Abstract
Altered lipid metabolism is a crucial jeopardy cause for developing non-alcoholic fatty liver disease (NAFLD). Among various lipid metrics, the ratio of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol (NHHR) has recently emerged as a promising indicator showing significant potential in predicting the prevalence of NAFLD. This study aimed to provide novel insights for the prevention of NAFLD by examining the relationship between NHHR and the prevalence of NAFLD. To identify the connection between NHHR and NAFLD, this study utilized descriptive analysis, multivariate logistic regression, and restricted cubic spline regression to investigate data collected by the National Health and Nutrition Examination Survey performed from 2017 to 2020. Furthermore, the connection between NHHR and the controlled attenuation parameter (CAP) was assessed using multiple linear regression, smoothed curve fitting, and threshold effect analysis. The NAFLD group showed higher NHHR levels than the non-NAFLD group (2.990 vs 2.240, P < 0.001). Multiple logistic and linear regression analyses indicated significant positive associations between NHHR and its quartiles with both the prevalence of NAFLD and CAP levels. Additionally, NHHR was positively associated with the prevalence of NAFLD in a linear dose–response relationship. Furthermore, smoothed curve fitting demonstrated a positive relationship between NHHR and CAP, with a threshold effect at an inflection point of 3.398. Higher NHHR levels were significantly associated with the prevalence of NAFLD and steatosis, and maintaining NHHR in the appropriate range may reduce these conditions.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is marked by widespread hepatocellular steatosis and fat accumulation within liver lobules, excluding alcohol consumption and other specific liver injuries1. NAFLD is predicted to affect 25.24% of people worldwide2. With increasing rates of obesity, NAFLD incidence is projected to reach 100.9 million by 20303. NAFLD has a broad spectrum of diseases, including non-alcoholic steatohepatitis, cirrhosis associated with NAFLD, and hepatocellular carcinoma4. As the second most prevalent liver disease in the twenty-first century, NAFLD is projected to become one of the major factors in end-stage liver disease, liver transplantation, and primary liver cancer within the next decade5. Given the absence of FDA-approved drugs for NAFLD, lifestyle interventions like diet and exercise are crucial for treatment. Consequently, preventing NAFLD is of paramount importance6.
Recently, the ratio of non-high-density lipoprotein cholesterol (Non-HDL-C) to high-density lipoprotein cholesterol (HDL-C), known as NHHR, has become a promising lipid index for evaluating atherosclerosis risk7. This index integrates Non-HDL-C, which promotes atherosclerosis, with HDL-C, known for its protective effects against atherosclerosis8. NHHR serves as a diagnostic marker for various disorders related to dyslipidemia. Abnormalities in NHHR reflect disruptions in lipid metabolism, including conditions like overweight9, insulin resistance10, and metabolic syndrome11, which are recognized as indicators of risk for NAFLD development. Excessive lipid accumulation in the liver produces reactive oxygen species (ROS), Which can cause oxidative stress that accelerates disease progression in NAFLD12. While traditional lipid indices such as triglycerides and total cholesterol have been demonstrated as risk factors for NAFLD, NHHR offers a non-invasive and easily measurable alternative that could enhance risk assessment for NAFLD13. There are no studies on the correlation between NHHR and NAFLD, and its relationship with NHHR and steatosis deserves further investigation.
This study investigates the correlation between NHHR and NAFLD disease progression, aiming to identify biomarkers of NAFLD and steatosis development from various perspectives. The objective is to develop preventive strategies at both individual and population levels and to provide reliable references for early detection and recognition indicators of NAFLD and steatosis.
Methods
Study population
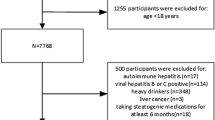
The National Health and Nutrition Examination Survey (NHANES) is a nationwide survey. The data used in this research was taken from the 2017–2020 NHANES database14. Inclusion criteria: participants aged 20 years and older (N = 9232). Exclusion criteria: (1) individuals lacking the controlled attenuation parameter (CAP) or liver stiffness measurement (LSM) data (N = 1310), (2) individuals with Hepatitis B or C infection (N = 128), (3) individuals consuming large amounts of alcohol (more than 30 g for male and more than 20 g for female per day) (N = 643), and (4) individuals lacking NHHR indicators (N = 500). The final analysis comprised 6651 participants. Figure 1 shows the detailed screening process.
Definition of NAFLD
Transient elastography has been extensively utilized for the detection of NAFLD15. A CAP value threshold of 274 dB/m or above was considered as the diagnostic criterion for NAFLD, with a sensitivity of 90% in identifying various degrees of hepatic steatosis16. In this study, the diagnosis of NAFLD was confirmed by a CAP value equal to or greater than 274 dB/m while excluding individuals with hepatitis B and C infections and alcoholic (For men, the limit is greater than 30 g/day, while for women, it is more than 20 g/day)17.
NHHR levels
NHANES measured total cholesterol (TC) and HDL-C through standardized enzymatic and chemiluminescent immunoassays in a controlled laboratory setting18. The NHHR is calculated by dividing non-HDL-C [mmol/L] by HDL-C [mmol/L], with non-HDL-C being obtained by subtracting HDL-C [mmol/L] from TC [mmol/L]19.
Assessment of covariates
Building on previous research on NAFLD, this study identified demographics, lifestyle factors, and health status as covariates for adjustment20. Demographic variables include age, gender, race, education, marriage, family poverty income ratio (FMPIR), and Body Mass Index (BMI). Lifestyle includes alcohol consumption and smoking status. Health status includes measurements of hypertension (HTN), hyperlipidemia (HL), and diabetes mellitus (DM), which are derived from questionnaires and measurement reports.
