Abstract
The purpose of this study is to investigate the association between the aortomesenteric angle (AMA) and the occurrence and image characteristics of spontaneous isolated superior mesenteric artery dissection (SISMAD). This is a single-centre retrospective case-control study. Between January 1 2013 and December 13 2022, consecutive patients with computed tomography angiography (CTA) confirmed symptomatic SISMAD were included. Controls were selected with 1:1 matches in patients with CTA of the superior mesenteric artery but without SISMAD using propensity score matching for age, sex, and body mass index. Patient demographics, symptoms, and dissection characteristics were recorded. Logistic regression was performed to assess the association between AMA and SISMAD. The study also evaluated the association between AMA and SISMAD using restricted cubic splines (RCS). The associations between AMA and the characteristics of SISMAD were evaluated. One hundred and five SISMAD patients (mean age, 54.8 ± 8.9 years) were included, and most patients were male (87.6%). Univariable analysis revealed hypertension, hyperlipemia, and AMA (all p < .001) were associated with SISMAD. An increasing AMA (adjusted OR, 1.03 per 1 ° increase in angulation) and hypertension (adjusted OR, 3.52) were identified as risk factors for SISMAD. Compared with small AMA level (< 50°), intermediate (50–71°) (adjusted OR, 2.62; 95% CI, 1.23–5.58; p = .013) and large angle level (> 71°) (adjusted OR, 4.50; 95% CI, 2.07–9.82; p < .001) were significantly associated with SISMAD. No obvious associations between AMA and the SISMAD imaging characteristics were found. Greater AMA and hypertension were independent risk factors for SISMAD.
Similar content being viewed by others
Introduction
Spontaneous isolated superior mesenteric artery dissection (SISMAD) was used to be considered a rare disease1. However, with the widespread utilisation of computed tomography angiography (CTA), SISMAD has been increasingly reported2. Although most SISMAD patients can be successfully managed with conservative treatment2,3, patients may still encounter dissecting aneurysm rupture, intestinal ischaemia, or even death4,5. Currently, there is no consensus on the most effective treatment regimen for SISMAD1. Besides, most previous studies focused on the characteristics or treatment of SISMAD. In contrast, the aetiology of SISMAD is yet unknown2. Arterial wall pathologies are frequently mentioned as possible causes of superior mesenteric artery (SMA) dissection2. However, none of these pathologies can be found in the majority of SISMAD patients6,7. Based on current evidence, male gender, hypertension, smoking, dyslipidaemia1,4, and large aortomesenteric angle (AMA)4 have been reported as potential risk factors for SISMAD.
Previous studies have demonstrated that large AMA may play an important role in the development of SISMAD4,6,8,9,10,11. The association between AMA and SISMAD was first evaluated by Park et al.6 using computational fluid dynamic models, and they found that SISMAD occurrence may be related to a haemodynamic force at convexity curvature following large AMA. This result was further demonstrated by Jia et al.8 and Wu et al.10. However, most studies had limited sample sizes6,10, or lacked control groups6. Unfortunately, no study has evaluated the association between AMA on a continuous scale and SISMAD risk. Moreover, the associations between AMA and the characteristics of SISMAD have not been evaluated.
The purpose of the present study is to evaluate whether an increasing AMA on a continuous scale is associated with an increasing risk of SISMAD. The impact of AMA levels (small, intermediate, and large) on the risk of SISMAD was evaluated. Moreover, the associations between AMA and image characteristics of SISMAD were evaluated.
