Abstract
It remains unclear whether exercises leading to different outcomes of delayed onset muscle soreness (DOMS) may also elicit different skin temperature responses. The aim of this study was to determine whether different intensities and volumes of a single-joint exercise influence the DOMS and skin temperature measurements differently in healthy adults. Thirty-nine men and women were randomly assigned to three groups performing different exercise of different intensities and volumes (Exhaustion, Fatigue, Submaximal) to induce DOMS in the biceps brachii. DOMS (numeric pain rate scale, NPRS), pressure pain threshold (PPT) and skin temperature (infrared thermography, IRT) were measured on exercise day and 48 h later. The different exercises resulted in lower PPT responses 48 h after exercise and different DOMS reported across the different groups. Skin temperature outcomes did not differ following the different protocols. We found an increased minimum skin temperature 48 h after exercise in groups performing more intense exercises, but such differences were found in both exercised and non-exercised arms. Differently of PPT outcomes, pain reported depended on exercise intensity, and skin temperature 48 h after exercise could not show acute exercise adaptations. Skin temperature responses are contingent upon the characteristics of the participants rather than exercise intensity.
Similar content being viewed by others
Introduction
Exercise-induced muscle damage is associated with inflammation and phagocyte infiltration into the muscle, an increase in reactive oxygen species, and an elevation of cytokines1. The exercise-induced muscle damage promotes the sensitization of nociceptor terminals, leading to a greater sensation of pain2. Such a condition called delayed-onset muscle soreness (DOMS) commonly originates from abrupt increases in exercise loads, exercises with unusual ranges of motion, and especially after performing exercises requiring eccentric actions3. The time course of DOMS varies from 24 h up to 7 days after the physical exercise1. It is accompanied by increased muscle stiffness and sensitivity of the muscle to touch or pressure, reduced range of motion, and transient strength losses4.
DOMS is considered an indirect indicator of muscle damage5,6, and therefore, its assessment has important field applications. An analog scale commonly assesses DOMS by allowing quick feedback based on the participant’s perception. However, these methods depend on familiarization with the instructions and are influenced by the subjective factor of the pain assessment7. Furthermore, when utilizing these scales to assess DOMS, the participant being evaluated must possess consciousness and awareness of their condition, which may only sometimes be feasible in specific clinical contexts. For this reason, there is interest in tools that complement and improve DOMS assessment. Many studies investigate whether skin temperature measured using infrared thermography (IRT) could provide relevant information8. IRT is a non-invasive distance imaging technique that allows measuring surface temperatures by recording infrared radiation emitted by bodies9. Although promising results of IRT were observed for injury detection in sports medicine9,10, its application for DOMS assessment is still limited5,11.
The hypothesis of an inverted U relationship between skin temperature and internal training loads could explain the variability of results12. Physical exercise with high loads but without leading to muscle damage or DOMS could increase skin temperature due to increased blood flow. However, vasoconstriction happens when load elicits muscle damage, and an opposite behavior would be observed. As a result, a reduction or no alteration in skin temperature would be observed. Blood flow restriction has been studied as a strategy to mitigate DOMS—with no clear relationship found between the two—but little is known about this relationship in the DOMS production mechanism itself13. The connection between the activation of sympathetic vasoconstrictor neurons in the skin and an escalation of pain sensation is recognized in complex regional pain syndromes14. Therefore, we estimate that this relationship can also manifest itself in the context of physical exercise.
We consider analyzing skin temperature responses 48 h after exercises eliciting different magnitudes of DOMS as a plausible strategy to discuss the proposed inverted U relationship between skin temperature and exercise load. Although a previous investigation has investigated skin temperature responses to two different intensities of exercise, a condition of maximal exercise was not considered15. Such context would have clarified the possible vasoconstrictor response induced by a high pain sensation. Therefore, we determine whether different configurations of physical exercise eliciting different submaximal and maximal intensities can influence the magnitude of DOMS and skin temperature responses 48 h after exercise in healthy men and women. We hypothesized that exercise configuration influences DOMS, with higher intensities resulting in higher DOMS. However, we do not anticipate any differences in skin temperature outcomes in response to higher intensities, while skin temperature is expected to increase after lower intensities exercise protocols.
Results
Table 1 describes the demographic characteristics of the 39 participants assigned to the different exercise groups.
