Abstract
Reduced muscle mass has been associated with the progression and prognosis of amyotrophic lateral sclerosis (ALS). However, it remains unclear whether decreased muscle mass is a risk factor for ALS or a consequence of motor neuron degeneration. Recently, serum creatinine-to-cystatin C ratio (CCR) have emerged as promising biomarkers for assessing muscle mass. We aimed to explore the association between CCR and the incidence of ALS using data from the UK Biobank. Between 2006 and 2010, 446,945 participants were included in the baseline. CCR was calculated as the ratio of serum creatinine to cystatin C. Cox regression models were used to analyze the relationship between CCR and ALS incidence. Furthermore, subgroup analyses were conducted to investigate potential covariates in these relationships. After adjusting for all covariates, the multivariate Cox regression analysis revealed a significant association between decreased CCR and an increased risk of ALS (hazard ratio (HR) = 0.990, 95% confidence interval (CI): 0.982–0.999, P = 0.026). Participants were stratified into groups based on CCR tertiles. Compared with participants in the highest tertiles of CCR, those in the lowest (HR = 1.388, 95% CI: 1.032–1.866, P = 0.030) and medium tertiles (HR = 1.348, 95% CI: 1.045–1.739, P = 0.021) had an increased risk of ALS incidence. Subgroup analysis showed that the relationship between CCR and ALS incidence was particularly significant among participants aged < 65 years (CCR tertile 1: HR = 1.916, 95% CI: 1.366–2.688, P < 0.001; CCR tertile 2: HR = 1.699, 95% CI: 1.267–2.278, P < 0.001). The present results demonstrate that lower CCR is significantly associated with a higher risk of ALS.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive muscle weakness resulting from motor neuron loss. This condition typically leads to death from respiratory failure within two to four years of diagnosis1. The etiology of ALS remains elusive, involving a complex interplay of genetic factors, chronic environmental exposures, and lifestyle influences. Furthermore, current therapeutic options are limited and unable to reverse disease progression. The poor prognosis of ALS imposes a significant burden on families and society. Therefore, early identification and management of ALS risk factors are critical for establishing effective prevention strategies.
Numerous observational studies have investigated the relationship between muscle mass and ALS2,3,4. Traditionally, ALS involves muscle denervation during disease progression, and reduced muscle mass at diagnosis is associated with accelerated disease progression and decreased survival5,6,7,8. However, recent studies have highlighted that skeletal muscle also plays a key role in the incidence of ALS9,10,11. The gold standard for muscle mass measurement includes dual-energy X-ray absorptiometry, computed tomography (CT), bioelectrical impedance analysis (BIA), and magnetic resonance imaging (MRI)12,13,14. However, these methods are not widely used due to their high cost, radioactivity and infeasibility15. Therefore, the use of accessible and cost-effective serum markers is particularly crucial. Recently, a novel serum creatinine-to-cystatin C ratio (CCR) developed by Kashani et al., has shown a positive relationship with muscle mass16. This ratio has demonstrated satisfactory diagnostic accuracy for muscle mass in various diseases17,18,19,20 and has been associated with the severity of muscle loss in a cohort of patients with ALS21. Despite this, it remains unclear whether lower CCR can increase the incidence of ALS. Given that CCR is influenced by factors such as age, sex, body weight, and renal function, we conducted stratified analyses for these covariates individually22,23.
Therefore, the main purpose of this study was to investigate the relationship between CCR and ALS incidence. And this was done by using survey data of the UK Biobank.
Methods
Study population
The study population was derived from the UK Biobank, a large-scale longitudinal cohort study. Over 500,000 participants aged 40–69 years were recruited between 2006–2010. The participants were from 22 baseline assessment centers in England (89%), Scotland (7%), and Wales (4%)24. During the baseline assessment, participants provided sociodemographic, lifestyle, and health-related information through touch-screen questionnaires and verbal interviews, underwent a series of physical examinations, and provided biological samples. Longitudinal samples were obtained through cohort-wide electronic medical records. All participants provided informed consent.
