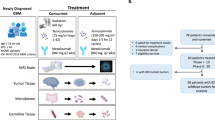
Abstract
Granulomatous mastitis (GM) poses challenges in diagnosis and treatment due to its similarities with other breast diseases like cancer. The comparative study evaluated the efficacy and safety of a vacuum-assisted biopsy device with minimally invasive excisions compared to traditional wide local excisions. The vacuum-assisted biopsy device technique offers benefits such as precise tissue removal, reduced damage to healthy tissue, shorter surgery and recovery times, and lower postoperative complication risks. The study found that the vacuum-assisted biopsy device had comparable efficacy to traditional wide local excision in treating GM with an overall effectiveness rate of 92.9% and a recurrence rate of 9.52%. The vacuum-assisted biopsy device group showed advantages in reduced hospitalization duration (2.83 days vs. 7.52 days), lower costs, and better cosmetic outcomes, with a 100% patient satisfaction rate compared to 80% in the control group. This study fills existing clinical evidence gaps regarding the effectiveness and safety of vacuum-assisted biopsy device in GM treatment. By providing evidence-based guidelines, it aims to assist clinicians in choosing the most appropriate treatment for GM patients, ultimately improving their quality of life and mental well-being. The research contributes valuable insights into GM therapy, potentially revolutionizing treatment approaches and enhancing patient outcomes.
Similar content being viewed by others
Introduction
Granulomatous mastitis (GM), a significant breast disease, is characterized by chronic inflammation in breast tissue. The literature has noted its association with various factors, including infections, corticosteroid changes, and immune responses1. GM presents with a range of symptoms, most commonly breast lumps, which often resemble those of other breast diseases such as breast cancer, leading to diagnostic challenges and sometimes ineffective treatment and recurrence2,3,4. Despite being a benign condition, GM’s timely and effective treatment is crucial due to its substantial impact on patients’ quality of life and potential for severe complications5.
In recent decades, there has been a significant shift in the treatment approaches for GM6. Traditional wide local excision, typically used for early-stage GM, often involves extensive removal of breast tissue. It can lead to marked changes in breast morphology and structure, adversely affecting patients’ mental well-being and quality of life7,8,9. Consequently, with advancements in medical technology, the focus has shifted to exploring minimally invasive excision methods as potential treatment alternatives10.
One such method, the Vacuum-assisted biopsy device minimally invasive excision, has received growing attention in recent years11. This technique utilizes specialized tools to remove affected tissue, minimizing damage to surrounding healthy tissue. Its benefits include reduced surgery and recovery times and a lower risk of postoperative complications12. However, despite its increasing application in various medical settings, there is a lack of substantial clinical evidence confirming its effectiveness and safety specifically for GM treatment13.
To fill this gap, we embarked on a comparative study to evaluate the efficacy and safety of Vacuum-assisted biopsy device minimally invasive excision against traditional wide local excision in the treatment of GM. Our objective is to provide clinicians with detailed, evidence-based guidelines for selecting the most suitable treatment approach for GM patients. This study is poised to contribute new insights into GM therapy, aiming to improve those affected’s quality of life and mental health.
Materials and methods
Ethical statement
This study was conducted in full compliance with the ethical guidelines set forth by the Medical Ethics Committee of Ningde Mindong Hospital formal ethical approval. All participants provided informed consent after being thoroughly informed about the study’s objectives, risks, and anticipated benefits.
Criteria for patient selection and study inclusion
We collected and analyzed the clinical data of patients diagnosed with GM at the Breast Surgery Department of Ningde Dong Hospital from January 2019 to January 2023. The primary presentation of patients was “breast lumps accompanied by pain,” and they were pathologically confirmed as GM following core needle biopsy with negative acid-fast and PAS staining.
Additional inclusion criteria included lack of treatment before definitive diagnosis and provision of signed informed consent for treatment. The criteria for selecting patients for surgical treatment were further specified: if patients had persistent lesions larger than 3 cm after conservative treatment (such as hormone-based local immune modulatory therapy and ultrasound-guided sealing solution injection), experienced lesion rupture, or showed intolerance to conservative treatment measures, they were selected for surgery.
