Abstract
The importance of collagen and elastin remains incompletely understood concerning tumor immunity in cancer tissues. This study explored the clinical significance of collagen and elastin deposition on tumor immunity in advanced colorectal cancer patients. The collagen and elastin contents were assessed simultaneously using elastic van Gieson (EVG) histochemical staining. Immunohistochemical staining was performed to measure the immune cell markers CD3, CD8, CD86, and CD163 in surgically resected primary tumors from 78 pT4 colorectal cancer patients. High collagen, elastin, and EVG scores are associated with aggressive characteristics and short disease-free survival. A high EVG score was identified as an independent predictor of poor disease-free survival. Furthermore, tumors with high collagen and EVG scores exhibited significantly fewer intratumoral CD3 + and CD8 + cells. Evaluating tumor fibrosis using the classical and straightforward EVG staining method could be a reliable predictor of recurrence in high-risk colorectal cancer patients with tumor immune tolerance.
Similar content being viewed by others
Introduction
Tumor progression involves substantial disruption of the extracellular matrix (ECM)1,2. Cancer cells exhibit increased ECM stiffness and fibrosis in the early phases. Eventually, the remodeled environment becomes crucial for tumor progression and treatment resistance in advanced phases3,4. Augmented fibrotic ECM has been correlated with treatment resistance, epithelial–mesenchymal transition (EMT), which is related to cancer aggressiveness, and poor prognosis in various cancer types5,6,7,8.
In addition to promoting tumor growth and survival, tumor fibrosis strongly influences the immune environment in cancer9,10. The importance of immune cell infiltration in prognosis and treatment response has been extensively studied11,12. In numerous cancer types, increased densities of tumor-infiltrating lymphocytes (TILs) are reportedly correlated with enhanced survival rates and heightened responsiveness to anticancer treatment regimens13,14,15,16. In contrast, different studies have reported different conclusions regarding macrophages, such as alternatively activated M2 macrophages17,18. M2-type macrophages exert tumor-promoting effects through increased TGF-β signaling, which eventually contributes to EMT and the activation of cancer-associated fibroblasts19,20. Thus, the importance of the immune system in the tumor microenvironment (TME) as both a suppressive factor and a tumor-promoting factor has been established.
CD86 is a macrophage marker indicating macrophages’ presence regardless of their activation state. It plays a crucial role in T cell activation and the immune response, suggesting that a higher density of CD86-positive macrophages (M1-like macrophages) may reflect an active immune environment within the tumor21. In contrast, CD163 is specifically associated with M2-like macrophages, often linked to immune suppression and tumor progression22. CD86 and CD163 provide a comprehensive understanding of macrophage polarization and innate immune status in TME.
In the ECM, collagen and elastin are the predominant fibrous proteins that contribute to tensile strength and significantly influence tumor characteristics23. Collagen is a fibrous component that supports structural integrity and is crucial for ECM stiffness24. Moreover, elevated collagen accumulation has been linked to enhanced tumor invasiveness and unfavorable prognosis in diverse types of cancers25,26. Furthermore, high collagen expression induces EMT and resistance to anticancer regimens in various types of cancers27,28,29,30. In contrast, elastin, another abundant fibrous protein in the ECM, was identified in relatively lower amounts than collagen31. However, it crosslinks with collagen and other fibrous elements to form a dense ECM matrix for cancer progression and invasion32. Recently, Fang et al. reported that elevated levels of intratumoral elastin are correlated with EMT and unfavorable prognosis in gastric cancer33. Moreover, elastin was found to significantly promote the proliferation and invasion of colorectal cancer (CRC)34. Nevertheless, the associations between collagen, elastin, and tumor immunity in advanced CRC remain elusive.
Based on previous reports on tumor fibrosis and immune cell infiltration as promising biomarkers of CRC, this study aimed to elucidate the relationships between collagen and elastin deposition and clinicopathological factors, including tumor-infiltrating immune cells, in patients with advanced CRC. To achieve this goal, we conducted elastic van Gieson (EVG) histochemical staining to evaluate collagen and elastin simultaneously on the same slides. Additionally, we performed immunohistochemical staining for immune cell markers, such as CD3 (a pan-T-cell marker), CD8 (an antitumoral cytotoxic T-cell marker), CD86 (an M1-like proinflammatory macrophage marker), and CD163 (an M2-like anti-inflammatory macrophage marker), on surgically resected specimens from patients diagnosed with pT4 CRC. Our data revealed that abundant tumor fibrosis with high EVG score based on high collagen and high elastin levels was associated with a more aggressive phenotype, low tumoral immune cell infiltration, and poorer prognosis in patients with pT4 CRC.