Rate of perceived exertion and exercise volume
Both the rate of perceived exertion (RPE, Fig. 1a) and exercise volume (Fig. 1b) differed between the exercise groups (Fig. 2). The Fatigue group showed higher RPE when compared to Exhaustion (F(2,14) = 6.57; p < 0.01, ESd = 0.00) but did not differ from Submaximal (p > 0.05). The Exhaustion group achieved higher exercise volume than Submaximal (p < 0.05; 308.00 IQR 463.5 AU vs. 120.00 IQR 120.00 AU, and ESW = 1.0). Exercise volume in Fatigue group did not differ of Exhaustion (p = 1.00) and Submaximal (p = 0.34).
Referred exertion and work volume. (a) Mean and standard deviation of rate of perceived exertion (RPE). (b) Median and interquartile range of work volume.Symbols identify differences between groups (†p < 0.05), and letters identify the magnitudes of effect sizes (small effect size ESdS or ESWS; moderate effect size ESdM or ESWM; large effect size ESdL or ESWL). Filled circles are the individual values of participants from each group.
Pain threshold results. (a) Mean and standard deviation of the delta (difference between 48 h after and pre-exercise) variation of pressure pain threshold (PPT) measured in the exercised arm. (b) PPT measures in the non-exercised arm in the three different groups assessed (Exhaustion, Fatigue, Submaximal). †Identifies the difference between groups (p < 0.05), and letters identify the magnitudes of effect sizes (small effect size ESFS; moderate effect size ESFM; large effect size ESFL). Filled circles are the individual values of participants from each group.
Delayed onset muscle soreness and pressure pain threshold
DOMS reported in the NPRS showed a group effect (p < 0.01). The Submaximal group showed higher values than Fatigue group 48 h after exercise (U = 28.500; p < 0.01, and ESr = 0.59). Comparisons between exercised and non-exercised arms were not considered in the analysis since participants consistently reported zero points for pain in the non-exercised arm.
Considering the three groups evaluated, DOMS increased 48 h after exercise in comparison to pre-exercise for the Exhaustion group (p < 0.01, 0.0 IQR 0.0 points vs. 6.00 IQR 4.00 points, and ESW = 1.0), Fatigue group (p < 0.01, 0.0 IQR 0.0 points vs 48 h 4.0 IQR 2.5 points and ESW = 1.0), and Submaximal group (p < 0.01, 0.0 IQR 0.0 points vs 6.5 IQR 1.3 points, and ESW = 1.0).
We determined PPT change considering the delta of difference between 48 h and the pre-exercise in the exercised arm (Fig. 2a) and found no differences were observed between groups (F(2.38) = 0.83; p = 0.44). In the non-exercised arm, the PPT did not differ across the different groups and time measures (Fig. 2b).
Skin temperature
The skin temperature outcomes in the non-exercised arm did not differ between moments (p > 0.05). No differences were found between groups considering the mean (p > 0.05; Fig. 3a) and maximum skin temperature (p > 0.05; Fig. 3c). Interestingly, non-exercised arm of the Exhaustion group showed higher minimum skin temperature than Submaximal group in pre-exercise (29.7 ± 1.4 °C vs. 27.7 ± 1.9 °C, F(2. 215) = 3.28; p < 0.05, ESF = 0.17, respectively; Fig. 3b) and 48 h post-exercise (30.4 ± 1.4 °C vs. 28.4 ± 1.8 °C, F(2. 215) = 3.78; p < 0.05, ESF = 0.18, respectively; Fig. 3b).
Absolute skin temperature values. Mean and standard deviation of (a) mean, (b) minimum and (c) maximum skin temperature outcomes in the exercised and non-exercised arms for groups Exhaustion, Fatigue, and Submaximal. †Identify the difference between groups at a moment (p < 0.05), and letters identify the magnitudes of effect sizes (small effect size ESFS; moderate effect size ESFM; large effect size ESFL). Filled circles are the individual values of participants from each group.