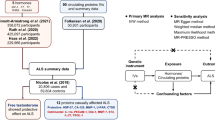
Of the 502,357 participants at baseline in the UK Biobank, 78 participants reported a personal history of ALS at baseline, 33,475 participants lacked information on serum creatinine and cystatin C, and 21,859 participants had an estimated glomerular filtration rate (eGFR) of < 60 mL/min/m2 using CKD-EPI Cystatin C equation25. After excluding these participants, data from 446,945 individuals were included in the primary analysis (Fig. 1).
Flowchart of the study. Participants with blood tests and traceable outcome data were eligible for Cox proportional hazards analysis after excluding those with prevalent ALS or eGFR < 60 mL/min/m2. ALS = amyotrophic lateral sclerosis; eGFR = estimated glomerular filtration rate; CCR = creatinine-to-cystatin C ratio; BMI = body mass index.
The UK Biobank study received ethical approval from the North West Multi-center Research Ethics Committee (https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics). Further information about the biobank data can be found at https://www.ukbiobank.ac.uk/. This study was conducted under the UK Biobank resource request number 108832.
Incident ALS
The primary outcome of this study, ALS events, was determined from hospitalized patient records in England, Scotland, and Wales. Hospital records were obtained from the Hospital Episode Statistics (HES) in England, the Scottish Morbidity Record in Scotland, and the Patient Episode Database in Wales. The diagnosis of ALS was classified according to the International Classification of Diseases, 10th Revision codes (ICD-10), G12.2 (motor neuron disease). ALS is used as an umbrella term for this group of motor neuron diseases. The reliability of identifying an ALS diagnosis in the UK biobank has been well established and with high positive predictive value estimates ranging from 70 to 91%26.
Serum creatinine-to-cystatin C ratio collection and calculation
The sample collection and processing procedure in the UK Biobank have been described in detail elsewhere. Briefly, blood samples were collected at baseline assessment centers, stored at -80 in two separate locations. Serum creatinine was measured by an enzymatic method on a Beckman Coulter AU5400 clinical chemistry analyzer using the manufacturer’s reagents and calibrators. Serum cystatin C was measured using a latex-enhanced immunoturbidimetric method on a Siemens Advia 18,005. Based on the serum creatinine and cystatin C measurements, CCR was calculated as CCR = creatinine / cystatin C.
Statistical analyses
Based on the tertile boundaries of CCR, participants were divided into 3 groups, with tertile 3 as the reference group27.
The baseline characteristics of participants were summarized for those with and without incident ALS, reported as medians and quartiles for continuous variables and as numbers and percentages for categorical variables.
A multivariate Cox proportional hazards regression model was used to estimate the relationship between CCR and ALS incidence. CCR was entered into the model as both a continuous and categorical variable. The study period spanned from the date of baseline assessment to the date of first diagnosis of ALS, death, loss to follow-up, or end of follow-up, whichever occurred first. The end of the follow-up was based on the availability of medical record data in the UK biobank. The end of follow-up was based on the availability of medical record data in the UK Biobank, which was reviewed on September 30, 2021, in England, July 31, 2021, in Scotland, and February 28, 2018, in Wales. Model 1 was adjusted for demographic variables including age at recruitment and sex. Model 2 was further adjusted for socioeconomics factors, including education and Townsend deprivation index (TDI). Model 3 was further adjusted for lifestyle factors (smoking status, drinking, body mass index (BMI)) and risk factors associated with ALS (diabetes28, serum glucose29,30, C-reactive protein (CRP)31,32, serum albumin33, and sleep duration34). Since ALS often takes more than a year to be diagnosed after the onset of the disease, Model 4 further excluding the ALS patients with less than 1 year of follow-up. We assessed multicollinearity using variance inflation factors (VIF).
Given the differences in CCR by sex, age, and BMI, subgroup analyses were performed by gender (male vs. female), age (< 65 years and ≥ 65 years), and BMI (< 25 kg/m2 and ≥ 25 kg/m2). Since CCR is calculated through two serum markers, both of which were used to assess the kidney function. We also examined the effect of eGFR (< 90 mL/min/m2 and ≥ 90 mL/min/m2) on CCR-ALS association. The above analyses were adjusted for age, sex, education, TDI, smoking status, drinking, BMI, diabetes, serum glucose, CRP, serum albumin, and sleep duration.