We excluded individuals who displayed acute skin reactions post-treatment, those who were pregnant or lactating, those with coagulation disorders, and patients whose lesions remained larger than 3 cm, experienced rupture, or were intolerant to the treatment protocols. These patients were advised to seek further medical evaluation and treatment at specialized centers, and those with severe systemic diseases making surgery untenable (Fig. 1).
Pregnant and lactating women were excluded because their conditions could affect the state of breast tissue and the metabolism and efficacy of drugs, thereby increasing complexity and risk. All pregnant and lactating women were excluded from the study to avoid potential risks to the fetus or infant. Potential female participants underwent pregnancy testing to confirm they were not pregnant at the time of study participation. Lactating women’s status was confirmed through inquiry and medical records, ensuring they had completely ceased breastfeeding for at least three months before participating in the study. Patients whose lesions remained larger than 3 cm after treatment, experienced rupture, or were intolerant to the treatment protocols were advised to seek further medical care elsewhere.
Randomization procedure
This study conducted a prospective randomized controlled trial and obtained approval from the Ethics Committee of Min Dong Hospital, affiliated with Fujian Medical University. The randomization occurred on the first day of patient enrollment at the breast surgery department and utilized a centralized computer-generated allocation sequence. We employed a block randomization method for random group assignment. The specific randomization process was as follows: Patients were ranked in order of their enrollment, and then each patient was assigned a unique random number sequentially. Subsequently, these random numbers were sorted in ascending order. After excluding patients based on individual factors, loss of follow-up contact, or cases where the reduction in tumor size was not substantial, the final allocation resulted in 42 patients being assigned to the observational group and 40 patients to the control group.
Ultrasound-guided local occlusive therapy
All patients receive initial hormone-based ultrasound-guided local immune modulatory therapy using this approach. The sealing solution is prepared according to the formula “lidocaine 100 mg (Specification: 100 mg * 1 vial, National Medical Products Administration Approval Number H20057816) + sodium chloride injection 90 mg (Specification: 90 mg * 1 vial, National Medical Products Administration Approval Number H20084468) + triamcinolone acetonide injection 40 mg (Specification: 40 mg * 1 vial, National Medical Products Administration Approval Number H20033525) + dexamethasone 5 mg (Specification: 5 mg * 1 vial, National Medical Products Administration Approval Number H37021969)”. Under ultrasound guidance, multiple local injections of the sealing solution are administered around the lesion location sites for targeted immune-modulating treatment (careful to avoid blood vessels). This procedure is performed weekly. In case of abscess formation during treatment, management follows the protocol above. The cumulative dose of triamcinolone acetonide does not exceed 400 mg, and dexamethasone does not exceed 50 mg. If acute infection symptoms such as localized redness, swelling, and increased pain occur during treatment, pus may be aspirated for bacterial culture and sensitivity testing. Pending sensitivity results, prophylactic use of second-generation cephalosporin antibiotics can be considered. Once sensitivity results are available, adjustments to the antimicrobial regimen are made accordingly. The average time between the injection of the sealing solution and the surgical procedure was four weeks. Among all patients, 28 benefited from the injection alone, showing significant pain reduction and lesion shrinkage. Five patients experienced minor complications after the injection alone, such as localized infection, which were managed conservatively. With the treatment above, significant alterations were observed in the histopathological examination of the afflicted tissues in patients (Supplementary Fig. 1).
Control group: traditional wide local excision
Traditional wide local excision is performed under general anesthesia in the control group (Fig. 2A). A suitable incision is made, the lesion is excised along with a margin of surrounding tissue, and sinus tracts are cleared. Techniques such as glandular flaps or intracavitary negative pressure drainage may be utilized to preserve breast aesthetics. Post-surgery compression bandages are applied to manage bleeding, and excised specimens are sent for pathological examination. Careful wound management, drainage tube removal, and suture removal complete the surgical process.