Methods
Study design
During the study period from January 1 2013 to December 31 2022, patients presented with sudden chest or abdominal pain and received aortic or SMA CTA to rule out aorta or visceral artery disease at the study hospital were screened. Consecutive hospitalised patients with CTA confirmed symptomatic SISMAD were included. During the study period, non-SISMAD patients who underwent CTA were allocated 1:1 to the control group based on propensity scores matching by age, sex, and body mass index. Patient demographics (age, sex, and body mass index), symptoms (abdominal pain, back pain, nausea/vomiting, diarrhoea, bloody stools, and syncope), comorbidities or risk factors (hypertension, diabetes mellitus, coronary heart disease, hyperlipaemia, peripheral artery disease, prior vascular dissection, prior aneurysm, cerebral infarction cancer, connective tissue disorder, smoking, and prior revascularization procedure), and laboratory findings (neutrophil count, lymphocyte count, D-dimer, triglyceride, and total cholesterol) were recorded and analysed. Dissection characteristics (AMA, dissection point, dissection length, dissection aneurysm, branch vessel involvement, and subtypes) were recorded. Arterial wall pathologies, including segmental arterial mediolysis, connective tissue disorders, cystic medial necrosis, vasculitis, and fibromuscular dysplasia, were not evaluated. The study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board of Nanjing First Hospital approved the study protocol on March 3 2023, and informed consent was waived due to the retrospective study design.
Diagnosis of SISMAD and measurement of AMA
CTA was performed using a 128-slice CT (Dual Source CT, SOMATOM Definition Flash, Siemens, Germany) that included contrast-enhanced 1.0 mm thick slices to allow for proper arterial analysis. SISMAD was diagnosed by CTA with the following criteria12: (a) intimal flap formation in SMA; (b) dual-lumen structure in SMA with contrast agent filling; (c) crescent intramural hematoma in SMA with or without ulcer-like projection communicating with the arterial lumen; and (d) crescent intramural hematoma in the SMA with arterial lumen occlusion. Dissecting aneurysm formation was defined as 1.5 times larger than the individual normal parent mesenteric artery13. Patients with coexisting dissection in other arteries were excluded. The subtype of SISMAD was categorised as I, IIa, IIb, and III according to Yun’s classification as follows14. Type I: Patent true and false lumen revealing entry and re-entry sites; Type II: Patent true lumen but no re-entry flow from the false lumen; Type IIa: Visible false lumen but not visible re-entry site; Type IIb: Not visible false luminal flow (thrombosed false lumen); Type III: Dissection with occlusion of SMA.
AMA was measured in the sagittal view of CTA. AMA was defined as the angle between the inferior SMA origin, a point 1 cm along the posterior wall of the SMA and a point 1 cm along the anterior wall of the distal aorta (Fig. 1)10,12. The diagnosis of SISMAD and measurement of the AMA was independently performed by two board-certificated radiologists blinded to the study purpose. A senior radiologist was consulted to reach a consensus when there was a disagreement between the two reviewers. Based on priorly measured AMA results, the patients were equally allocated into small, intermediate, and large angle levels, with 35 patients in each level.
Statistical analysis
The distribution of continuous data was tested by Kolmogorov–Smirnov test. Data with normal distribution were presented as mean ± standard deviation and 95% confidence interval. Data with asymmetric distribution were presented as the median and interquartile range (IQR). The intraclass correlation coefficient was calculated to evaluate inter-observer reliability. The difference between continuous data was compared using the Student t test, Mann–Whitney U test, or one-way variance analysis. The difference between categorical data was compared using the chi-square test or Fisher’s exact test. The Pearson correlation coefficient was used to evaluate the correlation between two variables. Univariate and multivariable logistic regression models were performed to estimate the association between the SISMAD and AMA levels with an odds ratio (OR) and 95% CI. Variables included in the multivariable regression model were based on a combination of clinical relevance and statistical significance. The association between SISMAD and AMA was evaluated on a continuous scale with restricted cubic splines (RCS) based on a logistic regression model. Knots are set at the 5th, 35th, 65th, and 95th percentiles of AMA15. All statistical analyses were performed by statistic software R (http://www.R-project.org; R Foundation for Statistical Computing, Vienna, Austria). A two-tailed p value < 0.050 was considered to be statistically significant.
