Abstract
Posaconazole enteric-coated tablet and oral suspension are two oral drugs in the treatment of invasive fungal infections (IFIs). This study compared the effectiveness and safety between posaconazole enteric-coated tablet and oral suspension, and provided a real world basis for the clinical practice. A retrospective cohort study was performed on IFIs patients treated with posaconazole enteric–coated tablet or oral suspension. The primary endpoints were in-hospital mortality, treatment discontinuation rate and clinical effective rate. The secondary endpoints were adverse events incidence (liver dysfunction, renal dysfunction and hypokalemia). One hundred and forty-four patients were totally included and divided into enteric-coated tablet group (n = 46) and oral suspension group (n = 98). There was no significant difference in effectiveness and safety between two groups. The female (OR = 0.130, P = 0.018) and diabetes mellitus (OR = 4.242, P = 0.003) were independently associated with combined in-hospital mortality/treatment discontinuation rate. The renal replacement therapy (OR = 10.071, P = 0.006), hypoalbuminemia (OR = 6.646, P = 0.002) and posaconazole duration (OR = 1.119, P = 0.002) were risk factors for liver dysfunction. The posaconazole enteric-coated tablet has comparable effectiveness and safety with oral suspension in IFIs, which need large-scale cases studies to confirm in the future.
Similar content being viewed by others
Introduction
Invasive fungal infections (IFIs) refer to the diseases in which fungi invades and grow in the human body, then causes inflammatory reaction and tissue damage1. IFIs are mostly found in immune compromised patients such as hematological diseases, malignancies and hematopoietic stem cell transplantation leading to the prolonged hospital stay and increased total treatment costs and mortality1. The incidence of IFIs in China patients with hematologic tumors is 8.3%, which may be lower than the actual value due to missed diagnosis2,3. Voriconazole, posaconazole and isavuconazole are the first-line recommended drugs for invasive aspergillosis4,5,6. As a second-generation triazole antifungal drug, posaconazole has broad-spectrum antifungal activity against most Aspergillus, Candida and Mucor7. At present, there are three available formulation of posaconazole in China including enteric-coated tablet listed in 2018, oral suspension listed in 2013 and injection listed in 2021.
Due to large individual pharmacokinetic differences, the plasma concentration of posaconazole oral suspension is influenced by various factors leading to the potential therapeutic failure8. The bioavailability of posaconazole oral suspension is greatly affected by foods, gastric pH and gastrointestinal motility, whereas enteric-coated tablet avoids these limitations9,10,11,12. Studies have reported that patients receiving posaconazole enteric-coated tablet reached higher plasma concentrations than those receiving oral suspension13,14,15,16,17. However, there is still lack of real world data comparing clinical effectiveness and safety between posaconazole enteric-coated tablet and oral suspension, which causes confusion in clinical practice. Therefore, the objective of this study was to retrospectively evaluate the effectiveness and safety of posaconazole enteric-coated tablet and oral suspension in patients with IFIs, and to provide evidence-based basis for clinical application.
Methods
Study design and participants
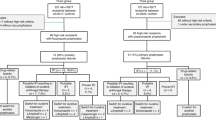
This study was a retrospective cohort study conducted in The First Affiliated Hospital of Shandong First Medical University, a tertiary university hospital in China. We screened patients of IFIs from June 2021 to March 2024. The data of this study was collected from Shandong Provincial Qianfoshan Hospital Healthcare Big Data Platform (SPQHHBDP). The SPQHHBDP integrates multi-source data from hospital information system (HIS), electronic medical records (EMR), laboratory information management system (LIS), picture archiving and communication system (PACS), nursing information system. The encrypted personal identification number was used as a unique identifier to interlink each person’s data information in the above-mentioned database. Patients inclusion criteria: (1) inpatients who meet the criteria for probable and proven diagnosis of IFIs18; (2) patients were administered with posaconazole enteric-coated tablet or oral suspension during hospitalization; (3) posaconazole duration more than 3 days. Patients exclusion criteria: (1) posaconazole enteric-coated tablet and oral suspension were used during the same hospitalization; (2) incomplete clinical information, such as absence of important laboratory tests; (3) pregnant and lactating patients.