Observational group: vacuum-assisted biopsy device minimally invasive excision
The Vacuum-assisted biopsy device, a minimally invasive core biopsy, is employed in the observational group. Local infiltration anesthesia is administered using a solution comprising lidocaine, normal saline, and epinephrine. The procedure involves making a small incision near the lesion, followed by ultrasound-guided infiltration of the anesthetic solution. A rotational excision needle is then directed into the lesion’s base, with careful adjustments made as needed (Fig. 2B). The procedure focuses on precise excision while avoiding damage to surrounding tissue. Negative pressure removes pus, and the surgical cavity is irrigated with saline. Post-surgery, specimen examination, wound care, and compression bandages are administered for optimal healing.
Postoperative follow-up
Patients are instructed to attend breast surgery outpatient follow-ups one-month post-surgery, with subsequent visits scheduled every three months post-surgery onward. A comprehensive medical history is obtained during these visits, and meticulous physical examinations are conducted. Breast ultrasound assessments are performed every three months, concurrently evaluating breast aesthetics, pain levels, and surgical recovery for both observational groups. The evaluation encompasses treatment effectiveness, recurrence rates, surgical complications, and perioperative metrics such as surgical duration, hospitalization period, costs, and pain profiles before treatment, preoperatively, and one-month post-surgery. Additionally, cosmetic outcomes following surgery are scrutinized.
Treatment efficacy assessment
Postoperative breast ultrasound reveals that lesions have substantially disappeared with no residual pain or discomfort, indicating a significant therapeutic effect. Lesions shrinking by more than 50% on ultrasound follow-up, without notable pain or discomfort, are considered to show treatment effectiveness. Conversely, lesions shrinking by less than 50% or causing prominent pain on follow-up are considered ineffective treatment. The overall effectiveness rate is calculated as the percentage of practical and effective outcomes14.
Recurrence determination
Within one year post-surgery, the appearance of new lesions within the surgical area or on the same side, confirmed via ultrasound examination and pathology, constitutes recurrence15.
Pain scoring
The visual Analog Scale (VAS) is utilized in both groups to evaluate pain levels before treatment, preoperatively, and one-month post-surgery. The scale ranges from 0 to 10: 0 represents no pain, 1–3 indicates mild pain, 4–6 signifies moderate pain, and 7–10 reflects severe pain16.
Postoperative cosmetic assessment
Liu’s aesthetic evaluation criteria for GM are applied to judge postoperative cosmetic outcomes17. The score of nine items ranges from 0 to 18, with scores ≥ 14 considered excellent, 8–13 as good, and scores below 8 as poor. For specific rating criteria, please refer to Table 1.
Surgical and perioperative indicators
Operative time, blood loss, hospitalization duration, and costs are recorded for both groups. Pain scores, assessed via the Visual Analog Scale (VAS), are recorded preoperatively, one day post-surgery, and at one and three months post-surgery. VAS scores range from 0 to 10, corresponding to no, mild, moderate, and severe pain.
Characteristics and time to remission target for nodule-stage GM
Characteristics of nodule-stage GM include a firm, irregularly shaped breast lump with indistinct borders. Affected breast movement may lead to pain, and the skin may appear slightly red. Localized skin temperature may be elevated while systemic fever is absent. Some cases show enlarged axillary lymph nodes. The “Time to Remission Target” refers to the time from GM diagnosis to meeting surgical criteria.
Statistical analysis
SPSS 26.0 software will be utilized for data analysis. Descriptive statistics represent normally distributed continuous variables, such as mean ± standard deviation. Independent samples t-test and one-way analysis of variance (ANOVA) will assess two-group and multiple-group comparisons for normally distributed continuous data. Categorical data will be presented as frequencies or percentages and compared using the chi-squared or Fisher’s exact rate test. Ordinal data will be analyzed using rank-sum tests. Statistical significance will be indicated by a P-value below 0.05.
