Abstract
In our recent study, we introduced a novel side-to-side (S-S) bypass technique for adult moyamoya disease (MMD) patients. We aimed to validate the potential of this technique in enhancing postoperative revascularization. Patients undergoing S-S or end-to-side (E-S) bypass were enrolled, clinical data and angiography parameters were collected and compared. We included 44 E-S and 40 S-S MMD patients. There were no significant differences in basic characteristics and postoperative Matsushima grades between the two groups. However, in the S-S group, fewer patients had Matsushima grade D (0%) compared to the E-S group (11.4%, P = 0.028). The S-S group showed a greater occipital artery (OA) participation in revascularization (87.5% vs. 56.8%, P = 0.002) and higher postoperative caliber change ratio (CCR) of the superficial temporal artery (STA)-frontal branch (1.26 ± 0.43 vs. 1.04 ± 0.53, P = 0.038) compared to the E-S group. This difference was more pronounced in the subgroup with poor revascularization outcomes: in the S-S group, the CCR of the STA-frontal branch and the degree of participation in revascularization were 1.163 ± 0.168 and 58.8%, respectively, while in the E-S group, they were only 0.798 ± 0.494 and 6.7%. The S-S group also had a higher OA CCR (1.133 ± 0.257) and participation (82.4%) compared to the E-S group (0.941 ± 0.216 and 37.5%, respectively). In conclusion, the S-S bypass technique effectively utilizes scalp arteries, particularly the STA-frontal branch and OA, for direct revascularization via the preserved distal STA in adult MMD patients. Scalp arteries can serve as a supplementary source of donor arteries, especially beneficial for patients with suboptimal revascularization outcomes.
Similar content being viewed by others
Introduction
Moyamoya disease (MMD) is a chronic cerebrovascular disorder characterized by the progressive stenosis or occlusion of the internal carotid artery (ICA) end and it’s proximal branches, such as the anterior cerebral artery (ACA) and middle cerebral artery (MCA), leading to inadequate cerebral perfusion, which can result in ischemic strokes and intracranial hemorrhages via the rupture of fragile collateral vessels known as “moyamoya” vessels1. Epidemiologically, MMD exhibits significant regional and ethnic differences, with the highest incidence observed in East Asia, particularly in Japan, Korea and China, where the prevalences have increased over time2,3,4. Studies show that MMD affects all age groups, with peak incidence occurring in children aged 5–9 years and adults aged 35–39 years2,5. Gender distribution also varies by region; in Japan, females are more frequently affected than males, while in China, the gender ratio is nearly balanced4,5. Additionally, MMD has a notable genetic component, with approximately 12.1% of patients having a family history of the disease5, and specific genetic markers, such as mutations in the RNF213 gene, are strongly associated with the condition in East Asian populations6,7.
MMD is one of the most common causes of cerebral ischemia and cerebral hemorrhage in young adults and children, with a high death and disability rate, which brings a huge burden to society8. Among the limited approaches to treating MMD, extracranial - to - intracranial vascular bypass surgery has emerged as the most effective therapeutic option. The primary objective of this surgical procedure is to recruit sufficient blood flow from donor artery, mainly the superficial temporal artery (STA) in the external carotid artery (ECA) system, to the affected intracranial regions9. In order to enhance the effectiveness of revascularization and mitigate postoperative complications, a multitude of innovative techniques and methods have been developed10,11,12. In our most recent study, we proposed a novel flow self-regulating STA-MCA bypass based on side-to-side (S-S) anastomosis for adult patients with MMD13. Benefit from the reservation of the distal STA, this S-S technique possesses self-regulating capabilities for bypass flow similar to the Dujiangyan Irrigation System for river flow, hence it is referred to as the “Dujiangyan-style” novel bypass procedure in China.