A series of sensitivity analyses were conducted to test the robustness of our results. 1) To evaluate the potential reverse causation biases, we performed the analyses by excluding the ALS patients with less than 3 years and 10 years of follow-up. 2) We repeated analyses after imputing missing covariates through multiple imputation. We used predictive mean matching (PMM) to handle missing data, setting the number of imputations to 5 (m = 5). This means that 5 different complete datasets were generated, each providing reasonable estimates for the missing values. This approach helps reduce bias due to missing data and enhances the reliability of the results. 3) We excluded extreme values of CCR (mean ± 3SD) for further analysis.
All statistical analyses were carried out using R version 4.2.1.
Results
Baseline characteristics of study participants
The baseline characteristics of the participants are presented based on incident ALS status (Table 1). Overall, the mean age of the participants was 56.21 years (± 8.07 years), and 204,331 (45.72%) were men. During the median follow-up period of 13.76 years, 589 participants developed ALS. Participants with ALS had higher cystatin C levels (P < 0.001) compared to those without ALS. Additionally, participants with ALS had lower CCR and eGFR (P < 0.05) than those without ALS. There was no significant difference in creatinine levels between participants with and without ALS.
Relation between CCR and ALS incidence
The correlation between CCR and ALS incidence was demonstrated by cox regression analyses. Lower CCR was significantly associated with a higher incidence of ALS when adjusting for age and sex in model 1 (hazard ratio (HR): 0.989, 95% confidence interval (CI): 0.982–0.996, P = 0.002). This association remained consistent in model 2 after further adjustment for socioeconomic indicators (education level and TDI) (HR: 0.990, 95%CI: 0.982–0.998, P = 0.011), and in model 3 after additional adjustment for smoking status, drinking, BMI, diabetes, serum glucose, C-reactive protein (CRP), serum albumin, and sleep duration (HR: 0.990, 95%CI: 0.982–0.999, P = 0.026). Participants were divided into three groups based on the tertile boundaries of CCR (73.776 and 86.191). In all three models, the risk of ALS incidence for CCR tertile 1 and CCR tertile 2 were 1.388 (95% CI: 1.032–1.866, P = 0.030) and 1.348 (95% CI: 1.045–1.739, P = 0.021) compared to those in CCR tertile 3. Model 4 remains consistent with previous results (Table 2). No multicollinearity among predictor variables.
Subgroup analyses and sensitivity analysis
We conducted subgroup analyses based on age, sex, BMI, and eGFR to explore the effects of variables on CCR and the incidence of ALS. We categorized individuals into two groups: young and middle-aged participants aged < 65 years, and older individuals aged ≥ 65 years. Among participants younger than 65 years, the risk of ALS incidence for those in CCR tertile 1 and CCR tertile 2 were 1.916 (95% CI: 1.366–2.688, P < 0.001) and 1.699 (95% CI: 1.267–2.278, P < 0.001) compared to those in CCR tertile 3. Moreover, smoking status, drinking, BMI, and diabetes do not affect the results (Table 3).
Sensitivity analyses were conducted to evaluate the robustness of the results. Participants with a short latency from initial sampling to diagnosis (3-year lag or 10-year lag) were excluded with the aim of excluding reverse causality, which did not substantially affect the results. Furthermore, the association between CCR and ALS risk was tested by repeating the analyses after imputing missing covariates or removing extreme values of CCR. The conclusion remained stable (Table 4).
Discussion
The main finding of this study was that lower CCR was significantly correlated with a higher incidence of ALS. Our study provided convincing results after adjusting for numerous potential confounders in Cox regression models. These findings were robust across a range of sensitivity analyses, indicating that they are unlikely to be influenced by confounders or reverse causality. Stratified analysis further revealed that the impact of CCR on ALS incidence is age-dependent.
Serum creatinine and cystatin C are markers used to assess glomerular filtration rate and kidney function35,36. Serum creatinine, primarily generated in muscle through the metabolism of phosphocreatine and creatine, is significantly influenced by the amount of skeletal muscle mass. Its concentration typically remains stable if muscle mass is unchanged. However, serum creatinine levels are also affected by kidney function, which limits its reliability as a sole indicator of muscle mass37,38. Cystatin C is also freely filtered in the glomeruli. Unlike creatinine, this protein is a small nonionic protein excreted by all nucleated cells and usually remains stable. Its production is less affected by muscle mass39. Given the characteristics of serum creatinine and cystatin C, the CCR was developed to assess muscle mass16. It has been validated as a more reliable measure of muscle mass changes in ALS compared to serum creatinine16. Both creatinine and cystatin C can be quickly measured by blood sampling, making CCR a significant advantage for risk monitoring in large populations.