Results
Patients
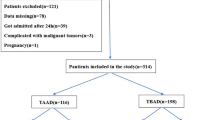
A total of 975 patients with abdominal or back pain underwent CTA of SMA. After excluding 7 patients with dissection of other arteries, 105 SISMAD patients were identified and assigned to the SISMAD group. The remaining patients without SMA dissection were 1:1 matched for the SISMAD group. Finally, 210 patients were included in this study, and the inclusion flowchart is presented in Fig. 2.
The aortomesenteric angle was determined as the angle between the posterior wall of the superior mesenteric artery and the anterior wall of the distal aorta. (A) The aortomesenteric angle in one patient with SISMAD was 102.11 °. (B) The aortomesenteric angle in one patient without SISMAD was 71.57 °. SISMAD = spontaneous isolated superior mesenteric artery dissection
The mean age of SISMAD patients was 54.8 ± 8.9 years, and most patients were male (87.6%). The mean BMI of SISMAD patients was 22.2 ± 3.7 kg/m2. Most SISMAD patients had abdominal pain with a median symptom-to-diagnosis time of 2.0 days. Other symptoms included back pain (6.7%), nausea/vomiting (9.5%), diarrhoea (9.5%), bloody stools (1.9%), and syncope (1.0%). The leading comorbidities of SISMAD patients were hypertension (53.3%) and hyperlipaemia (34.3%) (Table 1).
Characteristics and outcomes of SISMAD
All the entry tears of dissections were located at the anterior wall and around the convexity of the SMA. Based on Yun’s classification, type I, IIa, IIb, and III were noted in 10 (9.5%), 11 (10.5%), 74 (70.5%), and 10 (9.5%) patients, respectively. The median distance between the SMA ostium and dissection origin was 22.9 mm (IQR, 17.3–30.0 mm), and the median dissection length was 36.0 mm (IQR, 20.0–65.0 mm). Dissecting aneurysm was found in 37.1% (39/105) of patients. Branch vessel involvement and true lumen stenosis > 70% were noted in 33.3% (35/105) and 20.0% (21/105) of patients, respectively (Table 2).
The AMA of SISMAD patients ranged from 24.0 ° to 129.7 °, and the mean angle was 69.6 ± 23.1 °. As for all included patients, the AMA ranged from 18.1 ° to 129.7 °. The 33rd and 66th percentiles of overall AMA values were 50 ° and 71 °, respectively. Therefore, AMA was divided into small (< 50 °), intermediate (50–71 °), and large (> 71 °) angle levels with the same sample size.
All SISMAD patients achieved notable pain resolution after a median treatment time of 2.0 days. No patients experience bowel resection or SMA haemorrhage. After a median length of hospital stay of 9.0 days, all patients were discharged uneventfully.
Association between AMA and SISMAD incidence and characteristics
The intraclass correlation coefficient of AMA measured by the two radiologists was 0.90 (95% CI, 0.88–0.93). Figure 3 shows the density plots of AMA in SISMAD patients and non-SISMAD patients. The mean AMA of the SISMAD group was significantly larger than the Control group (69.6 ± 23.1 ° vs. 54.0 ± 22.3 °, p < .001). The univariant analysis also identified hypertension (p < .001) and hyperlipaemia (p < .001) were statistically significantly associated with SISMAD (Table 1).
Compared to small AMA level, univariate logistic regression analysis revealed intermediate levels were significantly associated with the SISMAD occurrence (Table 3).
The association remained after applying adjustment Model 2, which revealed that intermediate (adjusted OR, 2.62; 95% CI, 1.23–5.58; p = .013) and large (adjusted OR, 4.50; 95% CI, 2.07–9.82; p < .001) AMA levels significantly associated with SISMAD (Table 3). Model 2 was adjusted for age, sex, hypertension, and hyperlipidaemia. In model 2, hypertension was also identified as a risk factor (adjusted OR, 3.52; 95% CI, 1.88–6.56; p < .001). Besides, multivariant logistic regression analysis revealed that AMA was significantly associated with SISMAD (OR, 1.03 per 1 ° increase of AMA; 95% CI, 1.02–1.05; p < .001). RCS based on the logistic regression model adjusted by age, sex, hypertension, and hyperlipidaemia showed that the increasing AMA was associated with consistently increasing OR of SISMAD (overall p < .001, nonlinear p = .45). The OR of SISMAD was > 1.0 when the AMA was > 59.6 ° (Fig. 4).
