Abstract
Recent studies recommend sublobectomy as a surgical approach for non-small cell lung cancer (NSCLC) tumors that are 2 cm or smaller. However, it remains unclear whether NSCLC patients with squamous cell carcinoma (SCC) have comparable outcomes to those with adenocarcinoma (ADC) following sublobectomy. To that end, this study aims to compare the survival outcomes between SCC and ADC in patients with stage IA NSCLC (≤ 2 cm) who have undergone sublobectomy. We identified stage IA (≤ 2 cm) NSCLC patients diagnosed with lung squamous cell carcinoma or adenocarcinoma pathology and underwent sublobectomy from the Surveillance, Epidemiology, and End Results (SEER) database from 2004 to 2020. Overall survival (OS) was determined using the Kaplan–Meier method, and Cox proportional hazards regression was employed to identify risk factors for OS. A total of 9,831 patients diagnosed with stage IA NSCLC (≤ 2 cm) were evaluated. Of these, 2,078 patients met the inclusion criteria, including 1,565 with adenocarcinoma (ADC) and 513 with squamous cell carcinoma (SCC). Notably, SCC was associated with worse overall survival compared to ADC (HR: 2.02, 95% CI: 1.34–3.05, P = 0.03). Subgroup analyses revealed that SCC was comparable to ADC in terms of OS for tumors ≤ 1 cm (HR: 1.22, 95% CI: 0.47–3.18, P = 0.83), while patients with SCC displayed worse OS compared to ADC for tumors > 1 to 2 cm (HR: 2.05, 95% CI: 1.31–3.23, P = 0.002). Cox proportional hazards regression analysis identified female sex (HR: 1.53, 95% CI: 1.08–2.19, P = 0.017), high tumor grade (HR: 1.76, 95% CI: 1.02–3.03, P = 0.011), and SCC (HR: 1.58, 95% CI: 1.08–2.30, P = 0.017) as independent risk factors for OS. In patients with stage IA (≤ 2 cm) NSCLC who underwent sublobectomy, SCC is associated with worse overall survival compared to ADC. Furthermore, being female, having a high tumor grade, and SCC pathology are independent risk factors for OS in these patients.
Similar content being viewed by others
Introduction
Lung cancer has the highest morbidity and mortality rates among cancers worldwide. A recent report on the global cancer burden in 2022 indicated that there were 2.48 million new cases of lung cancer, accounting for 12.4% of all new malignancies globally. In the same year, approximately 1.8 million people died from lung cancer, representing 18.7% of all cancer deaths1. Importantly, the diagnosis and treatment of lung cancer continue to pose significant global health challenges.
Surgical treatment remains a crucial approach for lung cancer management. Historically, lobectomy and systematic mediastinal lymph node resection have been the standard surgical approach for lung cancer treatment2,3. While these procedures can reduce tumor recurrence, they also result in greater surgical trauma and damage to lung function. Recent studies have shown that sublobectomy can be an effective surgical option for patients with non-small cell lung cancer (NSCLC) lesions of 2 cm or less4.
Sublobectomy, which includes wedge resection and segmentectomy, has been evaluated in several studies. The JCOG0804 study revealed that sublobectomy is a feasible and effective surgical approach for patients with ground-glass opacity (GGO)-dominant and peripheral lung cancers ≤ 2 cm5,6. The JCOG0802 study compared the benefits of segmentectomy versus lobectomy for small peripheral NSCLC and found that segmentectomy had superior 5-year overall survival compared to lobectomy. Additionally, 5-year recurrence-free survival (RFS) and expiratory volume were similar between the two groups. These findings suggest that segmentectomy should be considered the standard surgical procedure for patients with clinical stage IA NSCLC (tumor diameter ≤ 2 cm; consolidation-to-tumor ratio > 0.5)7,8. Similarly, the CALGB140503 study compared sublobar resection and lobectomy in patients with clinically staged T1aN0 (tumor size ≤ 2 cm) and found that overall survival was comparable between the two procedures, with sublobar resection showing non-inferiority to lobectomy in terms of disease-free survival9.
Based on the above studies, the NCCN Guidelines version 1.2024 for NSCLC recommend sublobar resection for T1abN0M0 (tumor size ≤ 2 cm) and peripheral lung cancer. Nevertheless, there are no studies comparing oncologic outcomes between squamous cell carcinoma (SCC) and adenocarcinoma (ADC) in patients with pathologic stage IA (≤ 2 cm) who have undergone sublobectomy. Whether SCC yields outcomes equivalent to ADC in these patients remains unclear.