In the exercise arm, we also did not observe differences between moments to any skin temperature outcome (p > 0.05). In the group comparison, again the Exhaustion group showed higher minimum temperature than Submaximal in pre-exercise (29.3 ± 1.5 °C vs. 27.1 ± 2.2 °C, F(2. 215) = 4.20; p = 0.01, ESF = 0.19; Fig. 3b) and 48 h post-exercise (29.9 ± 2.0 vs. 27.6 ± 2.1 °C, F(2. 215) = 4.56; p = 0.01, ESF = 0.22; Fig. 3b). The delta of skin temperature [Δ48h = measure 48 h after – pre] did not differ between groups regarding mean (F(2.72) = 1.87; p = 0.16; Fig. 4a), minimum (F(2.72) = 0.31; p = 0.73; Fig. 4b) and maximum skin temperature (F(2,72) = 0.56; p = 0.57; Fig. 4c).
Variation in skin temperature. Delta of the skin temperature (ΔT, difference between 48 h post-exercise and pre-exercise) in the exercised arms for groups Exhaustion, Fatigue, and Submaximal and for control arm (Cont) and experimental arm (Expe). Delta was determined for (a) mean, (b) minimum, and (c) maximum temperature. Filled circles are the individual values of participants from each group.
The exploratory regression analysis showed no significant association between skin temperature variation at 48 h post-exercise and DOMS in the exercised arm (Fig. 5) for linear (p = 0.32) and quadratic regressions (p = 0.30).
Relationship between skin temperature and DOMS. Relationship between skin temperature variation (ΔT) at 48 h post-exercise and DOMS in the exercise arm Post48h, using linear regression (green line, results showed in the upper left side of the graphic) and quadratic regression (blue line, results showed in the upper right side of the graphic).
Discussion
In this study, we investigated whether various exercise configurations eliciting delayed-onset muscle soreness affect skin temperature and exercise-induced muscular pain outcomes differently. Although each exercise protocol led to a similar decrease of pressure pain threshold, we observed a difference in the pain perception, as measured by a numerical rating pain scale (NRPS). On the other hand, the different exercise protocols did not influence skin temperature 48 h after exercise, although we observed higher minimum skin temperature 48 h post-exercise following greater exercise intensity compared to lower intensities. The hypothetical U-inverted relationship between skin temperature variation at Post48h and DOMS was not significant in the exercised arm 48 h post-exercise.
One of our objectives of this study was to determine whether varying characteristics in the exercise configuration differently affect DOMS and skin temperature responses. The exercises varied in volume, consequently eliciting different intensities, as indicated by the RPE results. It subsequently allowed for the testing of hypotheses related to DOMS, skin temperature, and their associations with vasoconstriction mechanisms12. Our study extends findings from previous research in which maximal bouts of exercise were not considered15.
We found higher RPE in the Fatigue group than in the Exhaustion group. However, the RPE did not differ between the Fatigue and Submaximal groups. This behavior may result from the rest periods allowed between sets in the Exhaustion protocol, which could mitigate the perception of exertion. This is similar to the observed in an exercise involving high-intensity interval training (e.g., 20 s of exercise followed by 1-min recovery)16. A protocol consisting of one single set to exhaustion may lead to greater lactate accumulation17, which, without adequate time for metabolism, results in a burning sensation and increases the perceived exertion17,18.
Despite the differences in RPE, all exercise protocols were sufficiently demanding to produce DOMS, as depicted by the NRPS 48 h after exercise. But even with the high level of discomfort, participants were able to differentiate between the protocols. The NRPS is based on participant reports of overall discomfort. On the other hand, inducing DOMS in the same participants for all three protocols is not feasible. In such a scenario, the rebound effect of DOMS would hinder our ability to obtain reliable results2. Therefore, we also included a mechanical measure of pain, the pressure pain threshold (PPT). The PPT declined 48 h after exercise, with no difference in group comparison, what is expected considering the DOMS timeline3,19. This behavior in NRPS and PPT outcomes suggest that the various exercise configurations elicit distinct short-term adaptations and mechanisms, especially the type of receptors being stimulated in response to muscle damage.
The PPT outcomes decreased in response to different exercise protocols, but we did not observe differences in skin temperature 48 h after exercise in any group when compared to the measure pre-exercise. This may be attributed to the hypothesis that muscle damage causes muscle soreness but also suggests that muscle damage might induce peripherical vasoconstriction around the damaged muscles, potentially resulting in a cooling effect rather than warming20. In fact, we expected that the Submaximal group would show an increase in the skin temperature after exercise, which did not happen. The presence of the eccentric component, even in an exercise with low volume and intensity, was sufficient to induce muscle damage, as evidenced by the high sensation of DOMS experienced, which in a certain way may have depressed the skin temperature response. Our results demonstrated that changes in skin temperature might not be directly associated with the intensity of pain experienced.