Treatment and grouping
All patients were treated with posaconazole enteric-coated tablet or oral suspension according to clinical guidelines4,5,6,7. Patients were divided into posaconazole enteric-coated tablet group (POS-Tab group) and oral suspension group (POS-OS group). This study met the hospital ethics standards and had been approved by the Medical Ethics Committee of the First Afliated Hospital of Shandong First Medical University (Shandong Provincial Qianfoshan Hospital) (approval number: YXLL-KY-2023 (097)). The study is retrospective in nature, therefore the requirement for informed consent was waived by the Medical Ethics Committee of the First Afliated Hospital of Shandong First Medical University (Shandong Provincial Qianfoshan Hospital). And all procedures were conducted by the principles of the Declaration of Helsinki.
Evaluation of effectiveness and safety
The baseline characteristics, disease prognosis and laboratory tests were collected. The effectiveness endpoints of this study included in-hospital mortality, treatment discontinuation rate, combined in-hospital mortality/treatment discontinuation rate and clinical effective rate. In-hospital death was defined as the death due to disease deterioration or other reasons during hospitalization and death discharge diagnosis19. Treatment discontinuation was defined as the discontinue treatment due to the continuous disease aggravation20. Clinical effectiveness was defined as the recovery to normal, the improvement of clinical symptoms, microbiological examination and laboratory examination including white blood cell count, C-reactive protein and procalcitonin.
The safety endpoints of this study included liver dysfunction [elevations of alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin and γ-glutamyl transpeptidase (γ-GT)], renal dysfunction (elevations of blood creatinine and urea nitrogen) and hypokalemia. The evaluation criteria of safety endpoints referred to the adverse event evaluation criteria of CTCAE 5.0 version21。The multivariate logistic regression analyses were performed to correct and eliminate the impact of baseline difference on the effectiveness and safety. At the same time, univariate and multivariate logistic regression analyses were used to evaluate the influencing factors of combined in-hospital mortality/treatment discontinuation rate and liver dysfunction.
Statistical analysis
In this study, SPQHHBDP (based on R programming language) was used for statistical analysis. Continuous data were presented as mean and standard deviation (The independent-samples T test was used) if normally distributed otherwise median and range (The Wilcoxon rank-sum test was used). Categorical data were presented as numbers and percentages (The chi-square test was used).
Results
In this study, 494 patients were administered with posaconazole enteric-coated tablet or oral suspension more than 3 days during hospitalization. 144 patients with probable diagnosis and proven diagnosis of IFIs were finally included. In all included patients, 46 cases received posaconazole enteric-coated tablet (POS-Tab group) and 98 cases received posaconazole oral suspension (POS-OS group). The baseline characteristics of all included patients were shown in Table 1. The differences of age, leukemia, transplant status, diabetes mellitus, mechanical ventilation, neutropenia, combined immunosuppressant of cyclosporine and methotrexate, mean hospital stay and posaconazole daily dose between two groups were significant (P < 0.05).
Effectiveness outcomes
The total in-hospital mortality was 4.2% (6/144), including 4 cases in POS-Tab group and 2 cases in POS-OS group. The total treatment discontinuation rate was 12.5% (18/144), including 6 cases in POS-Tab group and 12 cases in POS-OS group. In total, the combined in-hospital mortality/treatment discontinuation rate was 16.7% (24/144). The total clinical effective rate was 54.2% (78/144).
After the multivariate logistic regression analysis correction, the in-hospital mortality, treatment discontinuation rate, combined in-hospital mortality/treatment discontinuation rate and clinical effective rate between the two groups showed no significant difference (P < 0.05) (Table 2).
The results of univariate and multivariate logistic regression analysis showed that female (OR = 0.130, P = 0.018) and diabetes mellitus (OR = 4.242, P = 0.003) were independently associated with combined in-hospital mortality/treatment discontinuation rate (P < 0.05) (Table 3).