In addition to improving postoperative cerebral hyperperfusion symptoms (CHS), in that study13, we suggested that the S-S technique has the potential to attract the reserved distal STA flow to contribute to postoperative cerebral revascularization through the anastomosis site. This means that the retained STA distal end may serve as an additional entry point to receive blood flow from other branches of the ECA, such as occipital artery (OA), for direct revascularization, which is completely different from the end-to-side (E-S) anastomosis that relies solely on the donor STA for direct revascularization. This phenomenon is crucial: Conventional E-S bypass surgery may face specific issues, such as intraoperative damage to the STA donor artery or local compression, leading to postoperative atrophy, stenosis, or even occlusion of the donor artery. As a result, MMD patients may struggle to benefit from direct revascularization and have to rely on the maturation of indirect revascularization to provide revascularization, putting them at risk of cerebral ischemia during this time window. If the potential of the S-S bypass to attract the reserved distal STA flow for enhancing postoperative cerebral revascularization can be confirmed, our research will offer new insights into optimizing surgical strategies and improving the effectiveness of MMD treatment.
In this study, we have further substantiated this potential and analyzed the contributions of the main branches of the ECA to postoperative revascularization following the S-S procedure. This research marks the first analysis of the donor blood flow sources after S-S bypass surgery, providing valuable insights into the effectiveness of S-S surgery in treating adult MMD.
Methods
Patient selection
Participants in the S-S group were selected from all patients admitted to our hospital’s Neurosurgery Department between October 2021 and July 2022. Inclusion criteria for adult MMD patients encompassed those who met the diagnostic standards14,15. Exclusion criteria covered: (1) missing preoperative/postoperative DSA electronic data at our facility; (2) absence of revascularization surgery; (3) lack of follow-up data; (4) incomplete data extraction (e.g., unmeasurable vessel diameter); (5) specific conditions like previous STA injury during hematoma removal craniotomy, radial artery bypass, occipital artery bypass, etc. We ultimately enrolled 40 patients in the S-S group; For the E-S group, we retrospectively selected patients from the hospital’s database, focusing on those who underwent E-S surgery between January 2021 and October 2021. We used the same inclusion and exclusion criteria as the S-S group to ensure consistency. Additionally, we matched the E-S group to the S-S group based on key baseline characteristics, including gender, age, type of onset (ischemic, hemorrhagic, asymptomatic), and follow-up time. Both groups of patients underwent similar preoperative preparation and postoperative management, with surgeries performed by the same neurosurgeons (JJ. Z. and JC. C.). Essential clinical data, encompassing gender, age, symptoms, etc., were retrieved from our Hospital Information System. Surgical particulars, like operation date, side, and revascularization type, were gleaned from electronic surgical records.
Ethics approval and consent to participate
This study protocol was approved by the Institutional Review Board at Zhongnan hospital of Wuhan University (approval number: Kelun-2023010) and was in accordance with the Declaration of Helsinki revised in 1983. Written informed consent was waived by the Institutional Review Board at Zhongnan hospital of Wuhan University (approval number: Kelun-2023010) for any data elements revealing personal information have been excluded.
Revascularization surgery
Surgical revascularization was guided by criteria targeting hemispheres with compromised cerebral blood flow or presenting ischemic/hemorrhagic symptoms15. This study utilized two procedures: a S-S combined bypass (S-S direct bypass + dural inversion) and a E-S combined bypass (E-S direct bypass + dural inversion). During vascular anastomosis, the STA parietal branch was chosen as the donor artery and M4 cortical artery was selected as recipient artery. Detailed surgical procedures can be found in previous studies13.
Digital subtraction angiography (DSA) parameter acquisition
DSA was performed in our hospital by the neurointerventional team. Procedures including injection of both ECA, ICA and vertebral arteries (VA), as well as assessment through the capillary and early venous phase to evaluate the collateral flow from all possible sources, were required for all recruited patients preoperatively and on follow-up. Angiography electronic data was stored in our Picture Archiving and Communication System. Two proficient assessors (J. Y. and M. H.), both of whom were unaware of the clinical particulars, meticulously evaluated all angiography images, in the event of any disparities in their evaluations, a unanimous agreement was reached through discussion.