The initial hypotheses suggesting a relationship between DOMS and increased skin temperature were predicated on the belief that muscle inflammation might lead to a rise in skin temperature after exercise. However, to date, no study has provided strong evidence to support this correlation8,20,21. We consider relevant to note that we evaluated participants who were physically active and submitted to a single session of exercise considering recruitment of a small muscle. It results in a different context compared to sports like soccer where different and larger muscle groups are recruited to perform the motor gesture. It is possible that this differences also reflects differences in IRT analyses22.
Skin temperature outcomes were generally consistent, except for the minimum skin temperature 48 h after exercise in the Exhaustion group when compared to the Submaximal. The minimum temperature appears to be more sensitive to changes in skin temperature induced by physical exercise. As we showed in a previous study involving an exercise protocol to induce cumulative fatigue, the minimum skin temperature may show a direct relationship with strength losses in elbow flexion strength after exercise (R2 = 0.25)23. However, here, we found the minimum skin temperature also demonstrated this difference in the non-exercised arm. We can explain this result due to a systemic effect of the thermoregulation24 along with body composition variability between subjects from each study25. Nevertheless, this difference was also observed in pre-exercise. It suggests that the participant’s characteristics such as body composition, fitness level and hydration level seem to influence the comparison between groups8,25,26. The measurement of skin temperature variation (Δ48h) did not show statistical differences between members and groups, and showed great variability between subjects. Future studies could explore whether any characteristic of the participants makes them “responders” in thermographic analysis.
Knowing that studies have not supported the relationship between a possible muscular inflammatory process and increased skin temperature, we constructed a protocol with different exercise intensities in order to explore the hypothesis of exercise dosage on the accuracy of IRT. The skin temperature measurements 48 h after exercise remained unchanged from pre-exercise levels in the different exercise groups, also for the mean temperature, which is a reliable metric for larger areas of interest and less likely to yield false positives or negatives due to varying temperature points27. One might note that in a previous study using a similar protocol to induce DOMS, authors observed an increase in skin temperature 24 h post-exercise5. However, despite the measure being taken before the expected time of peak for DOMS, the study also found temperature increases in both exercised and non-exercised arms, with no significant differences between them. The authors suggested a systemic inflammatory response occurring 24 h after exercise. But, from our perspective, this suggests that the exercise might not be responsible for the observed changes.
It is crucial to note that the complete mechanism of DOMS remains a topic of ongoing debate28. The connection between the activation of sympathetic vasoconstrictor neurons in the skin and the sensation of pain has been previously observed in pathological pain scenarios29. Although this relationship is still speculative in the context of DOMS, it aligns with findings from earlier studies that failed to establish a link between changes in skin temperature, muscle damage, and DOMS11,30. For instance, considering the gastrocnemius muscle and a similar approach of the Exhaustion group for DOMS induction, higher CK activity and DOMS were observed (p < 0.01), but changes in skin temperature were not significant 48 h after exercise and did not relate to the kinetics of CK activity11.
Future research should consider assessing the peripheral blood flow using laser Doppler. Combined with IRT measurements, this approach could yield valuable insights into the effectiveness of IRT in monitoring the acute adaptations triggered by exercise and understanding the etiology of delayed onset muscle soreness.
Our study has inherent limitations. We did not evaluate muscle strength and blood markers of muscle damage, such as CK, which could strengthen our understanding of the relationship between damage magnitude, DOMS, and skin temperature responses to different exercise intensities.
Different exercise intensities and volumes led to lower pressure pain threshold responses despite eliciting different reports of pain using a numerical pain rate scale. Changes in skin temperature after exercise did not differ between the different exercise configurations tested. Moreover, the minimum skin temperature was higher 48 h after high-intensity exercise than low-intensity exercise. Still, this behavior was observed in both exercised and non-exercise arms, limiting its association with the effect of exercise.