The OR of SISMAD versus aortomesenteric angle is modelled using logistic regressions with restricted cubic splines with 95% confidence limits (red ribbon). Analyses were adjusted for age, sex, hypertension, and hyperlipidaemia. The black dashed lines indicate reference lines for no association at an OR of 1.0. The arrow indicates the minimum angle for no association with SISMAD risk. OR = odds ratio; SISMAD = spontaneous isolated superior mesenteric artery dissection.
No correlation between AMA and dissection initial position (r = .030, p = .76) or dissection length (r = .065, p = .51) was noted. In addition, no significant difference in AMA of patients with and without dissecting aneurysm was found (70.2 ± 22.9 ° vs. 69.3 ± 23.4 °, p = .86) was noted. The mean AMA for type I, IIa, IIb, and III SISMAD dissection were 65.0 ± 17.5 °, 67.3 ± 13.6 °, 70.2 ± 24.2 °, and 73.0 ± 28.9 °, respectively. No significant difference was noted regarding AMA in different subtypes (p = .86).
Discussion
The present study found that hypertension and AMA were risk factors for SISMAD. Moreover, an increasing AMA represents an increasing SISMAD risk. Compared with the small AMA level, intermediate and large AMA levels were significantly associated with increased SISMAD risk.
The aetiology of SISMAD has not been well elucidated2,14. Hypertension, middle-aged male gender, dyslipidaemia, and smoking history were potential risk factors for SISMAD1, whereas few studies have evaluated these risk factors. Wu et al.10 reported that hypertension and smoking history were independent risk factors for SISMAD. The present study also identified hypertension (adjusted OR, 3.52) as a risk factor, whereas smoking history was not identified. Only 21% of SISMAD patients in our study had a smoking history. No other studies have validated smoking history as a risk factor for SISMAD. Moreover, the percentage of smoking reported by Wu et al.10 was much higher than the data from the European Society of Vascular Surgery guidelines (62% vs. 38%)1. The association between smoking and SISMAD still needs to be further investigated. Various arterial wall pathologies have been mentioned as possible causes of SISMAD2, whereas none of these can be found in the majority of SISMAD patients6,7. It has been suggested that the anatomical characteristics of the transitional zone in the SMA, from a fixed to an unfixed segment, maybe a potential underlying cause of SISMAD16. However, this speculation remains unverified by any study.
Previous studies have revealed that large AMA may play an important role in developing SISMAD4,6,8,9,10,11. In our study, the mean AMA of SISMAD patients was 69.6 ± 23.1 °, which was comparable to the results from Park et al.6 (estimated mean angle: 63.7 °), Wu et al.10 (73.0 ± 19.8 °), and Kim et al.11 (74.9 ± 17.5 °). The mean AMA (59.7 ± 21.4 °) reported by Jia et al.8. was slightly smaller than our results, which may be attributed to the different measurement methods. Previous studies consistently reported that SISMAD patients had larger AMA than non-SISMAD patients8,10,11. In one study including 37 SISMAD patients and 148 control patients, Wu et al.10 evaluated the association between AMA and SISMAD and found that the OR of SISMAD increased with increasing AMA (1, 10, 57, and 73 for < 50 °, 50–69 °, 70–90 °, and > 90 °, respectively; p < .05). Jia et al. reported that compared with AMA < 30 °, large AMA levels were significantly associated with increased OR of SISMAD (4.33 and 51.15 for 60–90 ° and > 90 °, respectively; p < .05)8. The present study also found that the intermediate (50–71 °) and large (> 71 °) AMA levels were significantly associated with SISMAD risk when compared to the small (< 50°) AMA level. The present study further revealed that an increasing AMA (adjusted OR, 1.03 per 1 ° increase in angulation) was identified as a risk factor for SISMAD. Furthermore, the association between AMA on a continuous scale and SISMAD based on RCS revealed that an increasing AMA was associated with a consistently increasing OR of SISMAD.