Results
Evaluation of collagen and elastin deposition in pT4 CRC samples
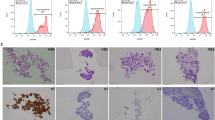
Using histochemical EVG staining, we quantitatively evaluated the distribution of collagen (red components) and elastin (dark blue to black components) in pathological T4 CRC samples (Fig. 1a). An examination of 19 samples comprising tumor and adjacent normal tissues on the same slides revealed that the proportions of collagen and elastin in tumor tissues were substantially greater than those in adjacent normal tissues (P < 0.0001 and P = 0.0016, respectively; Fig. 1c and d). Intratumoral collagen and elastin levels were further classified into high and low groups utilizing the threshold values ascertained from the ROC curves for cancer recurrence (Fig. 1e). Based on collagen deposition, 52 samples (66.7%) were categorized as collagen-low, and 26 tumors (33.3%) were assigned to the collagen-high group (Table 1). Based on the elastin level, 22 tumors (28.2%) were classified as elastin-high, and 56 tumors (71.8%) were classified as elastin-low (Table 1).
Image capture and quantitative evaluation of collagen, elastin, and immune cells. (a) For the image acquisition of EVG staining and the quantitative evaluation of collagen and elastin, five representative images were captured at 100× magnification. The percentage of collagen and elastin area was assessed using the Hybrid Cell Count system, and the calculation was performed by dividing the extracted area by the total area of each image. (b) For image acquisition of immunohistochemical staining and immune cell quantification (CD8+, CD3+, CD86+, and CD163+), four representative areas, each composed of nine images, were captured at 200× magnification. The number of stained immune cells per mm2 was semiautomatically counted using the Hybrid Cell Count system. Scale bar, 100 μm. (c) Representative images of EVG staining showing the distribution of collagen and elastin in adjacent normal and cancerous tissues. Images were captured at 100× magnification. Scale bar, 100 μm. (d) Comparison of collagen and elastin distribution between adjacent normal tissue and primary tumor tissue from 19 patients from whom the sections were available to evaluate cancerous and noncancerous areas on EVG-stained CRC sections simultaneously. The Wilcoxon matched-pairs tests analyzed the differences. (e) Categorization of samples according to collagen and elastin deposition. The categorization process of tumors was based on intratumoral collagen and elastin according to the cutoff values determined based on ROC curves for cancer recurrence. (f) The EVG scores were determined based on collagen and elastin deposition. CRC samples with high collagen and elastin levels were categorized as EVG-high. CRC samples were classified as EVG-low when collagen, elastin, or both were detected at low levels. The upper panel shows representative images of patients with low and high EVG scores.
Definition of the EVG score
The EVG score was estimated based on a combination of the observed collagen and elastin deposition in the tumors. Samples with a high distribution of both collagen and elastin were referred to as EVG-high, whereas the other samples were assigned as EVG-low (Fig. 1f). Eventually, 19 patients (24.4%) were categorized into the EVG-high group, and 59 patients (75.6%) were categorized into the EVG-low tumor group (Table 2).
Relationships of collagen, elastin, and the EVG score with clinicopathological factors
In the sample of 78 patients diagnosed with pT4 CRC, tumors with high collagen levels exhibited a correlation with smaller tumor size (P = 0.0225), immature or intermediate desmoplasia (P = 0.0010), positive resection margins (P = 0.0217), progression of lymph node metastasis (P = 0.0313), and distant metastasis (P = 0.0002; Table 1). Similarly, tumors with high elastin levels were found to be associated with a smaller tumor size (P = 0.0013), intermediate or immature desmoplasia (P = 0.0380), and progression to distant metastasis (P = 0.0112). Moreover, high EVG scores were correlated with decreased tumor size (P = 0.0035), intermediate or immature desmoplastic reactions (P = 0.0310), and progression to distant metastasis (P = 0.0031).
The prognostic value of collagen and elastin distribution and the EVG score in patients with pT4 CRC
We investigated the prognostic impact of collagen and elastin distribution and EVG score on disease-free survival (DFS) and overall survival (OS) in 78 patients who underwent surgical resection for pT4 CRC. Survival analyses were conducted using Kaplan–Meier curves with log-rank comparisons. The results revealed that patients with high levels of collagen and elastin had significantly shorter DFS than those with low levels of collagen and elastin (P = 0.0016, Fig. 2a, left panel; P = 0.0006, Fig. 2b, left panel). Additionally, patients with tumors exhibiting high EVG scores had significantly shorter DFS than did those with low EVG scores (P < 0.0001; Fig. 2c, left panel). However, the difference in OS between tumors with high collagen, elastin, and EVG scores and those with low collagen, elastin, and EVG scores was not significant (Fig. 2a, b, and c; right panels). The Cox regression model was used to analyze the risk factors for disease-free survival, and the results are presented in Table 3. Univariate analysis revealed that high levels of collagen (HR = 2.7911, 95% CI: 1.4326–5.4380; P = 0.0026), elastin (HR = 3.0351, 95% CI: 1.5565–5.9184; P = 0.0011), and EVG (HR = 3.7767, 95% CI: 1.9380–7.3597; P < 0.0001) were associated with poor DFS. Furthermore, multivariate analysis revealed that a high EVG score was an independent prognostic factor for an unfavorable DFS rate (HR = 2.4237, 95% CI: 1.0857–5.4108; P = 0.0307).