-
(1)
Diameter measurement of ECA main branches: on the lateral view of preoperative ECA selective angiography, the staining course was adjusted to make the target artery in the best state of staining. In this study, we chosen the STA trunk, STA frontal branch, STA parietal branch, posterior auricular artery (PAA), OA, deep temporal artery (DTA), and middle meningeal artery (MMA), as target arteries. The preoperative arterial diameters were measured using the length measuring tool within the PACS system at specific measurement sites (the anterior part of STA bifurcation, the start part of STA frontal & parietal branch, initiation of PAA, OA, DTA and MMA). Then, the ipsilateral postoperative ECA angiography from the same patient was selected, and the postoperative diameter of the corresponding arteries at the corresponding sties was measured by the same method. The postoperative caliber change ratios (CCRs) of each target artery were defined as: postoperative diameter / preoperative diameter.
-
(2)
Artery involvement. To determine whether the target artery was involved in the formation of EC compensation, we tracked the course of the target artery on the anteroposterior and lateral view phases to see whether its terminal branches formed intracranial collaterals or communicated with intracranial compensatory branches. It is judged as participating in the postoperative revascularization, otherwise it is not.
-
(3)
Matsushima grade. Postoperative revascularization was evaluated by Matsushima grading system16: A, the area supplied by the ECA collaterals covered more than 2/3 of the MCA distribution; B, the area supplied by the ECA collaterals covered 1/3 − 2/3 of the MCA distribution; and C, the area supplied by the ECA collaterals covered less than 1/3 MCA distribution. We additionally added grade D to indicate there was completely no compensatory condition17. Grade A and B was considered as “good” postoperative revascularization, while grade C and D were considered as “poor” postoperative revascularization.
Measurement of relative cerebral blood Flow (CBF) with SPECT
Patients underwent brain SPECT imaging with technetium-99m ethyl cysteinate dimer. Images were processed with a GE workstation. CBF was measured before and one day after surgery. Anastomosis sites were recorded during surgery and verified with MR angiography. ROIs were marked at the anastomosis and in the cerebellum. Relative CBF (rCBF) was calculated and compared before and after surgery to assess blood flow changes. CHS is defined as a marked increase in CBF exceeding 150% of the ipsilateral cerebellum at the anastomosis site, leading to severe headaches or focal neurological deficits, with no ischemic changes and exclusion of other causative conditions18,19.
Statistical analyses
IBM SPSS Statistics 26.0 was employed for statistical analysis in this study. Continuous variables were presented as mean ± standard deviation and analyzed using independent sample t-tests if they followed a normal distribution. Alternatively, if normal distribution was not met, variables were expressed as median (interquartile range [IQR] 25th percentile − 75th percentile) and analyzed using non-parametric tests (Whitney U test). The categorical variables were tested using chi-square tests. A significance level of P < 0.05 was considered statistically significant. Graphical illustrations were generated using GraphPad 8.0.2.
Results
Low incidence of postoperative Matsushima grade D and CHS in S-S group
A total of 40 hemispheres with MMD were included in S-S group and 44 in the E-S group. Basic characteristics including gender, age, onset, complications, Suzuki stages and follow-up time were found no difference between two groups. The overall postoperative Matsushima grades condition also showed no significant difference between the E-S group and the S-S group. (P = 0.076) However, hemispheres with Matsushima grade D in E-S group (11.4%) were significantly more than that in S-S group (0%). (P = 0.028) During the perioperative time, the portion of patients who developed CHS was also higher in E-S group (15%) than S-S group (6.8%). (P = 0.059). (Table 1; Fig. 1.)