Several observational studies have shown that ALS patients experience muscle mass loss compared to normal controls. Reduced muscle mass serves as an indicator of poor prognosis in ALS and is associated with rapid disease progression6,7,8. Adequate nutritional support may slow ALS progression by preventing muscle mass loss40,41,42. However, these studies cannot determine whether muscle mass loss is an effect of ALS or a result of motor neuron or peripheral nerve degeneration. To our knowledge, this is the first study to demonstrate a prospective relationship between low CCR and the risk of developing ALS decades before a clinical diagnosis. We hypothesized that the previously reported association between low BMI and the incidence of ALS43 may reflect early changes in muscle mass. Our study suggests that muscle mass plays a role in ALS pathogenesis. Targeting skeletal muscle is particularly important, and there have been some advances in this area. For example, mild to moderate physical activity has been shown to increase muscle mass, improve quality of life, and raise ALSFRS-R scores in ALS patients44,45,46. Additionally, preclinical studies have produced encouraging data for drugs that increase muscle mass, including creatine, anabolic androgenic steroids (AAS), and myostatin inhibitors40,41,42. These studies suggest that therapies targeting skeletal muscle in ALS are very promising. Therefore, further clinical trials are needed for additional support and validation.
The exact mechanism linking CCR and ALS remains unclear, but several possibilities exist. Firstly, serum creatinine is produced by the metabolism of creatine and phosphocreatine, which are essential for mitochondrial function as demonstrated in both in vivo and in vitro studies47. Mitochondrial dysfunction has been proved to contribute to ALS pathogenesis48. In addition, several studies show creatine has antioxidant capacity and neuroprotective function49,50. Therefore, CCR may influence ALS through mechanisms involving mitochondrial dysfunction and oxidative stress. Secondly, the neuromuscular junction (NMJ) connects muscle fibers and motor neurons, facilitating communication between them. ALS is the classic example of severely compromised communication between muscles and nerves51. Motor neuron activity regulates muscle physiology and function, while muscles, in turn, affect neuronal activity by sending retrograde signals that preserve NMJ functionality and structure52. The so-called “dying back” hypothesis suggests that retrograde signals from skeletal muscle contribute to the centripetal motor neuron degeneration in ALS53. Thirdly, a recent study indicated that muscle vesicles in ALS patients are toxic to motor neurons, suggesting that skeletal muscle might be a source of vesicle-mediated toxicity in ALS54.
In subgroup analyses, we found that reduced CCR was associated with an increased risk of ALS incidence in participants aged ≤ 65 years. There are few studies on the association between muscle mass and ALS across different age groups. There is a potential mechanism. In the elderly, reduced muscle mass is mainly due to impaired muscle anabolism, whereas in young adults, it is mainly due to increased muscle catabolism driven by various factors55. Hypermetabolism significantly impacts ALS development. Because muscle activity is a major contributor to whole-body energy metabolism, increased muscle catabolism may play a crucial role in ALS hypermetabolism56. However, due to the selection bias of age with 39 to 72 years in the UK Biobank, the age-dependent pattern in the correlation of CCR on ALS may need further exploration.
It is important to note that in the primary analyses, we corrected for conventional demographic variables, socioeconomic factors, and lifestyle factors, as well as ALS-related risk factors, including diabetes, serum glucose, CRP, serum albumin, and sleep duration. Firstly, disorders of glucose metabolism are now gaining great attention in ALS. These disorders usually occur early in the course of ALS, even before disease onset. Our team published a mendelian randomization study demonstrating that genetically predicted type 2 diabetes is associated with a significantly lower incidence of ALS28. Other studies have shown an association between higher blood glucose levels and a reduced risk of ALS29,30. Secondly, CRP, the most commonly studied biomarker of inflammation, has also been linked to ALS. Studies have found that elevated levels of CRP are associated with a higher risk of mortality and faster disease progression in ALS32. Furthermore, a recent prospective study has shown that higher CRP levels are associated with an increased risk of developing ALS31. Thirdly, albumin is a non-glycosylated plasma protein whose metabolism is affected by nutritional intake and inflammatory diseases. Serum albumin is reduced in patients with ALS compared with healthy controls57,58, and lower serum albumin levels at diagnosis are associated with a poorer prognosis in ALS33,59. Finally, several studies have reported a high prevalence of sleep disorders in ALS patients60. Our team has also previously demonstrated an association between sleep and the risk of developing ALS34.