K‒M curves of the data from patients with pT4 CRC based on collagen and elastin levels and the EVG score. (a) Kaplan–Meier survival analyses for disease-free survival (P = 0.0016, left panel) and overall survival (P = 0.2966, right panel) stratified according to collagen deposition. (b) Kaplan–Meier survival analyses for disease-free survival (P = 0.0006, left panel) and overall survival (P = 0.0793, right panel) stratified according to elastin deposition. (c) Kaplan–Meier survival analyses for disease-free survival (P < 0.0001, left panel) and overall survival (P = 0.0725, right panel) stratified according to the EVG score.
Correlations of collagen and elastin distribution and the EVG score with immune cell infiltration in 78 patients with pT4 CRC tumors
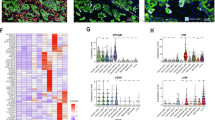
The numbers of CD3+ and CD8+ intratumoral lymphocytes in EVG-low and EVG-high tumors are presented in Fig. 3a. Tumors with high collagen and EVG scores were associated with a reduced number of intratumoral CD3+ and CD8+ lymphocytes (Fig. 3b and d). However, the elastin levels did not differ significantly with respect to lymphocyte infiltration (Fig. 3c). In contrast, intratumoral macrophages expressing CD86 and CD168 were not significantly associated with collagen or elastin levels or EVG scores (Supplementary Fig. 1).
Relationship of lymphocyte infiltration with collagen and elastin levels and the EVG score in CRC tissues. (a) Representative images of intratumoral lymphocyte cell density (CD3 + and CD8+) in EVG-low and EVG-high tumors. (b) Comparisons of intratumoral lymphocyte cell density (CD3 + and CD8+) in collagen-low and collagen-high tumors. (c) Comparisons of intratumoral lymphocyte cell density (CD3 + and CD8+) in elastin-low and elastin-high tumors. (d) Comparisons of intratumoral lymphocyte cell density (CD3 + and CD8+) in EVG-low and -high tumors. Images were captured at 200× magnification. Scale bar, 100 μm. The t-tests with Welch’s correction analyzed the differences.
Discussion
In this study, we utilized EVG staining to assess collagen and elastin deposition simultaneously in identical CRC sections and defined the EVG score based on both collagen and elastin densities. We found that high collagen and elastin levels and high EVG scores were associated with aggressive characteristics, such as intermediate or immature desmoplastic reactions, high rates of lymphatic and distant metastasis, and short DFS. Moreover, multivariate analysis revealed that a high EVG score is an independent predictor of poor DFS in patients with locally advanced pT4 CRC. Notably, tumors with high collagen and EVG scores exhibited significantly decreased intratumoral CD3+ and CD8+ cell counts. However, the presence of high elastin in tumors did not show any correlation with immune cell infiltration. Interestingly, the infiltration levels of CD86+ and CD163+ macrophages were not associated with collagen or elastin levels or with EVG scores in this study.
Fibrosis is also associated with the immune microenvironment in an intricate and multifaceted manner24,35. Collagen influences immune cell behavior and quantity through various mechanisms36,37,38. Notably, the dense deposition of collagen forms a physical barrier that impedes the infiltration of immune cells, including TILs39,40,41. Moreover, collagen can interact with integrins on the surface of immune cells, affecting their adherence, movement, and trafficking to tumors42,43,44. In the present study, high intratumoral collagen and EVG scores correlated with reduced TIL levels. This finding validates the negative effect of collagen on immune cell infiltration, particularly on that of TILs, even in advanced CRC. Unlike TILs, macrophages were not significantly correlated with collagen levels. Notably, macrophages secrete high levels of MMPs, such as MMP9 and MMP12, which are potent ECM-degrading proteases45,46. These findings may explain why the collagen/elastin/EVG ratio was not related to macrophage infiltration.
The level of fibrosis in the TME is a promising biomarker because it is strongly associated with cancer aggressiveness and immune cell infiltration47,48,49,50,51,52. The importance of stromal cells and proteins as biomarkers and therapeutic target molecules has been demonstrated by evaluating tumor stromal fibrosis and assessing cancer-associated fibroblasts and ECM proteins using different staining methods. The EVG staining technique used in this study is a classic, inexpensive, antibody-free tissue staining technique that can be easily performed in pathology laboratories worldwide. Fibrosis evaluation using EVG staining can be used to assess cancer severity and poor prognosis33,53. However, no studies have analyzed the relationship between the level of tumor-infiltrating immune cells, which strongly contribute to immunotherapy sensitivity, and fibrosis using EVG staining. This study revealed that patients with a high EVG score, i.e., those with a high amount of deposited collagen and elastin, had significantly more cases of distant metastasis and a greater risk of recurrence. Furthermore, patients with higher EVG scores and collagen levels had a significantly lower number of lymphocytes infiltrating the tumor tissue, suggesting an association with cold tumors (low immune cell infiltration and low expression of immune checkpoint proteins) that induce tumor immune tolerance54,55. In contrast, TGF-β signaling and other ECM factors that contribute to tumor fibrosis suppress tumor immunity and cause cold tumor status, contributing to immunotherapy resistance56. Moreover, such fibrotic ECM with active TGF-β signal has been known to cause the EMT regarding cancer aggressiveness and cancer stem cells with dormancy57,58. Our data indicated that the high EVG score linked to the fibrotic ECM was significantly associated with positive resection margins and small tumor size, suggesting the positive correlation of high EVG and EMT in locally advanced CRC. Thus, tumor fibrosis evaluation by simple EVG staining may be a promising method for predicting the recurrence of high-risk CRC patients with high metastatic potential and tumor immune tolerance caused by a cold TME.