In our opinion, ADC and SCC exhibit significant biological differences, particularly in their metastatic pathways. Notably, ADC tends to metastasize through the bloodstream, whereas SCC is more prone to lymphatic metastasis10. Based on this evidence, we hypothesize that there could be differences in recurrence and prognosis outcomes between ADC and SCC in NSCLC patients with tumors ≤ 2 cm who have undergone sublobar resection.
Herein, based on the SEER database, we compared survival outcomes between squamous cell carcinoma and adenocarcinoma among NSCLC patients with stage IA (≤ 2 cm) who underwent sublobectomy. The results indicated that SCC is associated with worse overall survival (OS) compared to ADC. Cox proportional hazards regression analysis identified female sex, high tumor grade, and SCC as independent risk factors for OS. This finding highlights the differences in OS between SCC and ADC and provides guidance for selecting patients who are suitable candidates for sublobectomy.
Methods
Patient population
The patients in this study were sourced from the Surveillance, Epidemiology, and End Results (SEER) database, a public population-based cancer registry encompassing approximately 28% of all diagnosed cancer cases in the United States. We included patients who were diagnosed with lung adenocarcinoma or squamous cell carcinoma and underwent sublobectomy for tumors ≤ 2 cm between 2000 and 2020. And patients never received other treatments, especially chemotherapy or radiotherapy (Fig. 1).The exclusion criteria were as follows: (a) patients who underwent surgeries other than sublobectomy; (b) patients who received additional treatments such as chemotherapy or radiotherapy; (c) patients with pathological types other than ADC or SCC; and (d) patients with multiple primary cancers. We collected clinical characteristics, including sociodemographic information (age, race, and gender), tumor characteristics (differentiation grade, primary site, and tumor size), treatment information (surgical procedures), and survival information.
Statistical analysis
The t-test or Pearson’s χ² test was used to compare variables between treatment groups, as appropriate. Survival analyses were conducted using the Kaplan-Meier method and assessed with the log-rank test. Cox proportional hazards regression analysis was employed to evaluate the independent effects on OS (The variables inclusion for multivariate analysis were selected from univariate analysis which P < 0.2). All statistical analyses were two-sided, with a significance level set at P < 0.05. The analyses were performed using SPSS statistical software (Statistical Product and Service Solutions software, version 26.0.0, https://www.ibm.com/spss) and GraphPad Prism (GraphPad Software, version 8.4.0, https://www.graphpad.com). Forest plots were created using ggplot2 in RStudio (RStudio Software, version 4.2.1, https://www.rstudio.com).
Results
A total of 9,831 patients diagnosed with stage IA (≤ 2 cm) NSCLC between 2000 and 2020 were evaluated. Among these, 2,078 patients met the inclusion criteria and received sublobectomy (ADC: 1,565 vs. SCC: 513). The baseline characteristics of the patients are summarized in Table 1. Adenocarcinoma was more common in females (62.7% vs. 50.5%) and in younger patients (> 65 years: 85.7% vs. 92.6%) compared to squamous cell carcinoma. Furthermore, patients with SCC who received sublobectomy had larger tumors (13.66 ± 3.95 mm vs. 12.03 ± 4.68 mm) and higher tumor grades (29.7% vs. 13.4%).
To further compare the differences between SCC and ADC, we conducted a subgroup analysis of clinical characteristics for tumors ≤ 1 cm and > 1 to 2 cm. Consistent with the findings for tumors ≤ 2 cm, adenocarcinoma was more common in females and younger patients in both the ≤ 1 cm and > 1 to 2 cm groups (Table 2).
Next, we performed survival analyses to compare outcomes between SCC and ADC. The survival analysis of the entire cohort showed that SCC has worse OS compared to ADC (HR: 2.02, 95% CI: 1.34–3.05, P = 0.03, Fig. 2A). Subgroup analyses revealed that SCC and ADC had similar OS for tumors ≤ 1 cm (HR: 1.22, 95% CI: 0.47–3.18, P = 0.83, Fig. 2B), but SCC had worse OS compared to ADC for tumors > 1 to 2 cm (HR: 2.05, 95% CI: 1.31–3.23, P = 0.002, Fig. 2C).
.
Univariate analysis identified gender, tumor size, tumor grade, surgical type, and pathological type as factors associated with OS in these patients. Cox proportional hazards regression analysis was also performed to evaluate the independent factors influencing OS. The results indicated that female sex (HR: 1.53, 95% CI: 1.08–2.19, P = 0.017), high tumor grade (HR: 1.76, 95% CI: 1.02–3.03, P = 0.011), and SCC (HR: 1.58, 95% CI: 1.08–2.30, P = 0.017) were independent risk factors for OS (Table 3) and (Fig. S1) .