Results
Comparative analysis of clinical characteristics
To comprehensively understand the patient demographics and clinical features, we closely examined the data from Table 2. All participants in the study were females, aged between 24 and 45 years, with a mean age of 31.92 ± 6.12 years. Notably, every participant had a history of childbirth and underwent steroid hormone treatment post-diagnosis. Leveraging the random number table method, they were categorized into an observational group of 42 cases and a control group comprising 40 cases. The observational group’s mean age was 32.18 ± 5.23 years, juxtaposed with the control group’s 31.26 ± 6.02 years, reflecting no statistically significant disparity (P = 0.462). Laterality examination revealed comparable distributions between the two cohorts: 47.6% left and 52.4% right for the observational group, and 52.5% left and 47.5% right for the control group (P = 0.659). The tumor diameter averaged 4.90 ± 1.62 cm in the observational group and 5.32 ± 2.19 cm in the control group (P = 0.325). In assessing BMI, both groups exhibited similar distributions across the various ranges, with a P-value of 0.717. Moreover, presenting symptoms such as painful masses and pain with erythema were uniformly distributed between the two groups (P = 0.938). Other clinical features, including enlarged axillary lymph nodes, nipple retraction, lactational mastitis, and local trauma, also mirrored this trend of unity.
In summary, the meticulous data scrutiny underscored the efficacy of the randomization process, confirming that both groups were well-matched in their demographic and clinical characteristics.
Equivalence in treatment outcomes and recurrence: a comparative insight
In evaluating the efficacy and recurrence rates between different treatment groups, a detailed examination of Table 3 unveils some insightful findings. Of the 42 patients in the observational group, 20 displayed evident effects, 19 were effective, and 3 were ineffective, culminating in an overall effectiveness rate of 92.9% and a recurrence rate of 9.52%. On the other hand, the control group, consisting of 40 patients, exhibited evident effects in 17 cases; 20 were deemed effective, and 3 were ineffective. It translates to an overall effectiveness rate of 92.5% and a recurrence rate of 12.5% (Fig. 3A). When juxtaposed, the difference in overall effectiveness between the two groups was not statistically significant (χ2 = 0.131, P > 0.05), suggesting comparable therapeutic outcomes. Similarly, the recurrence rates between the two cohorts also lacked significant disparity (χ2 = 0.006, P > 0.05), indicating analogous recurrence scenarios (Fig. 3B). Both groups manifested equivalent therapeutic efficacies and recurrence rates without any noteworthy statistical variances.
Comparative analysis of treatment efficacy and recurrence rates between observational and control groups. Note: (A) A stacked bar chart illustrating the proportional distribution of “Evident Effect,” “Effective,” and “Ineffective” outcomes between the study and control groups; (B) Another stacked bar chart showcasing the relative percentages of “Recurrence” and “Effective” outcomes within the study and control cohorts.
Superior surgical and postoperative outcomes in the observational group
To elucidate the distinctions between surgical procedures and postoperative outcomes, Table 3 presents a comprehensive evaluation of the observational group and control group. Starting with surgical metrics, the observational group displayed a pronounced advantage in terms of surgical duration (15.31 ± 5.12 min versus 29.52 ± 13.76 min), hospital stay duration (2.83 ± 1.12 days versus 7.52 ± 4.17 days), and hospitalization costs (¥6658.29 ± 861.26 versus ¥7819.21 ± 1362.32). All these variations were statistically significant with a χ2 value less than 0.05. Furthermore, postoperative complications were markedly reduced in the observational group, with only minor localized issues in contrast to the more severe complications in the control group. This difference was supported by a χ2 analysis yielding a value of 29.22. While preoperative pain assessments were comparable, the post-surgical phase had the observational group reporting lower VAS pain scores, especially 1 day and 1 month post-surgery, further emphasizing the enhanced efficacy of the procedures in the observational group.
In summation, the observational group demonstrated superior outcomes across multiple metrics, with the data solidifying its efficiency and effectiveness over the control group in both surgical and postoperative phases.
Positive aesthetic satisfaction in the observational group post-surgery
Upon delving into the aesthetic outcomes in Table 4, a clear delineation surfaces between the study and control groups. Out of all the patients assessed post-surgery, 8 were deemed unsatisfied with their cosmetic results, and notably, all were from the open surgery or control group. In stark contrast, the observational group achieved a flawless record with a satisfaction rate of 100% (42 out of 42 patients). The control group, though commendable, lagged slightly behind with a satisfaction rate of 80% (32 out of 40 patients) (Fig. 4). When pitted against each other, the aesthetic satisfaction between the two groups showcased a significant statistical divergence, affirmed by a χ2 value of 7.175 and a p-value less than 0.05 (Table 4). In essence, the postoperative aesthetic satisfaction in the observational group markedly surpassed that of the control group.