Safety outcomes
In this study, the total liver dysfunction rate was 20.8% (30/144), including 8 cases in POS-Tab group and 22 cases in POS-OS group. The total renal dysfunction rate was 11.8% (17/144), including 7 cases in POS-Tab group and 10 cases in POS-OS group. The total hypokalemia rate was 20.8% (30/144), including 7 cases in POS-Tab group and 23 cases in POS-OS group. There was no significant differences in safety endpoints between two groups (P > 0.05). In addition, the multivariate logistic regression analysis correction obtained the same statistical results (Table 4).
The results of univariate and multivariate logistic regression analysis showed that renal replacement therapy (OR = 10.071, P = 0.006), hypoalbuminemia (OR = 6.646, P = 0.002) and posaconazole duration (OR = 1.119, P = 0.002) were independently associated with liver dysfunction (Table 5).
Discussion
IFIs can lead to serious damage of body and function, and even serious life-threatening22,23. The triazole antifungals, especially posaconazole, play an important role in antifungal therapy and reduce the mortality of IFIs without increasing the risk of adverse reactions24. Compared to posaconazole oral solution, posaconazole solid formulations including tablets and capsules yielded higher mean blood concentration and oral bioavailability through increasing the intestinal solubility of posaconazole under fasted and fed conditions25. It has been shown that the overall mortality of IFIs can reach 30% after antifungal therapy alone26. Maertens et al. reported that the 6-week mortality of IFIs after posaconazole treatment was 15%, which was close to the combined in-hospital mortality/treatment discontinuation rate (16.7%) in this study27. In addition, the total clinical effective rate of posaconazole in this study was 54.2% and consistent with the reported result9,27. The clinical effective rate of posaconazole enteric-coated tablet and oral suspension was similar, which verified the excellent clinical efficacy of different posaconazole formulations.
In this study, the female and diabetes mellitus were independently correlated with poor prognosis. The combined in-hospital mortality/treatment discontinuation rate in female patients was lower than that in male patients. This is consistent with the results of recent studies that male was one of the risk factors for incidence and mortality of IFIs28,29. The diabetes mellitus has been verified as a risk factor for IFIs30,31,32. Based on the above results, the concomitant disease, especially diabetes mellitus, should be paid more attention to timely improve treatment measures and prognosis of IFIs.
Liver dysfunction is one of the most common adverse effect of posaconazole and the focus of attention during clinical treatment33. Maertens et al. reported that the incidence of elevated ALT, AST and total bilirubin were 8%, 6% and 3%, respectively27. Many studies showed that there was no significant difference in elevated ALT, AST and total bilirubin between posaconazole extended-release tablet and oral suspension15,34. In this study, there was also no significant difference in the liver dysfunction incidence between posaconazole enteric-coated tablet and oral suspension. The univariate and multivariate logistic regression analysis revealed that renal replacement therapy, hypoproteinemia and posaconazole duration were risk factors for liver dysfuntion of posaconazole. For patients with hypoproteinemia, elevated blood concentration of posaconazole is more likely to cause liver dysfunction35. In addition, excessive posaconazole duration will further aggravate the metabolic burden and increase the risk of liver dysfunction. Therefore, the liver function and blood concentration should be monitored to adjust posaconazole dose.
As a result of mainly fecal excretion, posaconazole rarely lead to renal dysfunction27. In this study, the incidence of renal dysfunction caused by posaconazole was low in the two groups. Another common adverse reaction of triazole antifungal drugs is hypokalemia, which may be related to pseudoaldosteronism36. The hypokalemia incidence of posaconazole can be up to 22%37. In this study, the overall incidence of hypokalaemia was 20.8%. The incidence of hypokalaemia between two groups was similar. This suggests that patients treated with posaconazole should focus on the monitoring of blood potassium and intervening as soon as possible.
Nonetheless, there are some limitations in our study. First, single-center retrospective study cannot represent real clinical situation in prospective studies because of research bias. Second, the sample size of posaconazole enteric-coated tablet was small due to the short application time in our hospital.
Conclusion
In conclusion, the posaconazole enteric-coated tablet has comparable effectiveness and safety with oral suspension in patients with IFIs. The male and diabetes mellitus are independent risk factors for treatment prognosis. The renal replacement therapy, hypoproteinemia and posaconazole duration are high risk factors for liver dysfunction of posaconazole. Additional large sample and multi-center studies are need in the future.