From our perspective, employing post-exercise skin temperature measurements to monitor muscle damage and DOMS in the biceps brachii might not be an alternative method to monitor acute exercise adaptations. The dependence of skin temperature on the participant’s characteristics and not on the magnitude of muscle damage and intensity of the exercise requires further investigation.
Material and methods
Participants and experimental design
A sample size of 39 participants, including men and women, was determined using the G* Power 3.1 software (University of Düsseldorf, Düsseldorf, Germany) considering a large effect size expected for mean skin temperature (effect size F = 0.4) in a repeated measures ANOVA design including three factors (arm with 2 levels, moment with 2 levels and intensity with 3 levels), with 80% power, and an error of 10%.
As inclusion criteria, the participants should self-report as healthy age, between 18 and 40 years old, not a smoker, not have a history of previous injury to the upper limbs in the last six months, and be classified as physically active according to the International Physical Activity Questionnaire31. In addition, the participants could not intake medications, supplements, and stimulants during their entire participation in the study. All participants performed their measurements and exercise in the afternoon to avoid the effects of the circadian cycle on physical performance, soreness and temperature measurements8,32.
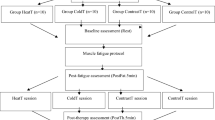
All participants were randomly distributed (1:1:1, using random.org) in three groups (see Table 1). Each group performed exercises with different intensity characteristics (exhaustion, fatigue, submaximal). They were evaluated pre-exercise and 48 h after exercise. The evaluations involved demographic measures for participants’ characterization, rate of perceived exertion (RPE), skin temperature, pain report using a numerical rate scale, and pressure pain threshold (PPT). All participants signed an informed consent form, agreeing to participate voluntarily. The local institution’s ethics committee approved this study (Federal University of Pampa, IRB #26037119.9.0000.5323), and all procedures complied with the Declaration of Helsinki.
Exercise protocols
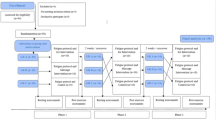
Three variations of a unilateral exercise protocol based on concentric and eccentric muscle actions were used to induce muscle damage and DOMS was used based on previous studies with similar goals23,33. The dumbbell biceps curl exercise involved elbow flexion (concentric phase) and extension (eccentric phase) performed with the non-preferred arm holding a dumbbell of 3 kg for men and 2 kg for women34. These magnitudes of dumbbell masses were defined according to a previous study23. Participants exercised seated on a bench, with the non-exercised arm relaxed along the body. The movement speed was set at 40 repetitions/min, controlled by a metronome. A researcher continuously provided verbal feedback regarding the quality of movement. The three exercise protocols are described below:
Exhaustion
All participants executed multiple sets to exhaustion. Initially, they performed the first set, aiming at the maximal repetition of bicep curls until exhaustion. After a rest period of equal duration to the first set of exercises, participants performed a second set of repetitions, which they should have performed at least 75% of the repetitions achieved in the first set. Additional series were completed until the participant could no longer perform more than 50% of the repetitions number from the first set or there was a significant loss in the movement quality. In these cases, the protocol ended33.
Fatigue
All participants completed one set aiming at the maximum voluntary repetitions until exhaustion. This protocol involved performing only the first phase of the protocol described for the exhaustion group. The protocol was finished when the participant could no longer perform the movements or when loss of movement quality was observed.
Submaximal
All participants executed multiple sets without reaching exhaustion. This protocol involved performing a fixed number of sets and repetitions. The participants were instructed to perform four sets of 15 repetitions for full elbow range of motion, with a 30-s between-set interval.
Assessment of perceived exertion and exercise volume
The rate of perceived exertion (RPE) was assessed immediately when the exercise ended using the 6 to 20-point Borg scale35. The exercise volume was estimated by the product between the number of repetitions x number of sets x external load23.
Muscle soreness and pressure pain threshold assessment
DOMS was quantified using a numeric rating pain scale (NPRS), where 0 represents (no pain), and 10 represents (extreme pain)36. All participants were instructed to sit on a chair with their muscles relaxed, feet flat on the ground, and knees shoulder-width apart. Upon returning to the laboratory 48 h after the exercise, they were requested to rate pain when they performed an elbow extension without external resistance with the previously exercised arm23.