Discussion
The rising incidence of GM in recent times presents a significant challenge in breast surgery, with no consensus yet on the most effective treatment modality18. The clinical presentation of GM often resembles that of other breast diseases, such as breast cancer, leading to initial diagnostic challenges19. Histopathology has become crucial in diagnosing GM, typically revealing lobular-centered granulomatous inflammation, micro-abscesses, and infiltration of inflammatory cells20.
Historically, traditional wide local excision was the preferred treatment for GM. However, recent medical advancements have shifted towards minimally invasive surgeries, such as the Vacuum-assisted biopsy device minimally invasive excision, gaining traction in the medical community21. Previous studies indicated a high relapse rate with hormone therapy alone, up to 50%. In contrast, our study demonstrated a substantial increase in efficacy, reaching 92.9%, which is an improvement over the 75% efficacy rate reported by Zhou et al.22. This increased effectiveness can be attributed to our use of multi-point steroid hormone injection closure treatment, which alleviates local symptoms and minimizes systemic side effects23. Using ultrasound guidance ensured precise delivery of the therapy directly to the affected tissues, maximizing therapeutic efficacy while minimizing systemic side effects24. The endpoint of this therapy was clearly defined: achieving a reduction in the maximum diameter of the tumor to less than 3 centimeters. This criterion was established based on the rationale that smaller lesions would allow for the use of minimally invasive surgical techniques, potentially improving patient outcomes by reducing operative times, accelerating recovery, decreasing the risk of complications, and enhancing cosmetic results post-surgery25,26. The selection of this endpoint was strategic, intending to optimize the condition of the breast tissue for subsequent surgical intervention and to establish a standardized criterion for assessing the readiness of the lesion for surgery27.
There were also differences in hospitalization duration. Patients who underwent Vacuum-assisted biopsy device surgery required an average hospital stay of 2.83 days, whereas those who underwent open surgery needed 7.52 days. The hospitalization duration was longer than anticipated, primarily due to comprehensive postoperative care protocols, including complication monitoring and adequate pain management. The longer hospital stay in the surgery group was also due to the need for more extensive wound care and management. Although both types of surgery can be performed as outpatient procedures under certain conditions, their invasiveness differs. Vacuum-assisted biopsy device surgery, being minimally invasive, typically results in less tissue damage and faster recovery, hence a shorter hospital stay. In contrast, traditional open surgery requires larger incisions, is more invasive, and may lead to more pain and complications, necessitating a longer hospital stay for postoperative monitoring and recovery.
Additionally, even though the surgeries themselves can be performed on an outpatient basis, the rate of postoperative recovery and the occurrence of complications are key factors affecting the length of hospitalization. For instance, if significant pain or complications such as bleeding occur after open surgery, the hospital stay may need to be extended for management and treatment. Overall, the shorter hospitalization duration for Vacuum-assisted biopsy device surgery patients reflects the advantages of minimally invasive procedures in postoperative recovery, while the longer duration for traditional open surgery patients may be related to more conservative monitoring and management strategies post-surgery. Future research should further explore the impact of different surgical types on hospitalization duration and how to optimize postoperative care processes to reduce unnecessary hospital stays, lower the economic burden on patients, and improve the efficiency of medical resource utilization.
In our research, Vacuum-assisted biopsy device minimally invasive excision showed comparable efficacy to traditional wide local excision in treating GM. Notably, it had advantages in reducing hospitalization duration, costs, and post-operative pain (Fig. 5). Additionally, our study focused solely on lump-type GM, which could introduce selection bias. Literature indicates that different types of GM may have significant differences in clinical presentation and treatment response19. Patients with lump-type GM typically present with distinct local lumps, which may respond better to localized treatment, whereas other types, such as abscess-type or fistula-type GM, may require more extensive surgical intervention20. Therefore, the observed superior results compared to the control group may partly be attributed to the selection of study subjects. This limitation should be considered when interpreting the results, and future studies should include more types of GM patients to comprehensively evaluate the effectiveness of different treatment methods. Preserving breast appearance and structure is vital for female patients’ psychological well-being and quality of life28. Our findings suggest that a vacuum-assisted biopsy device with minimally invasive excision more effectively maintains breast morphology, as evidenced by higher patient satisfaction with post-operative breast appearance29.