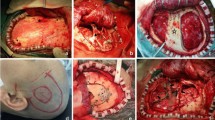
Distributions of postoperative Matsushima grades in groups with different anastomosis procedure. A intraoperative microscopic photographs showed the side-to-side (S-S) anastomosis between superficial temporal artery (STA) and the recipient artery (RA). The lower left panel showed the anastomosis after completion, and the lower right panel showed the indocyanine green fluorescence angiography corresponding to the surgical field, which showed the patency of the bypass. The dotted circle indicates the anastomosis site; B the bar chart showed the distribution of each postoperative Matsushima grades in end-to-side (E-S) group and S-S group, and the two groups have obvious differences in Matsushima grade D (P < 0.05); C, Illustrations of postoperative Matsushima grades A-C in the S-S group, and Matsushima grade D from E-S group. Red arrows showed the course of STA parietal branch, blue arrows indicated the RA, white dotted irregular rings inferred the areas of postoperative revascularization. DSA, digital subtraction angiography.
The participation and CCRs of ECA branches after bypass surgery
We next compared the participation and postoperative CCR of ECA main branches in two groups. We found that the OA was more involved in postoperative revascularization in the S-S group (87.5% VS. 56.8%, P = 0.002), but its CCR showed no significant difference compared to the E-S group (1.13 ± 0.20 VS. 1.05 ± 0.18, P = 0.056). The postoperative diameter dilation of the STA-frontal branch in the S-S group was significantly greater than in the E-S group (1.26 ± 0.43 VS. 1.04 ± 0.53, P = 0.038), but its participation in postoperative revascularization showed no statistical difference from the E-S group (37.5% VS. 25.0%, P = 0.216). No significant differences were observed between the two groups in the participation and CCRs of the STA-parietal branch, PAA, DTA, and MMA. (Table 2; Fig. 2A.)
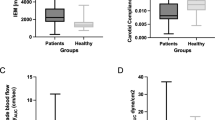
The postoperative caliber changes ratios (CCRs) of external carotid artery (ECA) branches. A postoperative CCRs of superficial temporal artery (STA) frontal / parietal branch, posterior auricular artery (PAA), occipital artery (OA), deep temporal artery (DTA) and middle meningeal artery (MMA) in end-to-side (E-S) group and side-to-side (S-S) group. The CCR of STA-frontal branch in S-S group was significantly larger than that in E-S group; B, the postoperative CCRs of STA-frontal branch and OA in S-S group with poor postoperative revascularization were both lager than those in E-S group with poor revascularization; C, there is no difference in postoperative CCRs of each ECA branches between S-S group and E-S group with good postoperative revascularization. * P < 0.05.
STA-frontal branch and OA were significantly mobilized in hemispheres with poor revascularization after S-S bypass.
To further explore the involvement of each ECA branch artery in hemispheres with different postoperative revascularization, we conducted a subgroup analysis based on the radiological effect of revascularization. Interestingly, we found that in the subgroup with poor revascularization outcomes (Matsushima grade C + D), patients who underwent S-S anastomosis had more involvement of the STA-frontal branch (58.8% VS. 6.7%, P = 0.001) and OA (82.4% VS. 37.5%, P = 0.008) than those who underwent E-S anastomosis. Additionally, the extent of caliber dilation for both the STA-frontal branch (1.163 ± 0.168 VS. 0.798 ± 0.494, P = 0.012) and OA (1.133 ± 0.257 VS. 0.941 ± 0.216, P = 0.027) were also greater than in the E-S group. (Table 2; Fig. 2B.) This phenomenon was not observed in the subgroup with good revascularization outcomes (Matsushima grade A + B). (Table 2; Fig. 2C.) More importantly, it was only in patients from the S-S group that the STA-frontal branch or the OA can be observed to enter the recipient vessels at the anastomotic site via the preserved STA-parietal branch, participating in direct revascularization (Fig. 3.)