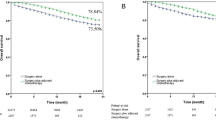
Univariate analysis indicated that surgical type was a risk factor for overall survival. We further performed a Kaplan-Meier plot analysis, showing that SCC had worse OS than ADC in the wedge resection group. However, this difference was not statistically significant in the segmentectomy group (Fig. 3).
Discussion
Although both ADC and SCC are pathological subtypes of NSCLC, they differ significantly in several aspects, including growth patterns, recurrence, metastasis, and (Tumor micro-environment)TME11,12,13. Previous studies have shown that ADC is more likely to occur in peripheral lung locations, whereas SCC tends to be found in central locations14. Moreover, ADC is prone to distant metastasis via the bloodstream, while SCC often exhibits invasive growth and is more likely to metastasize to lymph nodes through the lymphatic route10. Generally, ADC has a worse prognosis compared to SCC, attributed to its shorter doubling time and higher propensity for distant metastasis15,16.
Radical resection, including lobectomy and systematic mediastinal lymph node dissection, has traditionally been the main surgical approach for lung cancer. However, this method is more traumatic and can significantly impair lung function17. Researchers have been exploring less invasive surgical options that can still achieve curative outcomes. Sublobar resection has emerged as a preferable approach due to its reduced trauma and lesser impact on lung function18. Recent studies, including JCOG0804, JCOG0802, and CALGB140503, have demonstrated that sublobar resection can offer superior OS to radical resection for NSCLC patients with tumors ≤ 2 cm. Based on these findings, the NCCN guidelines recommend sublobar resection as a surgical option for patients with T1abN0M0 (tumor size ≤ 2 cm) and peripheral NSCLC5,6,7,8,9. While these studies focus on comparing sublobar resection to lobectomy, there is currently no evidence regarding whether the oncologic outcomes are consistent between ADC and SCC in patients who undergo sublobar resection.
In the present study, we compared the prognosis between ADC and SCC in NSCLC patients with stage IA (≤ 2 cm) who underwent sublobar resection. The results indicated that NSCLC patients with SCC were associated with poorer OS than those with ADC. Possible reasons for this include the central and invasive nature of SCC and its tendency for more localized recurrence patterns12. In addition, many patients with SCC have a long history of smoking, which may contribute to a higher incidence of comorbidities, such as chronic obstructive pulmonary disease, compared to patients with non-squamous NSCLC19,20. Furthermore, ADC can present as pure ground-glass opacity or mixed ground-glass opacity, both of which are associated with a better prognosis21,22. In contrast, SCC is often characterized by solid nodules, which may also contribute to the observed differences in prognosis. Histological type, gender, and tumor grade were identified as independent risk factors for OS following sublobar resection, consistent with previous studies11,23. Subgroup analysis revealed that sublobar resection for tumors ≤ 1.0 cm or segmentectomy for tumors ≤ 2 cm in SCC can yield outcomes comparable to those in ADC. However, sublobar resection for tumors 1–2 cm or wedge resection for tumors ≤ 2.0 cm in SCC were associated with poorer OS compared to ADC. These differences between lung adenocarcinoma and squamous cell carcinoma can assist clinicians in identifying patients who are suitable candidates for sublobar resection.
Conclusion
In conclusion, our study revealed that in NSCLC patients with stage IA (≤ 2 cm) who underwent sublobectomy, SCC is associated with worse OS compared to ADC. High tumor grade and SCC were identified as independent risk factors for OS in these patients. This study has inherent limitations, including its retrospective nature and the lack of data on RFS and tumor recurrence patterns between the two groups. Despite these limitations, in the absence of results from RCTs, our study provides valuable insights into the oncological outcomes of SCC versus ADC in stage IA lung cancer patients who received sublobar resection. We believe our findings may aid in the design of future studies.
Data availability
The data presented in this study can be obtained in online repositories: https://seer.cancer.gov. SEER data is publicly available and de-identified. And the datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SCC:
-
Squamous cell carcinoma
- ADC:
-
Adenocarcinoma
- NSCLC:
-
Non-small cell lung cancer
- SEER:
-
Surveillance, epidemiology, and end results database
- OS:
-
Overall survival
- RFS:
-
Relapse-free survival
- TME:
-
Tumor micro-environment
- RCT:
-
Randomized controlled trial
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263 (2024).
Cahan, W. G. Radical lobectomy. J. Thorac. Cardiovasc. Surg. 39, 555–572 (1960).