In summary, a vacuum-assisted biopsy device with minimally invasive excision emerges as a promising approach to treating GM, offering a potentially effective and patient-friendly alternative to traditional wide surgical excision. Despite its promising aspects, our study acknowledges several limitations that underscore the need for further research. These limitations include a small sample size and a relatively short one-year follow-up period, which may not sufficiently capture this technique’s long-term efficacy, safety, and recurrence rates. Moreover, the successful application of a vacuum-assisted biopsy device with minimally invasive excision demands considerable expertise in breast ultrasonography, highlighting a need for specialized training and experience among practitioners.
Another notable limitation is the non-double-blind design of the trial, which could introduce biases in the evaluation of treatment outcomes. Additionally, our study’s scope was limited to patients with lump-type GM, excluding those with other manifestations of the disease, such as abscess-type, sinus-type, or refractory GM30. While wide surgical excision is considered the last resort for GM, especially in severe cases where conservative treatments have failed, it involves the comprehensive removal of the affected area, including any fistula tracts and surrounding skin, to ensure margins are free of lesions. This approach aims to minimize the risk of recurrence by removing all potentially affected tissue. However, such extensive surgery can lead to significant cosmetic and functional consequences for the patient, including larger scars, changes in breast shape, and potential psychological impacts due to altered body image. These conditions indicate the necessity of expanding future research to encompass a wider range of GM presentations.
To address these challenges and build on the initial findings, future studies should aim for larger sample sizes and extended follow-up periods to more comprehensively assess the long-term outcomes of Vacuum-assisted biopsy device minimally invasive excision. Enhancing the technique and exploring its application across GM cases will be crucial for refining treatment strategies. Furthermore, adopting an interdisciplinary approach could provide valuable insights into the overall impact of this treatment on patients’ quality of life and mental health, considering both physical and psychological dimensions of recovery.
We anticipate advancements in this field and look forward to more extensive, multicentric, prospective, double-blind, randomized controlled trials. Such studies will be instrumental in providing deeper insights and establishing evidence-based guidelines for the most effective and safe treatment strategies for GM patients, ultimately aiming to improve patient outcomes and satisfaction with treatment.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Coombe, R. F. & Hamed, H. An update on granulomatous mastitis: a rare and complex condition. Br. J. Hosp. Med. (Lond). 82 (5), 1–7. https://doi.org/10.12968/hmed.2020.0718 (2021).
Soltany, A., Hraib, M., Alkhayer, M., Ibraheem, B. & Alshehabi, Z. Clinicopathological features of idiopathic granulomatous mastitis: A retrospective study & educational lessons from Syria. Ann Med Surg (Lond). ;77:103587. Published 2022 Apr 5. doi: (2022). https://doi.org/10.1016/j.amsu.2022.103587
Yin, L. et al. Differentiation Between Granulomatous Lobular Mastitis and Breast Cancer Using Quantitative Parameters on Contrast-Enhanced Ultrasound. Front Oncol. ;12:876487. Published 2022 Jul 15. doi: (2022). https://doi.org/10.3389/fonc.2022.876487
Naraynsingh, V., Hariharan, S., Dan, D., Harnarayan, P. & Teelucksingh, S. Conservative management for idiopathic granulomatous mastitis mimicking carcinoma: case reports and literature review. Breast Dis. 31 (1), 57–60. https://doi.org/10.3233/BD-2009-0294 (2010).
Jiao, Y., Chang, K., Jiang, Y. & Zhang, J. Identification of periductal mastitis and granulomatous lobular mastitis: a literature review. Ann. Transl Med. 11 (3), 158. https://doi.org/10.21037/atm-22-6473 (2023).