This study has several limitations. First, it must be acknowledged that this investigation was conducted retrospectively within a single institution and exclusively recruited patients with surgically resected pT4 CRC with abundant ECM compared to the superficial tumors. Second, we did not assess the status of mismatch repair proteins or microsatellite instability, which have been linked to tumor-infiltrating immune cells, treatment response, or overall prognosis in CRC patients. Third, although we examined the correlation between intratumoral fibrosis and tumor aggressiveness, immune cell infiltration, and prognosis in patients who underwent radical resection for CRC, it is important to note that our data may not fully capture the significance of EVG staining in all patients with CRC, including those who are considered inoperable in real-world settings. Fourth, we showed a significant relation between a high EVG score and low intratumoral immune cell infiltration. However, the spatial information between these factors based on whole slide images has not been evaluated. To validate the accuracy of our quantification method, we performed whole-tumor analyses using HALO® digital pathology software on twenty representative cases, including ten high EVG and ten low EVG samples (Supplementary Fig. 2). Using five representative fields, we then assessed the correlation between collagen and elastin deposition results from HALO® quantification and our previous method. Correlation analysis, using Pearson’s correlation coefficient (r), showed a strong positive correlation for collagen (r = 0.8, P < 0.0001, right panel) and elastin (r = 0.53, P = 0.0160, left panel). These data may suggest that our evaluation method provides reliable and reproducible results. Future research endeavors should aim to investigate whether increased deposition of collagen and elastin correlates not only with cancer aggressiveness and immune cell infiltration but also with drug sensitivity in all patients with CRC with stage I-IV disease by utilizing pretreatment biopsy samples and resected tumors.
In conclusion, this study clarified the correlation between tumor fibrosis levels based on classical EVG staining, cancer aggressiveness, and short disease-free survival in patients with locally advanced CRC. Moreover, high intratumoral fibrotic levels, such as high collagen and EVG scores, were found to be associated with decreased intratumoral lymphocyte infiltration. Our findings suggest that tumor fibrosis levels, which are based on collagen and elastin deposition, may play a pivotal role in the tumor immune microenvironment. Evaluation of tumor fibrosis using classical, inexpensive, and simple EVG staining could be a valuable predictor of the recurrence of high-risk CRC patients with high metastatic potential and tumor immune tolerance.
Materials and methods
Clinical samples
This study adhered to the principles outlined in the Declaration of Helsinki and received approval from the Institutional Review Board for Clinical Research of Gunma University Hospital (approval number: HS2023-056). Informed consent was obtained from the patients for this retrospective study using the opt-out method. A total of 78 patients with pathological T4 CRC who underwent surgery at Gunma University Hospital between July 2013 and February 2020 were included in the study (Supplementary Table 1). Patients who had received preoperative chemotherapy or radiation therapy, as well as those who had noncurative resection due to distant metastasis, were excluded. Fourteen patients had synchronous metastasis; however, these patients underwent surgical resection of both the primary tumor and the distant metastasis simultaneously. Patient information and relevant clinical data were collected from medical and surgical records. Follow-up was conducted until May 2023. The period between surgery and death from any cause was considered overall survival, while disease-free survival was defined as the duration between surgery and the first documented disease progression, including local recurrence, distant metastasis, or death from any cause.
Elastic Van Gieson histochemical staining
EVG staining is a histochemical technique commonly employed to visualize collagen and elastic fibers in histological sections. The EVG staining reagent was used per the manufacturer’s instructions (Product # 40321, Muto Pure Chemicals, Tokyo, Japan). Four-micrometer-thick sections of CRC specimens were deparaffinized, subjected to xylene removal, hydrated, and washed. To achieve intense staining, the sections were exposed to a resorcin-fuchsin solution for 60 min, followed by a 1-min treatment with 100% ethanol. The stained sections were then rinsed under running water for 5 min. Next, the sections were incubated in Weigert’s iron hematoxylin solution for 5 min and washed with tap water for 10 min. Afterwards, the samples were treated with van Gieson’s solution for 10 min. Elastic fibers, which possess a strong affinity for hematoxylin, appeared dark blue or black. The collagen fibers were highlighted as bright red fibers through counterstaining with van Gieson’s solution, which consists of acid fuchsin and picric acid.