Lardinois, D. et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 30 (5), 787–792 (2006).
Li, R. Z. & Qiu, B. JCOG lung cancer surgery trial series: review and interpretation. Zhonghua Zhong Liu Za Zhi 44 (7), 703–711 (2022).
Suzuki, K. et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J. Thorac. Cardiovasc. Surg. 163 (1), 289–301e282 (2022).
Yoshino, I. et al. Long-term outcome of patients with peripheral ground-glass opacity-dominant lung cancer after sublobar resections. J. Thorac. Cardiovasc. Surg. 166 (4), 1222–1231e1221 (2023).
Saji, H. et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399 (10335), 1607–1617 (2022).
Hattori, A. et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer with radiologically pure-solid appearance in Japan (JCOG0802/WJOG4607L): a post-hoc supplemental analysis of a multicentre, open-label, phase 3 trial. Lancet Respir Med. 12 (2), 105–116 (2024).
Altorki, N. et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N. Engl. J. Med. 388 (6), 489–498 (2023).
Popper, H. H. Progression and metastasis of lung cancer. Cancer Metast. Rev. 35 (1), 75–91 (2016).
Wang, B. Y. et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J. Cancer Res. Clin. Oncol. 146 (1), 43–52 (2020).
McAleese, J., Taylor, A., Walls, G. M. & Hanna, G. G. Differential relapse patterns for non-small cell lung cancer subtypes adenocarcinoma and squamous cell carcinoma: implications for radiation oncology. Clin. Oncol. (R. Coll. Radiol.) 31 (10), 711–719 (2019).
Wang, C. et al. The heterogeneous immune landscape between lung adenocarcinoma and squamous carcinoma revealed by single-cell RNA sequencing. Signal. Transduct. Target. Ther. 7 (1), 289 (2022).
Paris, C. et al. Smoking status, occupational asbestos exposure and bronchial location of lung cancer. Lung Cancer 40 (1), 17–24 (2003).
Chansky, K. et al. The International Association for the study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J. Thorac. Oncol. 4 (7), 792–801 (2009).
Pfannschmidt, J., Muley, T., Bülzebruck, H., Hoffmann, H. & Dienemann, H. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer 55 (3), 371–377 (2007).
Rami-Porta, R., Wittekind, C. & Goldstraw, P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 49 (1), 25–33 (2005).
Mamede, I., Ribeiro, L., Stecca, C., Escalante-Romero, L. & Cypel, M. Survival and pulmonary function in stage IA non-small cell lung cancer after sublobar resection versus lobectomy: an updated meta-analysis. J. Surg. Oncol. (2024).
Janssen-Heijnen, M. L., Schipper, R. M., Razenberg, P. P., Crommelin, M. A. & Coebergh, J. W. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung Cancer 21 (2), 105–113 (1998).
Papi, A. et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax 59 (8), 679–681 (2004).
Li, X. et al. Ten-year follow-up of lung cancer patients with resected stage IA invasive non-small cell lung cancer. Ann. Surg. Oncol. (2024).
Ding, L. et al. Prognostic implications of CT-defined ground glass opacity in clinical stage I-IIA grade 3 invasive non-mucinous pulmonary adenocarcinoma. Clin. Radiol. 79 (3), e353–e360 (2024).
Nakamura, H. et al. Difference in postsurgical prognostic factors between lung adenocarcinoma and squamous cell carcinoma. Ann. Thorac. Cardiovasc. Surg. 23 (6), 291–297 (2017).
Acknowledgements
The authors thank all patients and institutions involved in this study, especially the ability to have open access to the SEER database.
Author information
Authors and Affiliations
Contributions
Study concept and design: Jiannan Xu, Jian Zhang. Acquisition of data: Jiannan Xu, Yonghui Wu. Analysis and interpretation of data: Jiannan Xu, Kai Zhang, Yonghui Wu and Jian Zhang. Statistical analysis: Jiannan Xu, Kai Zhang and Yonghui Wu. Drafting of the manuscript: Jiannan Xu, Kai Zhang, Huiguo Chen, Weibin Wu and Xiaojun Li. Critical revision and final approval of the manuscript: Jiannan Xu, Yuanheng Huang and Jian Zhang. Study supervision: Jian Zhang. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, J., Zhang, K., Chen, H. et al. Squamous cell carcinoma predicts worse prognosis in stage IA (≤ 2 cm) non-small cell lung cancer patients following sublobectomy: a population-based study. Sci Rep 14, 30998 (2024). https://doi.org/10.1038/s41598-024-81965-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81965-z