Our study has several methodological strengths. First, because of the rarity of ALS, identifying potential risk factors requires a large cohort population. The sample size of the UK Biobank is sufficiently large to identify potential risk factors for ALS onset. In addition, most epidemiological studies on ALS risk factors are case–control studies, which carry inherent risks of referral, selection, and recall bias. The prospective cohort design of the UK Biobank mitigates these biases to some extent. However, some limitations must be acknowledged. Most participants in this study were of European descent, so the conclusions may not be generalizable to other ethnic groups. We hope to validate our results in other populations in the future. Moreover, most socio-demographic, lifestyle, and health-related factors were self-reported, which may obscure risk estimates due to recall bias. Although we adjusted for major confounders, residual confounding by unknown or unmeasured factors remains a possibility. Furthermore, despite excluding subjects with ALS at baseline and patients with disease onset within 3 and 10 years, residual confounding is likely in any observational study. Reverse causality remains possible, and causality cannot be deduced from the association. Finally, since the diagnosis of ALS was recorded in medical records, the positive predictive value may not reach 70–91%. Therefore, the estimated effect of the CCR was small and could be influenced by diagnostic accuracy.
In conclusion, our study indicates a significant association between decreased CCR and an increased risk of ALS. This evidence suggests that premorbid muscle mass may play a crucial role in the pathogenesis of ALS. Further investigation into the molecular basis of these changes could lead to the development of presymptomatic biomarkers and novel therapeutic targets.
Data availability
The data that support the findings of this study are available from UK Biobank but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding author upon reasonable request and with permission of UK Biobank.
References
Feldman, E. L. et al. Amyotrophic lateral sclerosis. Lancet 400, 1363–1380. https://doi.org/10.1016/s0140-6736(22)01272-7 (2022).
Yang, J., Jiang, F., Yang, M. & Chen, Z. Sarcopenia and nervous system disorders. J Neurol 269, 5787–5797. https://doi.org/10.1007/s00415-022-11268-8 (2022).
van Eijk, R. P. A. et al. Monitoring disease progression with plasma creatinine in amyotrophic lateral sclerosis clinical trials. J Neurol Neurosurg Psychiatry 89, 156–161. https://doi.org/10.1136/jnnp-2017-317077 (2018).
Ito, D. et al. Elevated serum creatine kinase in the early stage of sporadic amyotrophic lateral sclerosis. J Neurol 266, 2952–2961. https://doi.org/10.1007/s00415-019-09507-6 (2019).
Brown, R. H. & Al-Chalabi, A. Amyotrophic lateral sclerosis. N Engl J Med 377, 162–172. https://doi.org/10.1056/NEJMra1603471 (2017).
Kurihara, M. et al. Factors affecting energy metabolism and prognosis in patients with amyotrophic lateral sclerosis. Ann Nutr Metab 77, 236–243. https://doi.org/10.1159/000518908 (2021).
Vinciguerra, C. et al. Temporal muscle thickness and survival in patients with amyotrophic lateral sclerosis. Neurol Res 44, 1006–1010. https://doi.org/10.1080/01616412.2022.2096004 (2022).
Holdom, C. J. et al. Venous creatinine as a biomarker for loss of fat-free mass and disease progression in patients with amyotrophic lateral sclerosis. Eur J Neurol 28, 3615–3625. https://doi.org/10.1111/ene.15003 (2021).
Scaricamazza, S., Salvatori, I., Ferri, A. & Valle, C. Skeletal muscle in ALS: An unappreciated therapeutic opportunity? Cells 10, https://doi.org/10.3390/cells10030525 (2021).
