernatant was transferred to a new EP tube. Then, part of each sample was used to determine the protein concentration (Enhanced BCA Protein Assay Kit, Beyotime, China). The products were subjected to liquid chromatography‒tandem mass spectrometry (LC‒MS/MS) analysis. TMT was performed and analysed by Luming Biotechnology (Shanghai, China).
Quantitative real-time RT‒PCR
The total RNA was extracted with a Super FastPure Cell RNA Isolation Kit (Vazyme, Nanjing, China) as described in the manufacturer’s instructions. One microgram of RNA was subjected to cDNA synthesis with HiScript III RT SuperMix for qPCR (Vazyme, Nanjing, China) and ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). Quantitative real-time PCR was performed with the Bio-Rad iCycler real-time PCR detection system (Bio-Rad, Hercules, CA, USA) and the primers listed in Supplementary Tables 3, and the analysis was performed in triplicate in at least three independent experiments. Fold changes in the expression level of each gene were calculated using CT values. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize expression levels.
Immunomodulation assay
RAW264.7 cells (ATCC) were seeded in a 6-well plate at a density of 1 × 106 cells/well and incubated overnight. Then, 1 µg/mL lipopolysaccharide (LPS) (Sigma) was used to induce macrophage polarization for 24 h with or without co-culture with hAESCs or hWJMSCs. Then, RAW264.7 cells were collected for a qPCR analysis of proinflammatory factors, including IL-β, IL-6 and TNF-α, according to the manufacturer’s protocol (Lianke Bio). All primer sequences are presented in Supplementary Table 3.
Fibroblast activation assay
Human fibroblast IMR90 (Meisen Cell, Zhejiang, China) were starved overnight at 70% confluency in serum-free medium. The differentiation of myofibroblasts was stimulated with 10 ng/ml TGF-β1 (Abclonal, Wuhan, China), and then the cells were co-cultured with hAESCs or hWJMSCs. Following culture for 24 h, the cells were collected for qPCR (for α-SMA, COL1, CTGF and FN1). All primer sequences are presented in Supplementary Table 3.
Human endothelial cell tube formation assay
The basement membrane matrix Matrigel (Corning, Cat# 354277) was added to a 24-well plate and solidified by incubation at 37 °C for 30 min. Human umbilical vein endothelial cells (HUVECs) (4 × 104 cells per well) were plated onto a 24-well plate containing Matrigel with EGM-2 medium (Lonza, Cat# CC-3156) and incubated at 37 °C for 6 h in a humidified incubator. The experimental groups were co-cultured with hAESCs or hWJMSCs, and the tube structures were evaluated via microscopy. Tube formation was quantified by measuring the number of branches produced.
Wound healing assay
Endothelial cells (ECs) were seeded onto 6-well plates (10 × 104 cells/well) with EGM-2 medium and grown to confluence. A straight line was then scratched with a 1-mL pipette tip to conduct a “wound healing” assay. Subsequently, the experimental groups were co-cultured with hAESCs or hWJMSCs, and the migration area was digitally photographed at 0 and 24 h; the area of the cell-free gap was calculated using ImageJ (version 6.0, NIH). The extent of wound healing (% closure) was determined and reported as a percentage of closure relative to the initial wound size at 0 h.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. The mass spectrometry proteomic data have been deposited in the Proteome Xchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD044276 (https://www.iprox.cn//page/project.html? id=IPX0006849000).
References
Deus, I. A., Mano, J. F. & Custodio, C. A. Perinatal tissues and cells in tissue engineering and regenerative medicine. Acta Biomater. 110, 1–14 (2020).
Farmer, D. Placental stem cells: the promise of curing diseases before birth. Placenta. 59, 113–115 (2017).
Antoniadou, E. & David, A. L. Placental stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 31, 13–29 (2016).
Parolini, O. et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on Placenta derived stem cells. Stem Cells. 26, 300–311 (2008).
da Silva Meirelles, L., Chagastelles, P. C. & Nardi, N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell. Sci. 119, 2204–2213 (2006).
Abbaszadeh, H. et al. Regenerative potential of Wharton’s jelly-derived mesenchymal stem cells: a new horizon of stem cell therapy. J. Cell. Physiol. 235, 9230–9240 (2020).