According to previous studies, SISMAD typically begins at the superior convexity of SMA6,10,17,18,19. The present study found all entry tears of the dissections were located at the anterior wall around the convexity of the SMA, which was consistent with previous studies17,18. The development of SISMAD may be attributed to hemodynamic and histopathological changes in the SMA superior convexity. AMA plays an important role in haemodynamic changes at the SMA convexity. Wu et al.10 found that a larger AMA is associated with higher stress in the arterial wall and a higher oscillatory shear index at the SMA superior convexity. Jia et al.8 further demonstrated that larger AMA and SMA curvatures were associated with higher and more concentrated wall shear stress at the SMA superior convexity. Increased wall shear stress can cause the breakdown and disruption of cell-matrix connections20, as well as promote inflammation21, thus leading to the development of SISMAD. These studies may explain the consistent association between the increased AMA and increased SISMAD risk. Regarding the histopathological causes of SISMAD, Park et al.6 found no specific arterial wall pathology in the histopathologic examination. However, Jia et al.8 found a significantly smaller mean media thickness and area fractions of elastin and collagen in the anterior wall of the SMA curve compared to the posterior wall. Elastin and collagen provide elasticity and strength to the arterial wall22. The reduction of elastin and collagen at SMA superior convexity may impair the strength of the arterial wall, thus leading to SISMAD occurrence. Due to the impact of different AMA on haemodynamic changes, the imaging characteristic of SISMAD may be influenced. The present study evaluated the possible associations, whereas no meaningful finding was noted.
It is important to note that there are several limitations in the current study. The evidence level of the present study is limited by retrospective design and small sample size. SISMAD was found to be more prevalent in Asian countries as compared to European countries1. Thus, the results of the present study need to be verified by large-scale international studies. The retrospective study design may lead to selection bias, which may influence the final results. Despite these limitations, the present study is the first study to investigate AMA and SISMAD risk on a continuous scale. Future multicentre prospective studies, including Asian and Western countries, are needed to verify these findings.
In conclusion, an increasing AMA was consistently associated with an increasing risk of SISMAD. Intermediate and large AMA levels were associated with statistically significantly increased SISMAD risk compared to the small AMA level. AMA seems not to impact the image characteristics of SISMAD. These findings could aid in comprehending the significance of AMA in the development of SISMAD.
Data availability
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
Bjorck, M. et al. Editor (eds) ‘s Choice Management of the diseases of Mesenteric Arteries and veins: clinical practice guidelines of the European society of vascular surgery (ESVS). Eur. J. Vasc Endovasc Surg. 53 4 460–510 (2017).
Ullah, W. et al. Diagnosis and management of isolated Superior Mesenteric Artery dissection: a systematic review and Meta-analysis. Korean Circ. J. 49 (5), 400–418 (2019).
Zhu, Y. et al. Treatment strategies and outcomes of symptomatic spontaneous isolated Superior Mesenteric Artery dissection: a systematic review and Meta-analysis. J. Endovasc Ther. 25 (5), 640–648 (2018).
Acosta, S. & Goncalves, F. B. Management of spontaneous isolated mesenteric artery dissection: a systematic review. Scand. J. Surg. 110 (2), 130–138 (2021).
Becquemin, J. P. Management of the diseases of Mesenteric Arteries and veins: clinical practice guidelines of the European society for vascular surgery (ESVS) [corrected]. Eur. J. Vasc Endovasc Surg. 53 (4), 455–457 (2017).
Park, Y. J. et al. Inference from clinical and fluid dynamic studies about underlying cause of spontaneous isolated superior mesenteric artery dissection. J. Vasc Surg. 53 (1), 80–86 (2011).