Åberg, M. et al. Risk factors in Swedish young men for amyotrophic lateral sclerosis in adulthood. J Neurol 265, 460–470. https://doi.org/10.1007/s00415-017-8719-1 (2018).
Lépine, S., Castellanos-Montiel, M. J. & Durcan, T. M. TDP-43 dysregulation and neuromuscular junction disruption in amyotrophic lateral sclerosis. Transl Neurodegener 11, 56. https://doi.org/10.1186/s40035-022-00331-z (2022).
Cruz-Jentoft, A. J. & Sayer, A. A. Sarcopenia. Lancet 393, 2636–2646. https://doi.org/10.1016/s0140-6736(19)31138-9 (2019).
Cooper, C. et al. Tools in the assessment of sarcopenia. Calcif Tissue Int 93, 201–210. https://doi.org/10.1007/s00223-013-9757-z (2013).
Jones, K. I., Doleman, B., Scott, S., Lund, J. N. & Williams, J. P. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 17, O20-26. https://doi.org/10.1111/codi.12805 (2015).
Zheng, C. et al. Serum creatinine/cystatin C ratio as a screening tool for sarcopenia and prognostic indicator for patients with esophageal cancer. BMC Geriatr 22, 207. https://doi.org/10.1186/s12877-022-02925-8 (2022).
Kashani, K. B. et al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med 45, e23–e29. https://doi.org/10.1097/ccm.0000000000002013 (2017).
Hirai, K. et al. Serum creatinine/cystatin C ratio as a surrogate marker for sarcopenia in patients with chronic obstructive pulmonary disease. Clin Nutr 40, 1274–1280. https://doi.org/10.1016/j.clnu.2020.08.010 (2021).
Osaka, T. et al. Decreased the creatinine to cystatin C ratio is a surrogate marker of sarcopenia in patients with type 2 diabetes. Diabetes Res Clin Pract 139, 52–58. https://doi.org/10.1016/j.diabres.2018.02.025 (2018).
Tang, T. et al. Serum creatinine and cystatin C-based diagnostic indices for sarcopenia in advanced non-small cell lung cancer. J Cachexia Sarcopenia Muscle 13, 1800–1810. https://doi.org/10.1002/jcsm.12977 (2022).
Zhu, Y. et al. Sex differences in the relationship of serum creatinine to cystatin C ratio and depressive symptoms among middle-aged and older adults in China. J Affect Disord 319, 57–61. https://doi.org/10.1016/j.jad.2022.09.030 (2022).
Tetsuka, S., Morita, M., Ikeguchi, K. & Nakano, I. Creatinine/cystatin C ratio as a surrogate marker of residual muscle mass in amyotrophic lateral sclerosis. Neurol. Clin. Neurosci. 1, 32–37 (2013).
Kim, K. M., Jang, H. C. & Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med 31, 643–650. https://doi.org/10.3904/kjim.2016.015 (2016).
Wu, Y. et al. Sarcopenia index based on serum creatinine and cystatin C is associated with mortality in middle-aged and older adults in Chinese: A retrospective cohort study from the China Health and Retirement Longitudinal Study. Front Public Health 11, 1122922. https://doi.org/10.3389/fpubh.2023.1122922 (2023).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12, e1001779. https://doi.org/10.1371/journal.pmed.1001779 (2015).
Wang, J. et al. Lower creatinine to cystatin C ratio is associated with an increased risk of MASLD: A cross-sectional and prospective study of 368,634 UK Biobank participants. Clin Endocrinol (Oxf) 100, 116–123. https://doi.org/10.1111/cen.14990 (2024).
Nicoletti, A. et al. Sex and gender differences in Alzheimer’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis: A narrative review. Mech Ageing Dev 212, 111821. https://doi.org/10.1016/j.mad.2023.111821 (2023).
Li, S. et al. Serum creatinine-to-cystatin C ratio in the progression monitoring of non-alcoholic fatty liver disease. Front Physiol 12, 664100. https://doi.org/10.3389/fphys.2021.664100 (2021).
Zhang, L., Tang, L., Huang, T. & Fan, D. Association between type 2 diabetes and amyotrophic lateral sclerosis. Sci Rep 12, 2544. https://doi.org/10.1038/s41598-022-06463-6 (2022).