Illustrations of external carotid artery (ECA) branches involved in direct revascularization after side-side (S-S) bypasses. A preoperative digital subtraction angiography (DSA) from patient 1 showed the superficial temporal artery (STA) parietal (red arrows) and frontal (yellow arrows) branches, there was no obvious anastomosis between the two arteries. After S-S bypass, the DSA at postoperative 3 months showed the enlarged STA frontal branch (yellow thick arrows) contributed in the postoperative revascularization through the reserved distal end of STA parietal branch. Red dotted circle indicted the anastomosis site, blue arrow head indicated the filling of recipient artery, white dotted ring indicated the revascularization area; B, patient 2’s DSA showed obviously preoperative spontaneous anastomosis (white arrows) between the STA parietal branch (red arrows) and the occipital artery (OA) (green arrows) in scalp. 5 months after the S-S bypass (red dotted circle indicted the anastomosis site), the OA was enlarged and participated in postoperative revascularization via the reserved distal end of STA parietal branch, and archived moderate effect (green dotted oval). Blue arrow head indicated the filling of recipient artery; C, preoperative DSA from patient 3 showed no connection between the STA (red arrow) and OA (green arrow). 6 months after S-S bypass, spontaneous anastomoses (white arrows) were formed between the OA and distal donor STA, thus let OA flow entered the intracranial (white dotted oval); D, DSA at postoperative 12 months from patient 4 illustrated the co-participating of OA (green arrows) and STA frontal branch (yellow arrows) in postoperative revascularization (white dotted oval), although the STA parietal branch (red arrow) seemed weak even before surgery, the OA and STA frontal branch played the backup roles to direct revascularization and avoided the occurrence of complete no revascularization effect.
Discussion
Due to the complex hemodynamic changes following direct bypass surgery for MMD, patients may experience a range of postoperative complications, such as recurrent ischemia20, hemorrhage21, cognitive decline22, watershed shift23 and postoperative CHS24. In order to reduce postoperative complications and improve the safety of bypass surgery, our team introduced the S-S anastomosis bypass procedure in 202213. We demonstrated that patients who underwent S-S surgery exhibited milder and shorter-lasting CHS symptoms. More importantly, we believe that the S-S surgery owns the potential to facilitate self-regulation of blood flow, evoking scalp arteries as donor sources, and enabling direct revascularization through the distal branches of the STA. However, this phenomenon was not widely observed due to the relatively short follow-up period at that time. In this study, through a longer-term follow-up, we reported for the first time that after S-S surgery, scalp arteries can participate in direct revascularization through the preserved distal STA, a phenomenon we refer to as “scalp arteries hemodynamic diversion”.
ECA branches, such as STA, OA and PAA, are the main blood supplier for human scalp. They each have their respective primary supply areas on the scalp, but there is also rich terminal anastomosis between them, presenting potential sources for revascularization25. (Supplementary Fig. 1.) During the MMD process, some patients developed intracranial compensation collaterals from scalp arteries, reflecting a spontaneously initiated intracranial compensatory mechanism during the course of ischemia development12,26. (Supplementary Fig. 2.) The essence of bypass surgery for MMD is to expedite the intracranial-external carotid artery conversion27,28. The initial intent behind the S-S procedure was to minimize disruption to the established compensatory circulation and, ideally, to fully mobilize the third level of the intracranial-external carotid compensatory circulation to enhance the treatment of MMD patients. In our study, we observed that the OA and STA-frontal branch significantly participated in post-S-S revascularization. We carefully observed the course of the scalp arteries through DSA angiography, followed by measurements of their preoperative and postoperative vessel diameters, all of which showed consistent changes. While this phenomenon was also observed after E-S surgery, OA and STA-frontal branch mainly achieved gradual anastomosis around the craniotomy site to contribute to indirect revascularization, which is a slow process. In contrast, after S-S surgery, blood flow from the OA and STA-frontal branch directly entered the donor vessels through anastomosis with the reserved distal STA, facilitating direct revascularization (Fig. 2). This is crucial for ensuring postoperative safety before indirect revascularization becomes mature, thereby avoiding recurrent ischemic strokes.