Data availability
The datasets supporting the findings during current study are available upon reasonable request from the corresponding author.
References
Menzin, J. et al. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am. J. Health Syst. Pharm. 66, 1711–1717 (2009).
Sun, Y. Q. et al. Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: A multicenter, prospective, observational study in China. Tumour Biol. 36, 757–767 (2015).
Chamilos, G. et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003). Haematologica. 91, 986–989 (2006).
Medical Mycology Society of Chinese Medicine and Education Association & Chinese Society of Hematology, Chinese Medical Association. Chinese expert consensus for invasive fungal disease in patients after hematopoietic stem cell transplantation (2023). Chin. J. Hematol. 44, 92–97 (2023).
Ullmann, A. J. et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin. Microbiol. Infect. Suppl. 7, 53–67 (2012).
Mousset, S. et al. Treatment of invasive fungal infections in cancer patients-updated recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann. Hematol. 93, 13–32 (2014).
Working Group of Expert Consensus on Clinical Use of Posaconazole. Expert consensus on clinical use of posaconazole (2022 edition). Chin. J. Clin. Infect. Dis. 15, 321–332 (2022).
Akan, H. et al. Preventing invasive fungal disease in patients with haematological malignancies and the recipients of haematopoietic stem cell transplantation: practical aspects. J. Antimicrob. Chemother. 68(Suppl 3), iii5–16 (2013).
Panagopoulou, P. & Roilides, E. Evaluating posaconazole, its pharmacology, efficacy and safety for the prophylaxis and treatment of fungal infections. Expert Opin. Pharmacother. 23, 175–199 (2022).
Soysal, A. Prevention of invasive fungal infections in immunocompromised patients: The role of delayed-release posaconazole. Infect. Drug Resist. 8, 321–331 (2015).
Kersemaekers, W. M. et al. Effect of a high-fat meal on the pharmacokinetics of 300-milligram posaconazole in a solid oral tablet formulation. Antimicrob. Agents Chemother. 59, 3385–3389 (2015).
Wiederhold, N. P. Pharmacokinetics and safety of posaconazole delayed-release tablets for invasive fungal infections. Clin. Pharmacol. 8, 1–8 (2015).
Lenczuk, D. et al. Antifungal prophylaxis with Posaconazole Delayed-Release Tablet and oral suspension in a real-life setting: Plasma levels, efficacy, and tolerability. Antimicrob. Agents Chemother. 62, e02655–e02617 (2018).
Stelzer, D. et al. Posaconazole liquid vs tablet formulation in lung transplant recipients. Mycoses. 61, 186–194 (2018).
Wass, E. N., Hernandez, E. A. & Sierra, C. M. Comparison of the efficacy of Posaconazole delayed release tablets and suspension in Pediatric Hematology/Oncology patients. J. Pediatr. Pharmacol. Ther. 25, 47–52 (2020).
Chae, H. et al. Evaluation of posaconazole plasma concentrations achieved with the delayed-release tablets in Korean high-risk patients with haematologic malignancy. Mycoses. 63, 131–138 (2020).
Muehlberg, L. et al. Evaluation of Posaconazole serum concentrations achieved with delayed-release tablets and oral suspension in patients undergoing intensive chemotherapy for Acute myeloid leukemia and myelodysplastic syndrome. Open. Forum Infect. Dis. 11, ofae263 (2024).
Donnelly, J. P. et al. Revision and update of the Consensus definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 71, 1367–1376 (2020).
Writing Group of Guidance for Clinical Trials of Anti-bacterial Drugs &. Center for drug evaluation, China Food and Drug Administration. Guidance for clinical trials of anti-bacterial drugs. Chin. J. Clin. Pharmacol. 30, 844–856 (2014).
Wu, T. S. & Liao, L. J. Percutaneous catheter drainage for acute severe pancreatitis complicated with peripancreatic necrosis and infection. Chin. J. Gen. Surg. 25, 333–338 (2016).
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), v5.0. Preprint at (2017). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
Strickland, A. B. & Shi, M. P. Mechanisms of fungal dissemination. Cell. Mol. Life Sci. 78, 3219–3238 (2021).