Image acquisition and quantitative evaluation of collagen and elastin
In the EVG-stained slides, collagen and elastin deposition were separately assessed in five representative fields at 100× magnification (Fig. 1a). The total area covered during the analysis was 7.88 mm2 for each section. Intratumoral collagen, which appears as a red signal after EVG staining, was extracted using a semiautomatic Hybrid Cell Count System (BZ-X800; Keyence, Osaka, Japan). Initially, several pixels representing the target color were manually selected as references for training. Based on this, pixels with similar color intensities were extracted from the digital images, applying a color tolerance value of 20 to ensure optimal sensitivity. The color extraction settings were determined separately for collagen and elastin. Subsequently, a similar protocol was employed for elastin fibers, which are represented as dark blue to black components in EVG staining. The percentages of the collagen and elastin fiber areas were determined by dividing the collagen and elastin extraction areas by the total area in the field (Fig. 1a). A receiver operating characteristic (ROC) curve was used to identify the optimal collagen and elastin area percentage cutoff for cancer recurrence. The EVG score was defined based on the collagen and elastin density. The scoring table for EVG score determination is shown in Fig. 1f.
Immunohistochemical staining
Paraffin-embedded CRC specimens were sliced into 4 μm thick sections. These sections were incubated at 60 °C for 60 min and then deparaffinized using ClearPlus (FALMA, Tokyo, Japan). Rehydration was performed by subjecting the sections to a series of ethanol treatments, followed by antigen retrieval using Immunosaver (Nishin EM, Tokyo, Japan) at a temperature range of 98–100 °C for 45 min. To inhibit the activity of endogenous peroxidase, the sections were treated with a solution of 0.3% hydrogen peroxide in 100% methanol for 30 min at a temperature of 20–25 °C. Subsequently, the sections were blocked using Protein Block Serum-Free Reagent (Agilent, Santa Clara, USA) and incubated with primary antibodies against REAL Antibody Diluent (Agilent, Santa Clara, USA) at 4 °C for 24 h. The following primary antibodies were used: CD3 (1:1; Ventana, Tucson, USA; 790–4341), CD8 (1:400; Abcam, Cambridge, UK; ab4055), CD163 (1:500; Cell Signaling Technology, Danvers, USA; CST-93498 S), CD86 (1:400; Cell Signaling Technology, Danvers, USA; CST-91882 S), and CD31 (1:50; Agilent, Santa Clara, USA; M0823). The visualization of the primary antibody was conducted using a Histofine Simple Stain MAX-PO (Multi) Kit (Nichirei, Tokyo, Japan) following the instructions provided by the manufacturer. The chromogen 3,3’-diaminobenzidine tetrahydrochloride (DAB) was applied at a concentration of 0.02% in 50 mM ammonium acetate-citrate buffer (pH = 6.0) containing 0.005% hydrogen peroxide. Finally, Mayer’s hematoxylin was used for counterstaining. Negative controls were not exposed to the primary antibody and exhibited no observable staining.
Image acquisition and quantitative evaluation of immune cells
Four fields were selected from each CRC slide to quantify the presence of immune cells infiltrating the tumor (specifically, CD3+, CD8+, CD86+, and CD163+ cells). These fields comprised 36 images, covering an area of 9.07 mm² (Fig. 1b). An all-in-one microscope (BZ-X700; Keyence, Osaka, Japan) was utilized to capture the images. The numbers of CD3+, CD8+, CD86+, and CD163+ immune cells were subsequently enumerated using the Hybrid Cell Count System (BZ-X800; Keyence, Osaka, Japan), which is a semiautomatic image analysis software. The density of tumor-infiltrating immune cells was determined by dividing the number of cells by the total area (mm²), resulting in the cell density per mm².
Evaluation of desmoplastic reaction
An experienced pathologist, blinded to the patients’ clinical history and outcomes, reviewed the primary pT4 tumors to evaluate desmoplastic reaction according to the criteria59,60. The desmoplastic reaction was assessed based on the existence of keloid-like collagen and myxoid stroma, and the stroma was classified according to the most immature stromal area. A mature desmoplastic reaction was defined when fibrotic stroma comprised fine, mature collagen fibers and did not contain keloid-like collagen or myxoid stroma. An intermediate desmoplastic reaction was defined when keloid-like collagen was present with mature stroma. The immature desmoplastic reaction was determined when the stroma with myxoid changes was present.
Statistical analysis
The associations between categorical variables were examined using chi-square and Fisher’s exact tests, while the means of continuous variables across groups were compared using t tests with Welch’s correction. The Kaplan–Meier method was used to visualize overall and disease-free survival curves; differences between groups were assessed using the log-rank test. Univariate and multivariate analyses were conducted using Cox regression analysis. Statistical analyses were performed using JMP Pro 15 (SAS Institute, Cary, NC, USA) and GraphPad Prism 10 (DotMatics, Boston, MA, USA). A significance level of P < 0.05 was adopted.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author (TY) upon reasonable request.