In our analysis, we found that the " scalp arteries hemodynamic diversion” phenomenon did not appear in all MMD patients with S-S bypasses as we initially expected. It was mainly pronounced in cases where the revascularization effect was classified as “poor”, rather in “good”. Indeed, in S-S cases with postoperative Matsushima grade A and B, the STA-parietal branch was the main donor for direct revascularization and more expanded than OA and STA-frontal branch. We considered it was because the donor STA is sufficient to meet the intracranial blood supply needs. Hence, there’s no need for other scalp arteries to participate. However, when the postoperative revascularization effect is poor, especially when donor artery atrophy occurs, the S-S surgery can mobilize other scalp arteries as backups to participate in direct revascularization. This may explain the absence of Matsushima D grade in the S-S group. In contrast, in traditional E-S bypass procedures, when donor STA postoperatively atrophied, revascularization can only rely on indirect revascularization through the MMA and DTA29,30,31. From this perspective, in S-S bypass surgery, scalp arteries can be regarded as backups for direct revascularization (Fig. 4.)
We also found that the postoperative diameter dilation of the STA-frontal branch in the S-S poor group was significantly greater than in the E-S poor group (with a mean CCR of 0.798), which indicate that the STA-frontal branch’s diameter was reduced after E-S surgery in poor group. These findings suggested that there may be blood redistribution from the frontal to the parietal branch after revascularization. We hypothesize that after E-S surgery, if the STA donor artery (parietal branch) experiences atrophy or stenosis leading to poor revascularization, the blood flow from the STA trunk might compensate by diverting more flow through the bifurcation to the parietal branch, which could result in a reduction in the diameter of the frontal branch artery. This can serve as a kind of backup for the E-S bypass. However, if the parietal branch of the STA is completely occluded postoperatively, even if the frontal branch blood flow redistributes to compensate for the insufficient parietal branch flow, it may still fail to achieve effective revascularization, leading to Matsushima grade D. In contrast, in the S-S group, the preservation of the distal STA allows for collateral support from the STA frontal branch, even if it cannot retrogradely support the parietal branch through the bifurcation. This helps prevent extreme poor situations.
In this study, we observed that the postoperative occurrence rate of CHS in the S-S group (6.8%) was lower than in the E-S group (15.0%). We considered this was mainly due to the diversion effect of the S-S anastomosis, which prevented excessive blood flow from the STA into the intracranial. However, we also found that the proportion of patients with good postoperative revascularization in the S-S group (57.5%) was smaller than in the E-S group (63.6%), while the proportion of patients with a C grade was higher in the S-S group (42.5% vs. 25.0%), although the difference was not statistically significant. This suggested that due to the presence of distal outflow diversion, the input power of the donor STA in the S-S group may be relatively smaller compared to the input power of the donor STA in the E-S group, which may lead to a relatively reduced blood flow into the intracranial region after S-S surgery. This could be a contributing factor affecting postoperative S-S revascularization. As the S-S bypass is a relatively new surgical technique for treating adult MMD, clinical studies on its safety and effectiveness are still limited. Therefore, more qualified medical institutions need to participate in its verification. Indeed, we have already initiated a multicenter clinical study on the S-S bypass procedure for adult MMD treatment, and we will disseminate the results of this multicenter study in a timely manner when it complete.
In conclusion, the S-S bypass technique effectively utilizes scalp arteries, particularly the STA-frontal branch and OA, for direct revascularization via the preserved distal STA in adult MMD patients. Scalp arteries can serve as a supplementary source of donor arteries, especially beneficial for patients with suboptimal revascularization outcomes.