Barreto, D. S., Sedgwick, E. L., Nagi, C. S. & Benveniste, A. P. Granulomatous mastitis: etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Res. Treat. 171 (3), 527–534. https://doi.org/10.1007/s10549-018-4870-3 (2018).
Huang, J. et al. Dermis-retained breast dermo-glandular flap: a new surgical approach for granulomatous lobular mastitis. Front. Surg. 10, 1187811. https://doi.org/10.3389/fsurg.2023.1187811 (2023). Published 2023 Jun 16.
Zhang, C. et al. A clinical observation of stage I implant breast reconstruction for mass-like granulomatous lobular mastitis. Gland Surg. 10 (9), 2663–2672. https://doi.org/10.21037/gs-21-417 (2021).
Armstrong, V. L. et al. The impact of same-day discharge and enhanced recovery on patient quality of Life after Mastectomy with Implant Reconstruction. Ann. Surg. Oncol. 30 (5), 2873–2880. https://doi.org/10.1245/s10434-022-13019-5 (2023).
Himal, H. S. Minimally invasive (laparoscopic) surgery. Surg. Endosc. 16 (12), 1647–1652. https://doi.org/10.1007/s00464-001-8275-7 (2002).
Wang, Q., Zheng, J., Ren, Y. & Xu, H. Clinical effect of trans-areolar resection and minimally invasive mammotome biopsy in the treatment of breast fibroadenoma and its impact on the quality of life of patients. Am. J. Transl Res. 14 (5), 3539–3546 (2022). Published 2022 May 15.
Li, R. et al. Comparison of Curative Complications between Mammotome-Assisted Minimally Invasive Resection and Conventional Open Resection for Breast Neoplasm: A Retrospective Clinical Study [retracted in: Biomed Res Int. ;2024:9845801. doi: 10.1155/2024/9845801]. Biomed Res Int. 2021;2021:7739628. Published 2021 Nov 17. doi: (2024). https://doi.org/10.1155/2021/7739628
Zhu, Y. et al. Vacuum-assisted biopsy system for breast lesions: a potential therapeutic approach. Front. Oncol. 13, 1230083. https://doi.org/10.3389/fonc.2023.1230083 (2023). Published 2023 Aug 1.
Chen, K. et al. Ductal lavage for patients with nonlactational mastitis: a Single-Arm, proof-of-Concept Trial. J. Surg. Res. 235, 440–446. https://doi.org/10.1016/j.jss.2018.10.023 (2019).
Tan, Q. W. et al. Methylprednisolone for idiopathic granulomatous mastitis: a prospective observational cohort study. Gland Surg. 11 (9), 1538–1545. https://doi.org/10.21037/gs-22-484 (2022).
Hawker, G. A., Mian, S., Kendzerska, T. & French, M. Measures of adult pain: visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), short-form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), short Form-36 Bodily Pain Scale (SF-36 BPS), and measure of intermittent and constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. (Hoboken). 63 (Suppl 11), S240–S252. https://doi.org/10.1002/acr.20543 (2011).
Liu, P. Z. et al. A clinical study on the treatment of granulomatous lobular mastitis by the external application of the internal pus-expelling decoction and operation. Ann. Palliat. Med. 9 (5), 2631–2641. https://doi.org/10.21037/apm-19-684 (2020).
Lei, X. et al. Treatments for idiopathic granulomatous mastitis: systematic review and Meta-analysis. Breastfeed. Med. 12 (7), 415–421. https://doi.org/10.1089/bfm.2017.0030 (2017).
Gurleyik, G., Aktekin, A., Aker, F., Karagulle, H. & Saglamc, A. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J. Breast Cancer. 15 (1), 119–123. https://doi.org/10.4048/jbc.2012.15.1.119 (2012).
Azzam, M. I. et al. Idiopathic granulomatous mastitis: clinical, histopathological, and radiological characteristics and management approaches. Rheumatol. Int. 43 (10), 1859–1869. https://doi.org/10.1007/s00296-023-05375-6 (2023).