References
Popova, N. V. & Jücker, M. The functional role of extracellular matrix proteins in cancer. Cancers. 14 https://doi.org/10.3390/cancers14010238 (2022).
Chandler, C., Liu, T., Buckanovich, R. & Coffman, L. G. The double edge sword of fibrosis in cancer. Transl Res. 209, 55–67. https://doi.org/10.1016/j.trsl.2019.02.006 (2019).
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J. & Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120. https://doi.org/10.1038/s41467-020-18794-x (2020).
Deng, B. et al. Biological role of matrix stiffness in tumor growth and treatment. J. Transl Med. 20, 540. https://doi.org/10.1186/s12967-022-03768-y (2022).
Fattet, L. et al. Matrix rigidity controls epithelial-mesenchymal plasticity and tumor metastasis via a mechanoresponsive EPHA2/LYN complex. Dev Cell 54, 302–316.e307 https://doi.org/10.1016/j.devcel.2020.05.031 (2020).
Kai, F., Drain, A. P. & Weaver, V. M. The extracellular matrix modulates the metastatic journey. Dev. Cell. 49, 332–346. https://doi.org/10.1016/j.devcel.2019.03.026 (2019).
Bauer, J. et al. Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling. Sci. Rep. 10, 50. https://doi.org/10.1038/s41598-019-55687-6 (2020).
Calitz, C. et al. Influence of extracellular matrix composition on tumour cell behaviour in a biomimetic in vitro model for hepatocellular carcinoma. Sci. Rep. 13, 748. https://doi.org/10.1038/s41598-023-27997-3 (2023).
Jiang, H., Hegde, S. & DeNardo, D. G. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol. Immunother. 66, 1037–1048. https://doi.org/10.1007/s00262-017-2003-1 (2017).
Herzog, B. H. et al. Tumor-associated fibrosis impairs immune surveillance and response to immune checkpoint blockade in non-small cell lung cancer. Sci. Transl Med. 15, eadh8005. https://doi.org/10.1126/scitranslmed.adh8005 (2023).
Barnes, T. A. & Amir, E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br. J. Cancer. 117, 451–460. https://doi.org/10.1038/bjc.2017.220 (2017).
Zuo, S., Wei, M., Wang, S., Dong, J. & Wei, J. Pan-cancer analysis of immune cell infiltration identifies a prognostic immune-cell characteristic score (ICCS) in lung adenocarcinoma. Front. Immunol. 11, 1218. https://doi.org/10.3389/fimmu.2020.01218 (2020).
Pages, F. et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391, 2128–2139. https://doi.org/10.1016/S0140-6736(18)30789-X (2018).
Asambaev, A. A., Finkel, E. A., Akulov, V. D. & Morozov, V. L. Effectiveness of the treatment of experimental tuberculosis with BCG vaccine and tuberculin. Probl. Tuberk 44–47 (1987).
Shang, B., Liu, Y., Jiang, S. J. & Liu, Y. Prognostic value of tumor-infiltrating FoxP3 + regulatory T cells in cancers: a systematic review and meta-analysis. Sci. Rep. 5, 15179. https://doi.org/10.1038/srep15179 (2015).
Yeong, J. et al. Intratumoral CD39(+)CD8(+) T cells predict response to programmed cell death Protein-1 or programmed death Ligand-1 blockade in patients with NSCLC. J. Thorac. Oncol. 16, 1349–1358. https://doi.org/10.1016/j.jtho.2021.04.016 (2021).
Zhang, Y., Zhao, Y., Li, Q. & Wang, Y. Macrophages, as a promising strategy to targeted treatment for colorectal cancer metastasis in tumor immune microenvironment. Front. Immunol. 12, 685978. https://doi.org/10.3389/fimmu.2021.685978 (2021).
Kinoshita, J. et al. Prognostic value of tumor-infiltrating CD163(+)macrophage in patients with metastatic gastric cancer undergoing multidisciplinary treatment. BMC Cancer. 22, 608. https://doi.org/10.1186/s12885-022-09713-y (2022).
Tamborindeguy, C., Huot, O. B., Ibanez, F. & Levy, J. The influence of bacteria on multitrophic interactions among plants, psyllids, and pathogen. Insect Sci. 24, 961–974. https://doi.org/10.1111/1744-7917.12474 (2017).
Li, X., Chen, L., Peng, X. & Zhan, X. Progress of tumor-associated macrophages in the epithelial-mesenchymal transition of tumor. Front. Oncol. 12, 911410. https://doi.org/10.3389/fonc.2022.911410 (2022).
Guerriero, J. L. Macrophages: their untold story in T cell activation and function. Int. Rev. Cell. Mol. Biol. 342, 73–93. https://doi.org/10.1016/bs.ircmb.2018.07.001 (2019).
Ma, S. et al. CD163 as a potential biomarker in colorectal cancer for tumor microenvironment and cancer prognosis: a Swedish study from tissue microarrays to big data analyses. Cancers. 14. https://doi.org/10.3390/cancers14246166 (2022).