Afterward, the pressure pain threshold (PPT) was quantified bilaterally in response to a mechanical pressure stimulus in the central portion of the biceps brachii muscle belly. PPT was measured using a digital algometer with a resolution of 0.01 N and a flat tip measuring 1 cm2. The algometer tip was positioned perpendicular to the region of interest and pressed against the skin always by the same researcher, following the procedures previously described37. All participants were positioned on a stretcher in a prone position, being asked to report the moment when the pressure sensation became a pain sensation. Both DOMS and Pain Threshold assessments were evaluated prior to any other assessment (except for IRT) on days 1 (pre-DOMS induction) and 48 h post-exercise.
Skin temperature assessment
Skin temperature was determined using an infrared thermal camera with a resolution of 320 × 240 pixels (FLIR model E-60, Flir Systems Inc., Wilsonville, OR, USA) with noise-equivalent temperature difference (NETD) < 0.05 °C, and measurement uncertainty of ± 2 °C or 2%. The camera was turned on for 10 min before capturing the images to ensure its stabilization8. For image capture, the camera was positioned 1 m away from the participant, with the lens aligned perpendicularly to the regions of interest (ROIs). Images were captured before any other evaluation to avoid the effect of skin contact. The participant was standing, wearing comfortable clothes, with ROIs exposed after a 10-min room thermal adaptation38. An anti-reflective panel was placed behind the participant to minimize the effects of infrared radiation reflected by a non-neutral background38. All thermal images were taken in an air-conditioned and controlled environment: room temperature (day 1, pre-exercise): 18.7 ± 1.9 °C and (day 2, 48 h post-exercise): 19.4 ± 1.6 °C (p = 0.076) and relative humidity (day 1, pre-exercise): 67.6 ± 8.9% and (day 2, 48 h post-exercise): 68.2 ± 9.5% (p = 0.334). Data was collected in the afternoon (between 02:00 and 06:00 PM). The thermal image capture was performed based on all data collection procedures described in the TISEM checklist38.
The ROIs were defined considering the anterior region of the arm of both limbs (Fig. 6). The axillary cleavage was taken as a proximal reference to determine the ROIs. Posteriorly, the evaluator drew a horizontal line on the lateral edge of the skin, skirting the skin distally to the arm and meeting the upper line of the antecubital fossa (anterior cleavage of the elbow); then, a transverse line was drawn to the medial border of the arm; and finally, the arm was circumvented proximally until the starting point was found23. Skin temperature data were obtained using commercial software (ThermaCAM Researcher Pro 2.10, FLIR, Wilsonville, OR, USA), considering mean, maximum, minimum temperature, amplitude (maximum-minimum), and standard deviation, using an emissivity of 0.98. The difference between 48 h post-exercise and pre-exercise (Δ48h-pre) was determined.
Example of two thermograms. Representation of the two regions of interest (ROIs) considered for the skin temperature data extraction. The image presents the exercised arm (left arm) and non-exercise arm (right arm), and it is representative of all participants. A. Represents pre-exercise, B. represents post-exercise.
Statistical analysis
The normality of data distribution was checked using the Shapiro–Wilk test. Parametric data are reported with mean and standard deviation and non-parametric data are reported with median and interquartile range (IQR). DOMS, temperature, relative humidity and exercise volume showed non-parametric distribution. Pairs were tested with Mann–Whitney test. The Kruskal–Wallis test was performed to evaluate the differences between groups with Mann–Whitney U post-hoc to evaluate the differences between groups for each moment, and the Friedman test with Dunn-Bonferroni post-hoc was performed to evaluate differences between the moments for each group. The effect size r (ESr) was determined for Kruskal–Wallis tests and classified as small (ESr > 0.10), medium (ESr > 0.30) and large (ESr > 0.50)39. Also, Kendall’s coefficient of concordance [Kendall’s W; ESW] was computed for the pair significant non-parametric differences (p < 0.05) and classified as small (ESW 0.2–0.4), moderate (ESW 0.5–0.7), or large (ESW > 0.8)39. The skin temperature outcomes were compared between the exercise groups with a two-way analysis of variance ANOVA with Bonferroni corrections considering three factors: arm (non-exercise x exercised), moments (pre, and 48 h after exercise), and group (Exhaustion, Fatigue, Submaximal). The effect size index f (ESF) was determined for the ANOVAs and classified as large (ESFL > 0.4), medium (ESFM of 0.26–0.4), and small (ESFS of 0.1–0.25)39. The variation of PPT and RPE were compared between groups with one-way ANOVA with the Tukey post hoc test. Also, for significant pair parametric differences, Cohen’s d effect sizes (ESd) were computed and classified as small (ESdS 0.2–0.4), moderate (ESdM 0.5–0.7), or large (ESdL > 0.8)39. Finally, an exploratory analysis was performed to assess the hypothetical U-inverted relationship between skin temperature variation at Post48h and DOMS in the exercised arm at Post48h, using linear regression and quadratic regression. All analyses were performed using SPSS version 21 software (SPSS Inc., Chicago, IL, USA) and RStudio (version 2022.02.03), considering an alpha set at 5%.