Brown, G. D., Denning, D. W., Gow, N. A., Levitz, S. M. & Netea, M. G. &White, T. C. Hidden killers: Human fungal infections. Sci. Transl Med. 4, 165rv13 (2012).
Boutin, C. A. & Luong, M. L. Update on therapeutic approaches for invasive fungal infections in adults. Ther. Adv. Infect. Dis. 11, 20499361231224980 (2024).
Krishna, G., Ma, L., Martinho, M. & O’Mara, E. Single-dose phase I study to evaluate the pharmacokinetics of posaconazole in new tablet and capsule formulations relative to oral suspension. Antimicrob. Agents Chemother. 56, 4196–4201 (2012).
Sprute, R., Grothe, J. H., Heringer, S. & Cornely, O. A. Reason and reality identifying barriers to patient enrolment for clinical trials in invasive candidiasis. J. Antimicrob. Chemother. 77, 3475–3481 (2022).
Maertens, J. A. et al. Posaconazole versus Voriconazole for primary treatment of invasive aspergillosis: A phase 3, randomised, controlled, non-inferiority trial. Lancet. 397, 499–509 (2021).
Ting, J. Y. et al. Invasive fungal infections in neonates in Canada: Epidemiology and outcomes. Pediatr. Infect. Dis. J. 37, 1154–1159 (2018).
Almutairi, A. et al. The prevalence of acute kidney injury in patients with community-acquired pneumonia who required mechanical ventilation. Ann. Saudi Med. 44, 104–110 (2024).
Seok, H. et al. Invasive fungal diseases in kidney transplant recipients: Risk factors for mortality. J. Clin. Med. 9, 1824 (2020).
Leitheiser, S. et al. Risk factors Associated with invasive fungal infections in kidney transplant patients. Am. J. Med. Sci. 359, 108–116 (2020).
Muskett, H. et al. Risk factors for invasive fungal disease in critically ill adult patients: A systematic review. Crit. Care. 15, R287 (2011).
O’Flynn, R., Zhou, Y. P., Waskin, H., Leong, R. & Straus, W. Hepatic safety of the antifungal triazole agent posaconazole: Characterization of adverse event reports in a manufacturer’s safety database. Expert Opin. Drug Saf. 21, 1113–1120 (2022).
Chen, L. et al. Pharmacokinetics and pharmacodynamics of Posaconazole. Drugs. 80, 671–695 (2020).
Zheng, C. Y. et al. Development and validation of a clinical risk score to Predict Immune-mediated liver Injury caused by Sintilimab: assessed for causality using updated RUCAM. J. Clin. Transl Hepatol. 11, 1387–1396 (2023).
Benitez, L. L. & Carver, P. L. Adverse effects Associated with Long-Term Administration of Azole Antifungal agents. Drugs. 79, 833–853 (2019).
Nguyen, M. H. et al. Posaconazole serum drug levels Associated with Pseudohyperaldosteronism. Clin. Infect. Dis. 70, 2593–2598 (2020).
Acknowledgements
We want to express our gratitude to the staff at the Center for Big Data Research in Health and Medicine, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, for their valuable contribution.
Funding
This study was supported by grants from Pharmacovigilance youth project in medical institutions of Shandong Pharmaceutical Association (No. 2024ywjj04) and ADR monitoring research project of Shandong Province (No.2024-SDADR-019).
Author information
Authors and Affiliations
Contributions
Y.Y. and R.Y. wrote the main manuscript text. Q.Y. processed the data. Y.H. and Y.L. prepared Tables 1, 2, 3, 4 and 5. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, YL., Yi, QY., Han, Y. et al. The effectiveness and safety of posaconazole enteric-coated tablet versus oral suspension in invasive fungal infections. Sci Rep 14, 27887 (2024). https://doi.org/10.1038/s41598-024-79512-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79512-x
Keywords
This article is cited by
-
Design and Assessment of Posaconazole-Loaded Transfersomal Gel for Antifungal Efficacy Against Candida Albicans and Aspergillus Niger
Journal of Pharmaceutical Innovation (2026)
-
Hepatobiliary disorders associated with triazole antifungal agents: a disproportionality and Bayesian analysis based on the FAERS database
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)