Muiznieks, L. D. & Keeley, F. W. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim. Biophys. Acta. 1832, 866–875. https://doi.org/10.1016/j.bbadis.2012.11.022 (2013).
De Martino, D. & Bravo-Cordero, J. J. Collagens in cancer: structural regulators and guardians of cancer progression. Cancer Res. 83, 1386–1392. https://doi.org/10.1158/0008-5472.CAN-22-2034 (2023).
Song, K. et al. Collagen remodeling along cancer progression providing a novel opportunity for cancer diagnosis and treatment. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms231810509 (2022).
Necula, L. et al. Collagen family as promising biomarkers and therapeutic targets in Cancer. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms232012415 (2022).
Medici, D. & Nawshad, A. Type I collagen promotes epithelial-mesenchymal transition through ILK-dependent activation of NF-kappaB and LEF-1. Matrix Biol. 29, 161–165. https://doi.org/10.1016/j.matbio.2009.12.003 (2010).
Shintani, Y., Maeda, M., Chaika, N., Johnson, K. R. & Wheelock, M. J. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am. J. Respir Cell. Mol. Biol. 38, 95–104. https://doi.org/10.1165/rcmb.2007-0071OC (2008).
Gilles, C., Polette, M., Seiki, M., Birembaut, P. & Thompson, E. W. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab. Invest. 76, 651–660 (1997).
Koenig, A., Mueller, C., Hasel, C., Adler, G. & Menke, A. Collagen type I induces disruption of e-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 66, 4662–4671. https://doi.org/10.1158/0008-5472.CAN-05-2804 (2006).
Wang, Y., Song, E. C. & Resnick, M. B. Elastin in the tumor microenvironment. Adv. Exp. Med. Biol. 1272, 1–16. https://doi.org/10.1007/978-3-030-48457-6_1 (2020).
Ye, M. et al. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol. Ther. 215, 107633. https://doi.org/10.1016/j.pharmthera.2020.107633 (2020).
Fang, T. et al. The prognostic marker elastin correlates with epithelial-mesenchymal transition and vimentin-positive fibroblasts in gastric cancer. J. Pathol. Clin. Res. 9, 56–72. https://doi.org/10.1002/cjp2.298 (2023).
Li, J. et al. Elastin is a key factor of tumor development in colorectal cancer. BMC Cancer. 20, 217. https://doi.org/10.1186/s12885-020-6686-x (2020).
Fang, M., Yuan, J., Peng, C. & Li, Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 35, 2871–2882. https://doi.org/10.1007/s13277-013-1511-7 (2014).
Larsen, A. M. H. et al. Collagen Density modulates the immunosuppressive functions of macrophages. J. Immunol. 205, 1461–1472. https://doi.org/10.4049/jimmunol.1900789 (2020).
Rygiel, T. P., Stolte, E. H., de Ruiter, T., van de Weijer, M. L. & Meyaard, L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol. Immunol. 49, 402–406. https://doi.org/10.1016/j.molimm.2011.09.006 (2011).
Romer, A. M. A., Thorseth, M. L. & Madsen, D. H. Immune modulatory properties of collagen in cancer. Front. Immunol. 12, 791453. https://doi.org/10.3389/fimmu.2021.791453 (2021).
Kuczek, D. E. et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother Cancer. 7, 68. https://doi.org/10.1186/s40425-019-0556-6 (2019).
Sun, X. et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 599, 673–678. https://doi.org/10.1038/s41586-021-04057-2 (2021).
Gao, H. et al. 3D collagen fiber concentration regulates Treg cell infiltration in triple negative breast cancer. Front. Immunol. 13, 904418. https://doi.org/10.3389/fimmu.2022.904418 (2022).
Chen, Y. et al. Oncogenic collagen I homotrimers from cancer cells bind to alpha3beta1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell. 40, 818–834.e819 https://doi.org/10.1016/j.ccell.2022.06.011 (2022).
Wu, X., Cai, J., Zuo, Z. & Li, J. Collagen facilitates the colorectal cancer stemness and metastasis through an integrin/PI3K/AKT/Snail signaling pathway. Biomed. Pharmacother. 114, 108708. https://doi.org/10.1016/j.biopha.2019.108708 (2019).
Lv, Y. et al. Type I collagen promotes tumor progression of integrin beta1 positive gastric cancer through a BCL9L/beta-catenin signaling pathway. Aging (Albany NY). 13, 19064–19076. https://doi.org/10.18632/aging.203355 (2021).
Tekin, C. et al. Macrophage-secreted MMP9 induces mesenchymal transition in pancreatic cancer cells via PAR1 activation. Cell. Oncol. (Dordr). 43, 1161–1174. https://doi.org/10.1007/s13402-020-00549-x (2020).
Aristorena, M. et al. MMP-12, secreted by pro-inflammatory macrophages, targets endoglin in Human macrophages and endothelial cells. Int. J. Mol. Sci. 20 https://doi.org/10.3390/ijms20123107 (2019).