Naji, A. et al. Biological functions of mesenchymal stem cells and clinical implications. Cell. Mol. Life Sci. 76, 3323–3348 (2019).
Can, A. & Karahuseyinoglu, S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 25, 2886–2895 (2007).
Miki, T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am. J. Reprod. Immunol. 80, e13003 (2018).
Karahuseyinoglu, S. et al. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 25, 319–331 (2007).
Yang, P. J. et al. Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation. Acta Pharmacol. Sin. 39, 1305–1316 (2018).
Li, J. et al. Subretinal Transplantation of Human Amniotic Epithelial Cells in the treatment of Autoimmune Uveitis in rats. Cell. Transpl. 27, 1504–1514 (2018).
Tan, B. et al. Therapeutic effect of human amniotic epithelial cells in murine models of Hashimoto’s thyroiditis and systemic lupus erythematosus. Cytotherapy. 20, 1247–1258 (2018).
Li, J. et al. Human amniotic epithelial stem cell-derived retinal pigment epithelium cells repair retinal degeneration. Front. Cell. Dev. Biol. 9, 737242 (2021).
Koike, C. et al. Characterization of amniotic stem cells. Cell. Reprogram. 16, 298–305 (2014).
Ghamari, S. H., Abbasi-Kangevari, M., Tayebi, T., Bahrami, S. & Niknejad, H. The bottlenecks in translating placenta-derived amniotic epithelial and mesenchymal stromal cells into the clinic: current discrepancies in marker reports. Front. Bioeng. Biotechnol. 8, 180 (2020).
Qiu, C., Ge, Z., Cui, W., Yu, L. & Li, J. Human amniotic epithelial stem cells: a promising seed cell for clinical applications. Int. J. Mol. Sci. 21,7730 (2020).
Rosado, J. D. & Rodriguez-Sosa, M. Macrophage Migration Inhibitory factor (MIF): a key player in Protozoan infections. Int. J. Biol. Sci. 7, 1239–1256 (2011).
Ito, T. et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J. Invest. Dermatol. 128, 1196–1206 (2008).
Cheng, K. Y. et al. Follistatin-like protein 1 suppressed pro-inflammatory cytokines expression during neuroinflammation induced by lipopolysaccharide. J. Mol. Histol. 48, 63–72 (2017).
Du, L. et al. IGF-2 Preprograms Maturing macrophages to acquire oxidative phosphorylation-dependent anti-inflammatory properties. Cell. Metab. 29, 1363–1375e1368 (2019).
Niu, X. et al. EDIL3 influenced the αvβ3-FAK/MEK/ERK axis of endothelial cells in psoriasis. J. Cell. Mol. Med. 26, 5202–5212 (2022).
Gong, T. et al. EphrinB2/EphB4 signaling regulates DPSCs to induce sprouting angiogenesis of endothelial cells. J. Dent. Res. 98, 803–812 (2019).
Stavely, R. & Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med. 9, 985–1006 (2020).
Valle-Prieto, A. & Conget, P. A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem. Cells. Dev. 19, 1885–1893 (2010).
Han, L. G. et al. Differential response of immortalized human amnion mesenchymal and epithelial cells against oxidative stress. Free Radical Bio Med. 135, 79–86 (2019).
Zhang, J. et al. Human amniotic epithelial cells alleviate a mouse model of Parkinson’s Disease mainly by Neuroprotective, anti-oxidative and anti-inflammatory factors. J. Neuroimmune Pharmacol. 16, 620–633 (2021).
Silini, A. R., Masserdotti, A., Papait, A. & Parolini, O. Shaping the future of Perinatal cells: lessons from the past and interpretations of the Present. Front. Bioeng. Biotechnol. 7, 75 (2019).
Zhou, T. et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 14, 24 (2021).
Munoz-Perez, E., Gonzalez-Pujana, A., Igartua, M., Santos-Vizcaino, E. & Hernandez, R. M. Mesenchymal Stromal Cell Secretome for the Treatment of Immune-Mediated Inflammatory Diseases: Latest Trends in Isolation, Content Optimization and Delivery Avenues. Pharmaceutics 13, 1802 (2021).