Mariosa, D. et al. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: A more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol 81, 718–728. https://doi.org/10.1002/ana.24936 (2017).
Xia, K. et al. Mutation-specific metabolic profiles in presymptomatic amyotrophic lateral sclerosis. Eur J Neurol 30, 87–95. https://doi.org/10.1111/ene.15584 (2023).
Batty, G. D., Kivimäki, M., Frank, P., Gale, C. R. & Wright, L. Systemic inflammation and subsequent risk of amyotrophic lateral sclerosis: Prospective cohort study. Brain Behav Immun 114, 46–51. https://doi.org/10.1016/j.bbi.2023.07.026 (2023).
Lunetta, C. et al. Serum C-reactive protein as a prognostic biomarker in amyotrophic lateral sclerosis. JAMA Neurol 74, 660–667. https://doi.org/10.1001/jamaneurol.2016.6179 (2017).
Sun, J. et al. Blood biomarkers and prognosis of amyotrophic lateral sclerosis. Eur J Neurol 27, 2125–2133. https://doi.org/10.1111/ene.14409 (2020).
Zhang, G. et al. Daytime sleepiness might increase the risk of ALS: A 2-sample Mendelian randomization study. J Neurol 268, 4332–4339. https://doi.org/10.1007/s00415-021-10564-z (2021).
Kim, S. W. et al. A new equation to estimate muscle mass from creatinine and cystatin C. PLoS One 11, e0148495. https://doi.org/10.1371/journal.pone.0148495 (2016).
Suzuki, K. et al. Utility of creatinine/cystatin C ratio as a predictive marker for adverse effects of chemotherapy in lung cancer: A retrospective study. J Int Med Res 43, 573–582. https://doi.org/10.1177/0300060515579116 (2015).
Sime, F. B., Udy, A. A. & Roberts, J. A. Augmented renal clearance in critically ill patients: etiology, definition and implications for beta-lactam dose optimization. Curr Opin Pharmacol 24, 1–6. https://doi.org/10.1016/j.coph.2015.06.002 (2015).
Thongprayoon, C., Cheungpasitporn, W. & Kashani, K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis 8, E305-311. https://doi.org/10.21037/jtd.2016.03.62 (2016).
Randers, E. & Erlandsen, E. J. Serum cystatin C as an endogenous marker of the renal function–a review. Clin Chem Lab Med 37, 389–395. https://doi.org/10.1515/cclm.1999.064 (1999).
Klivenyi, P. et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med 5, 347–350. https://doi.org/10.1038/6568 (1999).
Yoo, Y. E. & Ko, C. P. Dihydrotestosterone ameliorates degeneration in muscle, axons and motoneurons and improves motor function in amyotrophic lateral sclerosis model mice. PLoS One 7, e37258. https://doi.org/10.1371/journal.pone.0037258 (2012).
Holzbaur, E. L. et al. Myostatin inhibition slows muscle atrophy in rodent models of amyotrophic lateral sclerosis. Neurobiol Dis 23, 697–707. https://doi.org/10.1016/j.nbd.2006.05.009 (2006).
Nakken, O., Meyer, H. E., Stigum, H. & Holmøy, T. High BMI is associated with low ALS risk: A population-based study. Neurology 93, e424–e432. https://doi.org/10.1212/wnl.0000000000007861 (2019).
Tsitkanou, S., Della Gatta, P., Foletta, V. & Russell, A. The role of exercise as a non-pharmacological therapeutic approach for amyotrophic lateral sclerosis: Beneficial or detrimental?. Front Neurol 10, 783. https://doi.org/10.3389/fneur.2019.00783 (2019).
Sanjak, M., Bravver, E., Bockenek, W. L., Norton, H. J. & Brooks, B. R. Supported treadmill ambulation for amyotrophic lateral sclerosis: A pilot study. Arch Phys Med Rehabil 91, 1920–1929. https://doi.org/10.1016/j.apmr.2010.08.009 (2010).
Bello-Haas, V. D. et al. A randomized controlled trial of resistance exercise in individuals with ALS. Neurology 68, 2003–2007. https://doi.org/10.1212/01.wnl.0000264418.92308.a4 (2007).