Akimoto, N. et al. Desmoplastic reaction, immune cell response, and prognosis in colorectal cancer. Front. Immunol. 13, 840198. https://doi.org/10.3389/fimmu.2022.840198 (2022).
Han, S. et al. Intratumoral fibrosis and patterns of immune infiltration in clear cell renal cell carcinoma. BMC Cancer. 22, 661. https://doi.org/10.1186/s12885-022-09765-0 (2022).
Peng, D. H. et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion. Nat. Commun. 11, 4520. https://doi.org/10.1038/s41467-020-18298-8 (2020).
Liang, Y. et al. Prognostic significance of abnormal matrix collagen remodeling in colorectal cancer based on histologic and bioinformatics analysis. Oncol. Rep. 44, 1671–1685. https://doi.org/10.3892/or.2020.7729 (2020).
Dong, S. et al. Development and validation of a collagen signature to predict the prognosis of patients with stage II/III colorectal cancer. iScience 26, 106746. https://doi.org/10.1016/j.isci.2023.106746 (2023).
Mak, T. K. et al. The cancer-associated fibroblast-related signature predicts prognosis and indicates immune microenvironment infiltration in gastric cancer. Front. Immunol. 13, 951214. https://doi.org/10.3389/fimmu.2022.951214 (2022).
Maehara, J. et al. Quantification of intratumoral collagen and elastin fibers within hepatocellular carcinoma tissues finds correlations with clinico-patho-radiological features. Hepatol. Res. 50, 607–619. https://doi.org/10.1111/hepr.13484 (2020).
Guo, L., Wang, C., Qiu, X., Pu, X. & Chang, P. Colorectal cancer imune infiltrates: significance in patient prognosis and immunotherapeutic efficacy. Front. Immunol. 11, 1052. https://doi.org/10.3389/fimmu.2020.01052 (2020).
Naik, A. & Leask, A. Tumor-associated fibrosis impairs the response to immunotherapy. Matrix Biol. 119, 125–140. https://doi.org/10.1016/j.matbio.2023.04.002 (2023).
Ding, J. H. et al. Integrated analysis reveals the molecular features of fibrosis in triple-negative breast cancer. Mol. Ther. Oncolytics. 24, 624–635. https://doi.org/10.1016/j.omto.2022.02.003 (2022).
Fasano, M. et al. TGF-beta modulated pathways in Colorectal Cancer: new potential therapeutic opportunities. Int. J. Mol. Sci. 25 https://doi.org/10.3390/ijms25137400 (2024).
Tauriello, D. V., Calon, A., Lonardo, E. & Batlle, E. Determinants of metastatic competency in colorectal cancer. Mol. Oncol. 11, 97–119. https://doi.org/10.1002/1878-0261.12018 (2017).
Ueno, H. et al. Histopathological atlas of desmoplastic reaction characterization in colorectal cancer. Jpn J. Clin. Oncol. 51, 1004–1012. https://doi.org/10.1093/jjco/hyab040 (2021).
Shiraishi, T. et al. Association of tumor size in pathological T4 colorectal cancer with desmoplastic reaction and prognosis. Ann. Gastroenterol. Surg. 6, 667–678. https://doi.org/10.1002/ags3.12571 (2022).
Acknowledgements
The authors would like to thank Ms. Mariko Nakamura, Ms. Kao Abe (Department of General Surgical Science, Graduate School of Medicine, Gunma University, Maebashi, Japan), and Ms. Yukiko Suto (Laboratory for Analytical Instruments, Gunma University, Maebashi, Japan) for their assistance with EVG and immunohistochemical staining. The authors would also like to thank Ms. Miyoko Suzuki, Ms. Chiho Noguchi, and Ms. Saori Suto (Division of Integrated Oncology Research, Gunma University Initiative for Advanced Research, Maebashi, Japan) and Ms. Sayaka Okada and Ms. Harumi Kanai (Department of General Surgical Science, Graduate School of Medicine, Gunma University, Maebashi, Japan) for their administrative support.
Funding
This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS; grant numbers 23K08147, 23K14610, 22H02912, 22K08766, 22K16480, 21K08749, and 24K19348).
Author information
Authors and Affiliations
Contributions
GD and TS contributed equally to this study. GD, BE, and HO collected and evaluated the image data. GD, BEO, TS, and TY analyzed and interpreted the data. TS, MSO, and TY verified the authenticity of the raw data. GD, BEO, TS, and TY composed the manuscript. MSO, TS, BEO, TY, KS, and HS conceptualized the study. TS, AY, IS, CK, NN, NO, YS, TOK, AK, TOY, KO, AS, MSA, HO, and MSO participated in sample collection and data analysis. All authors have reviewed and endorsed the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dorjkhorloo, G., Shiraishi, T., Erkhem-Ochir, B. et al. High levels of fibrotic tumor components are associated with recurrence and intratumoral immune status in advanced colorectal cancer patients. Sci Rep 14, 30735 (2024). https://doi.org/10.1038/s41598-024-80489-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80489-w