Data availability
The datasets generated during and/or analysed during the current study are available in the ResearchGate repository, https://doi.org/10.13140/RG.2.2.10727.05284.
References
Aoi, W. et al. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic. Biol. Med. 37, 480–487 (2004).
Mizumura, K. & Taguchi, T. Delayed onset muscle soreness: Involvement of neurotrophic factors. J. Physiol. Sci. 66, 43–52 (2016).
Cheung, K., Hume, P. A. & Maxwell, L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med. 33, 145–164 (2003).
Fernandes, T. L., Pedrinelli, A. & Hernandez, A. J. Muscle injury: Physiopathology, diagnosis, treatment and clinical presentation. Rev. Bras. Ortop. Engl. Ed. 46, 247–255 (2011).
Stewart, I. B. et al. Thermal infrared imaging can differentiate skin temperature changes associated with intense single leg exercise, but not with delayed onset of muscle soreness. J. Sports Sci. Med. 19, 469–477 (2020).
Chalchat, E. et al. Appropriateness of indirect markers of muscle damage following lower limbs eccentric-biased exercises: A systematic review with meta-analysis. PLoS ONE 17, e0271233 (2022).
Katz, J. & Melzack, R. Measurement of pain. Surg. Clin. North Am. 79, 231–252 (1999).
Application of Infrared Thermography in Sports Science (Springer International Publishing, 2017). https://doi.org/10.1007/978-3-319-47410-6.
Hildebrandt, C., Raschner, C. & Ammer, K. An overview of recent application of medical infrared thermography in sports medicine in Austria. Sensors 10, 4700–4715 (2010).
Priego-Quesada, J. I., Oficial-Casado, F., Gandia-Soriano, A. & Carpes, F. P. A preliminary investigation about the observation of regional skin temperatures following cumulative training loads in triathletes during training camp. J. Therm. Biol. 84, 431–438 (2019).
da Silva, W. et al. Can exercise-induced muscle damage be related to changes in skin temperature?. Physiol. Meas. 39, 104007 (2018).
Priego-Quesada, J. I. et al. Effect of a marathon on skin temperature response after a cold-stress test and its relationship with perceptive, performance, and oxidative-stress biomarkers. Int. J. Sports Physiol. Perform. 15, 1467–1475 (2020).
Rodrigues, S. et al. Effect of blood flow restriction technique on delayed onset muscle soreness: A systematic review. Medicina 58, 1154 (2022).
Baron, R., Schattschneider, J., Binder, A., Siebrecht, D. & Wasner, G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: A case-control study. The Lancet 359, 1655–1660 (2002).
Barboza, J. A. M. et al. Can skin temperature be altered after different magnitudes of eccentric exercise-induced muscle damage?. Res. Q. Exerc. Sport 93, 702–709 (2022).
De Salles, B. F. et al. Rest interval between sets in strength training. Sports Med. 39, 765–777 (2009).
Rabinowitz, J. D. & Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2, 566–571 (2020).
Jorfeldt, L., Juhlin-Dannfelt, A. & Karlsson, J. Lactate release in relation to tissue lactate in human skeletal muscle during exercise. J. Appl. Physiol. 44, 350–352 (1978).
Lacourpaille, L. et al. Time-course effect of exercise-induced muscle damage on localized muscle mechanical properties assessed using elastography. Acta Physiol. 211, 135–146 (2014).