Torre, P. & Flores, A. I. Current status and future prospects of perinatal stem cells. Genes (Basel) 12, 6 (2020).
Gauthier, B. R. et al., Human Omental Mesothelial Cells Impart an Immunomodulatory Landscape Impeding B- and T-Cell Activation. Int. J. Mol. Sci. 23, 5942 (2022).
Han, Y. Y. et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal. Transduct. Tar 7, 92 (2022).
Mounayar, M. et al. PI3kalpha and STAT1 interplay regulates human mesenchymal stem cell Immune polarization. Stem Cells. 33, 1892–1901 (2015).
Kehl, D. et al. Proteomic analysis of human mesenchymal stromal cell secretomes: a systematic comparison of the angiogenic potential. NPJ Regen Med. 4, 8 (2019).
Xia, C., Dai, Z., Jin, Y. & Chen, P. Emerging antioxidant paradigm of mesenchymal stem cell-derived exosome therapy. Front. Endocrinol. (Lausanne). 12, 727272 (2021).
Shin, S. et al. Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton’s Jelly. Int. J. Mol. Sci. 22, 845 (2021).
Pires, A. O. et al. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: a proteomic analysis. Stem. Cells. Dev. 25, 1073–1083 (2016).
Naeem, A. et al. A Survey and critical evaluation of isolation, culture, and Cryopreservation methods of human amniotic epithelial cells. Cell. Cycle. 21, 655–673 (2022).
Banerjee, A. et al. Different metabolic activity in placental and reflected regions of the human amniotic membrane. Placenta. 36, 1329–1332 (2015).
Barboni, B. et al. Gestational stage affects amniotic epithelial cells phenotype, methylation status, immunomodulatory and stemness properties. Stem Cell. Reviews Rep. 10, 725–741 (2014).
Li, J. et al. Repair of Retinal Degeneration by Human Amniotic Epithelial Stem Cell-Derived Photoreceptor-like Cells. Int. J. Mol. Sci. 23, 8722 (2022).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms and viruses. Protein Sci. 30, 185–185 (2021).
Acknowledgements
This work was supported by the Zhejiang Provincial Key R&D Program of China (2022C03097 to L.Y.), the National Natural Science Foundation of China (82370450 to L.Y., 82300296 to J.L.), National Key R&D Program of China (2018YFA0800504 to L.Y.), the China Manned Space Flight Technology Project Chinese Space Station (YYWT-0901-EXP-06 to L.Y.), the Zhejiang Provincial Natural Science Foundation of China (LZ20H020002 to L.Y.), the Medical and Health Science and Technology Program of Zhejiang Provincial Health Commission of China (2022KY725 to Z.G.), the Basic Public Welfare Research Program of Zhejiang Province (LTGD24H020002 to Z.G.), the China Postdoctoral Science Foundation (2019M662036 to J.L.) and the Fundamental Research Funds for the Central Universities of China. We thank Shanghai OE Biotech Co., Ltd., for the proteomic characterization and bioinformatics analysis.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.Y. and J.L.; methodology and investigation, Z.G., C.Q., W.Y. and T.J.; data acquisition: Z.G., J.L., C.Q., J.Z. and Z.Y.; writing—original draft preparation, Z.G., J.L. and C.Q.; writing—review and editing, J.L. and L.Y.; funding acquisition, L.Y., J.L. and Z.G. All the authors have read and agreed with the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ge, Z., Qiu, C., Zhou, J. et al. Proteomic analysis of human Wharton’s jelly mesenchymal stem/stromal cells and human amniotic epithelial stem cells: a comparison of therapeutic potential. Sci Rep 14, 28061 (2024). https://doi.org/10.1038/s41598-024-79063-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79063-1
Keywords
This article is cited by
-
Comparative evaluation of the therapeutic efficacy between human amniotic epithelial cells and human umbilical cord mesenchymal stem cells in premature ovarian insufficiency
Stem Cell Research & Therapy (2025)
-
Umbilical cord mesenchymal stem cells protect against obstetric deep venous thrombosis in rats by suppressing ferroptosis
Stem Cell Research & Therapy (2025)
-
Precision medicine in premature ovarian insufficiency: a focus on the precision therapeutic strategies for mesenchymal stem cells
Stem Cell Research & Therapy (2025)