Limitations
This study has several limitations that should be considered. First, the relatively small sample size and short follow-up period may limit the ability to fully assess postoperative outcomes and potential vascular events, emphasizing the need for larger, multi-center studies and longer observation periods to yield more definitive conclusions. Nonetheless, our findings are valuable given the rarity of MMD and the challenges inherent to long-term clinical and angiographic follow-up. We intend to continue tracking this patient cohort for further insights. The study’s retrospective design introduces potential selection bias; however, efforts were made to minimize this by involving a single surgeon and including patients only from 2021 onward. Additionally, vascular diameter measurements in this study were relatively coarse, highlighting the importance of more precise techniques in future research to improve evaluation accuracy and deepen understanding of vascular changes. There are also limitations in using SPECT imaging and the rCBF ratio to assess postoperative cerebral blood flow, as SPECT’s lower spatial resolution and quantification challenges can impact the accuracy of rCBF measurements. Finally, while our study offers practical clinical insights, the underlying mechanisms of observed phenomena remain open for further investigation.
Data availability
Data and material are available when suitable asking the corresponding author for information.
References
Ihara, M. et al. Moyamoya disease: diagnosis and interventions. Lancet Neurol. 21 (8), 747–758 (2022).
Ahn, I. M. et al. Incidence, prevalence, and survival of moyamoya disease in Korea: a nationwide, population-based study. Stroke 45 (4), 1090–1095 (2014).
Kuriyama, S. et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke 39 (1), 42–47 (2008).
Zhang, D. et al. Epidemiology of Moyamoya disease in China: A nationwide hospital-based study. Lancet Reg. Health West. Pac. 18, 100331 (2022).
Wakai, K. et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin. Neurol. Neurosurg. 99 (Suppl 2), S1–5 (1997).
Lee, M. J. et al. The impact of Moyamoya Disease and RNF213 mutations on the Spectrum of plasma protein and MicroRNA. J. Clin. Med. 8(10). (2019).
Lin, J. & Sheng, W. RNF213 Variant Diversity Predisposes Distinct Populations to Dissimilar Cerebrovascular Diseases. Biomed Res Int 2018:6359174. (2018).
Gonzalez, N. R. et al. Adult Moyamoya Disease and Syndrome: Current Perspectives and Future Directions: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke 54(10):e465-e479. (2023).
Nguyen, V. N. et al. Direct, Indirect, and combined extracranial-to-intracranial bypass for adult Moyamoya Disease: an updated systematic review and Meta-analysis. Stroke 53 (12), 3572–3582 (2022).
Machida, T. et al. Sagittal splitting of the temporalis muscle for encephalo-myo-synangiosis to prevent ischemic complications due to a swollen temporalis muscle without inhibiting collateral developments in patients with moyamoya disease. J. Neurosurg. 2018:1–8 .
Hu, M., Yu, J., Zhang, J. & Chen, J. Designing a flow-controlled STA-MCA anastomosis based on the Hagen-Poiseuille law for preventing postoperative hyperperfusion in adult moyamoya disease. Ther. Adv. Chronic Dis. 14, 20406223231181492 (2023).
Khan, N. R. et al. One-donor, two-recipient extracranial-intracranial bypass series for moyamoya and cerebral occlusive disease: rationale, clinical and angiographic outcomes, and intraoperative blood flow analysis. J. Neurosurg. 136 (3), 627–636 (2022).
Zhang, J. et al. A flow self-regulating superficial temporal artery-middle cerebral artery bypass based on side-to-side anastomosis for adult patients with moyamoya disease. J. Neurosurg. 138 (5), 1347–1356 (2023).
Guidelines for diagnosis. And treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol. Med. Chir. 52 (5), 245–266 (2012).
Fukui, M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on spontaneous occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin. Neurol. Neurosurg. 99 (Suppl 2), S238–240 (1997).
Matsushima, T. et al. Surgical treatment of moyamoya disease in pediatric patients–comparison between the results of indirect and direct revascularization procedures. Neurosurgery 31 (3), 401–405 (1992).
Zhao, Y. et al. Predictors of neoangiogenesis after indirect revascularization in moyamoya disease: a multicenter retrospective study. J. Neurosurg. :1–11. (2019).
Fujimura, M. et al. Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after extracranial-intracranial bypass for moyamoya disease: comparative study with non-moyamoya patients using N-isopropyl-p-[(123)I]iodoamphetamine single-photon emission computed tomography. Neurosurgery 68(4). (2011).