Kim, Y. W. Current understandings of spontaneous isolated Superior Mesenteric Artery Dissection. Vasc Specialist Int. 32 (2), 37–43 (2016).
Jia, Z. et al. The pathogenesis of superior mesenteric artery dissection: an in-depth study based on fluid-structure interaction and histology analysis. Comput. Methods Programs Biomed. 226, 107187 (2022).
Dou, L. et al. Isolated superior mesenteric artery dissection: CTA features and clinical relevance. Abdom. Radiol. (NY). 45 (9), 2879–2885 (2020).
Wu, Z. et al. The significance of the Angle between Superior Mesenteric Artery and aorta in spontaneous isolated Superior Mesenteric Artery Dissection. Ann. Vasc Surg. 45, 117–126 (2017).
Kim, H. & Labropoulos, N. The role of aortomesenteric angle in occurrence of spontaneous isolated superior mesenteric artery dissection. Int. Angiol. 39 (2), 125–130 (2020).
Arthurs, O. J., Mehta, U. & Set, P. A. Nutcracker and SMA syndromes: what is the normal SMA angle in children? Eur. J. Radiol. 81 (8), e854–e861 (2012).
Jeong, M. J. et al. Clinical outcomes of conservative treatment in patients with symptomatic isolated spontaneous renal artery dissection and comparison with Superior Mesenteric Artery Dissection. Eur. J. Vasc Endovasc Surg. 56 (2), 291–297 (2018).
Yun, W. S. et al. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur. J. Vasc Endovasc Surg. 37 (5), 572–577 (2009).
Shi, Y. et al. Impact of Common Iliac Vein Compression on the incidence of pulmonary embolism in patients with Acute Deep vein thrombosis. Eur. J. Vasc Endovasc Surg. 65 (6), 887–894 (2023).
Park, Y. J. et al. Natural history of spontaneous isolated superior mesenteric artery dissection derived from follow-up after conservative treatment. J. Vasc Surg. 54 (6), 1727–1733 (2011).
Luan, J. Y., Li, X., Li, T. R., Zhai, G. J. & Han, J. T. Vasodilator and endovascular therapy for isolated superior mesenteric artery dissection. J. Vasc Surg. 57 (6), 1612–1620 (2013).
Wen, D. et al. Endovascular stent-graft repair of spontaneous isolated dissection of the Superior Mesenteric Artery. Cardiovasc. Intervent Radiol. 41 (5), 692–698 (2018).
Ye, M. et al. Conservative Versus Endovascular treatment for spontaneous isolated Superior Mesenteric Artery Dissection: A Clinical and Imaging follow-up study. J. Endovasc Ther. :15266028231163733. (2023).
Salmasi, M. Y. et al. High Wall Shear stress can predict Wall Degradation in ascending aortic aneurysms: an Integrated Biomechanics Study. Front. Bioeng. Biotechnol. 9, 750656 (2021).
Burris, N. S., Hoff, B. A., Kazerooni, E. A. & Ross, B. D. Vascular deformation mapping (VDM) of thoracic aortic enlargement in Aneurysmal Disease and Dissection. Tomography 3 (3), 163–173 (2017).
Cocciolone, A. J. et al. Elastin, arterial mechanics, and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 315 (2), H189–H205 (2018).
Author information
Authors and Affiliations
Contributions
H.S. and Y.S. designed the study; Y.Z. and Y.Y. collected clinical data; Y.S., Y.Z. and Y.Y. interpreted data; L.C. and X.H. analyzed the data; Y.S. wrote the initial paper; L.C., X.H. and H.S. revised the paper; H.S. had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, Y., Zhou, Y., Yuan, Y. et al. Association between aortomesenteric angle and symptomatic spontaneous isolated superior mesenteric artery dissection. Sci Rep 15, 8605 (2025). https://doi.org/10.1038/s41598-024-81103-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81103-9