Beal, M. F. Neuroprotective effects of creatine. Amino Acids 40, 1305–1313. https://doi.org/10.1007/s00726-011-0851-0 (2011).
Cozzolino, M. & Carrì, M. T. Mitochondrial dysfunction in ALS. Prog Neurobiol 97, 54–66. https://doi.org/10.1016/j.pneurobio.2011.06.003 (2012).
Guimarães-Ferreira, L. et al. Short-term creatine supplementation decreases reactive oxygen species content with no changes in expression and activity of antioxidant enzymes in skeletal muscle. Eur J Appl Physiol 112, 3905–3911. https://doi.org/10.1007/s00421-012-2378-9 (2012).
Sestili, P. et al. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med 40, 837–849. https://doi.org/10.1016/j.freeradbiomed.2005.10.035 (2006).
Lepore, E., Casola, I., Dobrowolny, G. & Musarò, A. Neuromuscular junction as an entity of nerve-muscle communication. Cells 8, https://doi.org/10.3390/cells8080906 (2019).
Heckman, C. J. & Enoka, R. M. Motor unit. Compr Physiol 2, 2629–2682. https://doi.org/10.1002/cphy.c100087 (2012).
Dadon-Nachum, M., Melamed, E. & Offen, D. The, “dying-back” phenomenon of motor neurons in ALS. J Mol Neurosci 43, 470–477. https://doi.org/10.1007/s12031-010-9467-1 (2011).
Le Gall, L. et al. Muscle cells of sporadic amyotrophic lateral sclerosis patients secrete neurotoxic vesicles. J Cachexia Sarcopenia Muscle 13, 1385–1402. https://doi.org/10.1002/jcsm.12945 (2022).
Jung, H. N., Jung, C. H. & Hwang, Y. C. Sarcopenia in youth. Metabolism 144, 155557. https://doi.org/10.1016/j.metabol.2023.155557 (2023).
Quessada, C. et al. Skeletal muscle metabolism: Origin or prognostic factor for amyotrophic lateral sclerosis (ALS) development? Cells 10, https://doi.org/10.3390/cells10061449 (2021).
Ghasemzadeh, N., Nyberg, F. & Hjertén, S. Highly selective artificial gel antibodies for detection and quantification of biomarkers in clinical samples. II. Albumin in body fluids of patients with neurological disorders. J Sep Sci 31, 3954–3958, https://doi.org/10.1002/jssc.200800386 (2008).
Yang, J. W. et al. Hypolipidemia in patients with amyotrophic lateral sclerosis: A possible gender difference?. J Clin Neurol 9, 125–129. https://doi.org/10.3988/jcn.2013.9.2.125 (2013).
Chiò, A. et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: a population-based study. JAMA Neurol 71, 1134–1142. https://doi.org/10.1001/jamaneurol.2014.1129 (2014).
Liu, S. et al. Excessive daytime sleepiness in Chinese patients with sporadic amyotrophic lateral sclerosis and its association with cognitive and behavioural impairments. J Neurol Neurosurg Psychiatry 89, 1038–1043. https://doi.org/10.1136/jnnp-2018-318810 (2018).
Acknowledgements
This research was funded by the National Natural Science Foundation of China (81873784, 82071426) and Clinical Cohort Construction Program of Peking University Third Hospital (BYSYDL2019002) and key Program of Peking University Third Hospital (BYSYZD2021004). This study was conducted using UK Biobank resource (application number, 108832). We wish to express our sincere thanks to the participants of the UK Biobank and the members of the survey, development, and management teams involved in this project.
Author information
Authors and Affiliations
Contributions
B.B.D. and D.S.F. contributed to the conception and design of the study. Z.Y.W. and W.C. contributed to the acquisition and analysis of data. Z.Y.W. contributed to drafting the text or preparing the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical declarations
All participants have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study. Details that might disclose the identity of the subjects under study should be omitted.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Z., Cao, W., Deng, B. et al. Lower creatinine-to-cystatin c ratio associated with increased risk of incident amyotrophic lateral sclerosis in the prospective UK biobank cohort. Sci Rep 14, 28289 (2024). https://doi.org/10.1038/s41598-024-79910-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79910-1