Uchino, H. et al. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke 43 (10), 2610–2616 (2012).
Zhao, M. et al. Risk factors for and outcomes of postoperative complications in adult patients with moyamoya disease. J. Neurosurg. 2018:1–12 .
Tokairin, K. et al. Postoperative intracerebral hemorrhage after combined revascularization surgery in Moyamoya Disease: profiles and Clinical associations. World Neurosurg. 120, e593–e600 (2018).
Yanagihara, W. et al. Impact of cerebral blood flow changes due to arterial bypass surgery on cognitive function in adult patients with symptomatic ischemic moyamoya disease. J. Neurosurg. 131 (6), 1716–1724 (2018).
Tashiro, R. et al. Incidence and risk factors of the Watershed Shift Phenomenon after superficial temporal artery-middle cerebral artery anastomosis for adult Moyamoya Disease. Cerebrovasc. Dis. 47 (3–4), 178–187 (2019).
Bersano, A. et al. European Stroke Organisation (ESO) guidelines on Moyamoya Angiopathy endorsed by vascular European Reference Network (VASCERN). Eur. Stroke J. 8 (1), 55–84 (2023).
Lee, S., Kim, S. K. & Phi, J. H. Anatomic variation of the superficial temporal artery and posterior auricular artery in a Pediatric Moyamoya Disease Population. AJNR Am. J. Neuroradiol. 42 (6), 1157–1162 (2021).
Liebeskind, D. S. Collateral circulation. Stroke 34 (9), 2279–2284 (2003).
Fujimura, M. & Tominaga, T. Lessons learned from moyamoya disease: outcome of direct/indirect revascularization surgery for 150 affected hemispheres. Neurol. Med. Chir. (Tokyo). 52 (5), 327–332 (2012).
Fujimura, M. & Tominaga, T. Current status of revascularization surgery for Moyamoya disease: special consideration for its ‘internal carotid-external carotid (IC-EC) conversion’ as the physiological reorganization system. Tohoku J. Exp. Med. 236 (1), 45–53 (2015).
Honda, M. et al. Magnetic resonance angiography evaluation of external carotid artery tributaries in moyamoya disease. Surg. Neurol. 64 (4), 325–330 (2005).
Xu, D. et al. Outcomes after superficial temporal artery-middle cerebral artery anastomosis combined with multiple burr hole surgery and dural inversion synangiosis for moyamoya disease in adults. Front. Surg. 9, 1047727 (2022).
Houkin, K., Kuroda, S., Ishikawa, T. & Abe, H. Neovascularization (angiogenesis) after revascularization in moyamoya disease. Which technique is most useful for moyamoya disease? Acta Neurochir. 142 (3), 269–276 (2000).
Acknowledgements
Authors thanks all participants in this study.
Funding
This study was funded by National Natural Science Foundation of China (Funding No. 82401553) and Hubei Provincial Natural Science Foundation of China (2024AFB308).
Author information
Authors and Affiliations
Contributions
Jin Yu: Methodology; Validation; Formal analysis; Investigation; Writing – original draft preparation; Visualization; Writing – review and editing; Funding acquisitionQian Du: Methodology; Validation; Formal analysis; Investigation; Writing – original draft preparation; Visualization; Writing – review and editing; Miao Hu: Methodology; Validation; Investigation; Writing – original draft preparation; Tianshu Tao & Guiping Wan: Writing – review and editing; Funding acquisition; Visualization; Jianjian Zhang: Conceptualization; Formal analysis; Investigation; Resources; Supervision; Writing – review and editingJincao Chen: Conceptualization; Resources; Supervision; Writing – review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors in this study approve the publication of this paper in this journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, J., Du, Q., Hu, M. et al. Role of scalp arteries in revascularization after side to side anastomosis in moyamoya disease patients. Sci Rep 14, 29961 (2024). https://doi.org/10.1038/s41598-024-81362-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81362-6