Priego-Quesada, J. I. et al. Relationship between Skin temperature, electrical manifestations of muscle fatigue, and exercise-induced delayed onset muscle soreness for dynamic contractions: A preliminary study. Int. J. Environ. Res. Public. Health 17, 6817 (2020).
Escamilla-Galindo, V. L. et al. Skin temperature response to unilateral training measured with infrared thermography. J. Exerc. Rehabil. 13, 526–534 (2017).
Majano, C. et al. Association between physical demands, skin temperature and wellbeing status in elite football players. Sci. Rep. 13, 13780 (2023).
Machado, A. S. et al. Green tea supplementation favors exercise volume in untrained men under cumulative fatigue. Sci. Sports 38, 385–393 (2023).
Kenny, G. P. Human thermoregulation: Separating thermal and nonthermal effects on heat loss. Front. Biosci. 15, 259 (2010).
Korman, P. et al. Exploring the correlation of skin temperature and body composition in athletes undergoing exhaustive physical exercise. J. Therm. Biol. 123, 103918 (2024).
Charkoudian, N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J. Appl. Physiol. 109, 1221–1228 (2010).
Machado, Á. S. et al. Influence of infrared camera model and evaluator reproducibility in the assessment of skin temperature responses to physical exercise. J. Therm. Biol. 98, 102913 (2021).
Markus, I., Constantini, K., Hoffman, J. R., Bartolomei, S. & Gepner, Y. Exercise-induced muscle damage: Mechanism, assessment and nutritional factors to accelerate recovery. Eur. J. Appl. Physiol. 121, 969–992 (2021).
Chalacheva, P. et al. High levels of peripheral vasoconstriction detected by polysomnography predict more acute severe pain episodes in children with sickle cell anemia. Blood 134, 894–894 (2019).
Doménech-García, V., Boudreau, S. A., Giner-Nicolás, R. & Bellosta-López, P. Skin temperature normalizes faster than pressure pain thresholds, pain intensity, and pain distribution during recovery from eccentric exercise. J. Therm. Biol. 111, 103423 (2023).
Matsudo, S. et al. Questionário internacional de atividade física (IPAQ): Estudo de validade e reprodutibilidade no Brasil. Rev. Bras. Atividade Física Saúde 6, 5–18 (2012).
Bumgarner, J. R., Walker, W. H. & Nelson, R. J. Circadian rhythms and pain. Neurosci. Biobehav. Rev. 129, 296–306 (2021).
Thedon, T., Mandrick, K., Foissac, M., Mottet, D. & Perrey, S. Degraded postural performance after muscle fatigue can be compensated by skin stimulation. Gait Posture 33, 686–689 (2011).
Rodrigues, F., Jacinto, M., Figueiredo, N., Monteiro, A. M. & Forte, P. Effects of a 24-week low-cost multicomponent exercise program on health-related functional fitness in the community-dwelling aged and Older adults. Medicina 59, 371 (2023).
Borg, G. A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14, 377–381 (1982).
Hawker, G. A., Mian, S., Kendzerska, T. & French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF). Arthritis Care Res. 63, S240–S252 (2011).
Pöntinen, P. J. Reliability, Validity, Reproducibility of Algometry in Diagnosis of Active and Latent Tender Spots and Trigger Points. J. Musculoskelet. Pain 6, 61–71 (1998).
Moreira, D. G. et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement of human skin temperature. J. Therm. Biol. 69, 155–162 (2017).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Academic Press, 1977).
Acknowledgements
This study was partially funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES, Financing Code 001) funding WS, by the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERG) with an undergraduate research funding ACLM, and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico funding FPC (CNPq, process 406715/2018-10) and ASM (process 169461/2023-8).
Author information
Authors and Affiliations
Contributions
ACL: Methodology, Validation, Formal analysis, Data curation, Writing, Review and editing. ASM: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing, Review and editing. MEF: Methodology, Formal analysis, Writing, Review and editing. WS: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing, Review and editing. JIPQ: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing, Review and editing, Supervision. FPC: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing, Review and editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
de Morais, A.C.L., Machado, Á.S., Pereira, M.E.F. et al. Intensity and volume of physical exercise influence DOMS and skin temperature differently in healthy adults. Sci Rep 14, 30282 (2024). https://doi.org/10.1038/s41598-024-79785-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79785-2