Atak, T., Sagiroglu, J., Eren, T., Ali Özemir, I. & Alimoglu, O. Strategies to treat idiopathic granulomatous mastitis: retrospective analysis of 40 patients. Breast Dis. 35 (1), 19–24. https://doi.org/10.3233/BD-140373 (2015).
Zhou, F. et al. Comparison of Conservative versus Surgical Treatment protocols in treating idiopathic granulomatous mastitis: a Meta-analysis. Breast Care (Basel). 15 (4), 415–420. https://doi.org/10.1159/000503602 (2020).
Yildirim, E. et al. Comparison of the efficiency of systemic therapy and intralesional steroid administration in the treatment of idiopathic granulomatous mastitis. The novel treatment for Granulomatous Mastitis. Ann. Ital. Chir. 92, 234–241 (2021).
Niesen, A. D., Jacob, A. K., Law, L. A., Sviggum, H. P. & Johnson, R. L. Complication rate of ultrasound-guided paravertebral block for breast surgery. Reg. Anesth. Pain Med. 45 (10), 813–817. https://doi.org/10.1136/rapm-2020-101402 (2020).
Sanderink, W. B. G. et al. Minimally invasive breast cancer excision using the breast lesion excision system under ultrasound guidance. Breast Cancer Res. Treat. 184 (1), 37–43. https://doi.org/10.1007/s10549-020-05814-z (2020).
Karakas, H. M. & Yildirim, G. Minimally Invasive Excision of Breast Masses under Ultrasound Guidance: A Single Center’s Five-Year Experience on the Breast Lesion Excision System. Breast J. 2022;2022:1888726. Published 2022 Feb 4. https://doi.org/10.1155/2022/1888726
Sanderink, W. B. G. & Mann, R. M. Advances in breast intervention: where are we now and where should we be? Clin. Radiol. 73 (8), 724–734. https://doi.org/10.1016/j.crad.2017.10.018 (2018).
Liao, H. et al. Ultrasound classification-guided minimally invasive rotary cutting in granulomatous lobular mastitis. BMC Womens Health. 20 (1), 252. https://doi.org/10.1186/s12905-020-01118-y (2020). Published 2020 Nov 16.
Wang, Y., Song, J., Tu, Y., Chen, C. & Sun, S. Minimally invasive comprehensive treatment for granulomatous lobular mastitis. BMC Surg. 20 (1), 34. https://doi.org/10.1186/s12893-020-00696-w (2020). Published 2020 Feb 22.
Yuan, Q. Q. et al. Management of granulomatous lobular mastitis: an international multidisciplinary consensus (2021 edition) [published correction appears in Mil Med Res. ;9(1):47. doi: 10.1186/s40779-022-00408-w]. Mil Med Res. 2022;9(1):20. Published 2022 Apr 26. doi: (2022). https://doi.org/10.1186/s40779-022-00380-5
Acknowledgements
None.
Funding
This work was supported by Startup Fund for scientific research, Fujian Medical University ( Grant number:2021QH1208 ).
Author information
Authors and Affiliations
Contributions
F.M.Z. played a pivotal role in conceptualizing the research topic and methodology. He also contributed significantly to the drafting of the original manuscript. C.Y.L. was responsible for the curation and analysis of data and creating visual representations of the research findings. X.Q.W. contributed to developing software tools used in the research and was instrumental in validating results. K.L.M. provided essential resources and supervision throughout the research project, ensuring that all processes adhered to high standards. Z.Z. was involved in reviewing and editing the manuscript for intellectual content and was responsible for the overall project administration. J.Z. played a crucial role in acquiring funding for the research and shared responsibilities in the administration of the project.
Corresponding author
Ethics declarations
Ethical statement
This study was conducted strictly according to the guiding principles of the Medical Ethics Committee of Ningde Mindong Hospital and has received ethical approval (Approval No.: 2022012602 K). All patients participating in the study provided informed consent after being fully apprised of the study’s objectives, risks, and benefits.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, F., Li, C., Wu, X. et al. Non-inferiority of minimally invasive rotational cutting in granulomatous mastitis treatment: a comparative trial. Sci Rep 15, 728 (2025). https://doi.org/10.1038/s41598-024-79778-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79778-1