Statistical analysis
R software (version 4.2.2) and Empower software were utilized by the researcher to support the current statistical analysis. If continuous variables conform to the normal distribution, it is expressed as mean ± standard deviation (Mean ± SD), and if it is skewed, it is represented by Median (P25, P75). Categorical variables were reported as rates and component ratios. The relationship between NAFLD and CAP with NHHR was examined using multiple logistic and linear regression models. NHHR was examined both as a continuous variable and as a categorical variable, with odds ratios (OR), beta coefficients (β), and 95% confidence intervals (CI) calculated in three models. Model 1 did not include any covariates. Model 2 took age, gender, and race into account; and Model 3 further took into account factors like education, marriage, FMPIR, BMI, smoking, drinking, DM, HTN, and HL. To explore potential non-linear associations between NHHR and NAFLD, we employed restricted cubic spline (RCS) modeling. Additionally, smooth curve fitting and generalized additive models (GAM) were used to further assess the non-linear relationship between NHHR and CAP. To identify any threshold effects, threshold effect analysis was conducted, and a two-part linear regression model was applied to estimate the inflection point. Significance was determined at P < 0.050. All methods were performed by the NHANES guidelines and regulations.
Result
General demographic of the study
There were 6,651 participants in the study, and their median age was 52 years. There were 51.92% female participants and 48.08% male participants. Additionally, The ethnic composition was 12.64% Mexican American, 28.04% from other races, 34.01% non-Hispanic white, and 25.30% non-Hispanic black. The median NHHR value was 2.560 mmol/L.
In comparison to the non-NAFLD group, the NAFLD group was more inclined to be male, older, less than high school, smoking more than 100 cigarettes, being married or living with a partner, and having higher waist circumferences (WC) and BMI. Additionally, they were more likely to have HTN, HL, or DM. Laboratory measurements revealed elevated levels of systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides (TG), alanine aminotransferase (ALT), aspartate aminotransferase (AST), CAP, LSM, TC, Non-HDLC, and NHHR were elevated, while HDL cholesterol (HDLC) was low. Please refer to Table 1 for detailed information.
Analysis of the NHHR with NAFLD and CAP using multiple logistic regression and linear regression
We utilized multiple logistic and linear regression models to delve into the interrelation among NHHR, NAFLD, and CAP. The prevalence of NAFLD increased by 132.80% (OR = 2.328; 95% CI: 2.166–2.503), 130.30% (OR = 2.303; 95% CI: 2.135–2.483), and 73.60% (OR = 1.736;95%CI: 1.587–1.899) in Models 1,2, and 3, respectively, for every unit increase in NHHR. Simultaneously, CAP increased by units of measurement equal to 19.769 (β = 19.769; 95%CI:18.557 -20.981),16.761(β = 16 0.761; 95%CI:17 0.432–19.888), and 9.041(β = 9.041; 95%CI:7.902 -10.181) in each respective model.
Analyzing NHHR as an interquartile metric, Model 3 revealed that the NHHR Q3 (OR = 1.781; 95% CI: 1.485–2.137) and Q4 (OR = 2.795; 95% CI: 2.314–3.381) groups had higher prevalences of NAFLD, with increases of 78.10% and 179.50%, respectively, when compared to the lowest NHHR group, Q1. Additionally, CAP values for the NHHR Q2, Q3, and Q4 groups were higher by 3.396 dB/m (β = 3.396; 95% CI: 0.092–6.699), 16.761 dB/m (β = 16.761; 95% CI: 13.357–20.164), and 25.069 dB/m (β = 25.069; 95% CI: 21.395–28.743), respectively. Please refer to Tables 2 and 3 for detailed information.
Dose–response relationship between NHHR and NAFLD
To fully consider the potential role of covariates, we made adjustments for age, gender, race, education, marriage, FMPIR, BMI, smoking, drinking, DM, HTN, and HL. Using RCS regression, we discovered a linear relationship (Pnonlinear = 0.075) and a positive dose–response relationship (P < 0.001) between NHHR and the prevalence of NAFLD. When NHHR was below 2.570 mmol/L, it acted as a protective factor for NAFLD, with the protection decreasing as NHHR levels increased. Conversely, when NHHR exceeded 2.570 mmol/L, it became a risk factor for NAFLD, with the risk increasing as NHHR levels rose. Please refer to Fig. 2 for detailed information.
Analysis of threshold effects and smoothed curve fitting for NHHR and CAP
Smooth curve fitting and generalized additive models were used to further evaluate whether there was a nonlinear association between NHHR and CAP, and threshold effect analysis was used to find the inflection point. A two-part linear regression model was applied to estimate threshold effects. We found a positive smooth curve correlation between NHHR and CAP with an inflection point of 3.398. Below the inflection point (SIRI ≤ 3.398), CAP increased by 10.919 dB/m for every unit increase in NHHR. Beyond this inflection point, CAP increased by 6.321 dB/m for every unit increase in NHHR. Please refer to Fig. 3 and Table 4 for detailed information.
Discussion
The study, involving 6651 participants, found that NHHR levels were much higher in those with NAFLD compared to those without. By constructing multivariate logistic and linear regression models, we analyzed NHHR as a continuous indicator and quartile indicator, respectively. Our results indicated the prevalence of NAFLD and the levels of CAP were positively correlated with NHHR. NHHR exhibited a positive linear dose–response relationship with the prevalence of NAFLD. Additionally, there was a positive smoothed curve-fit relationship between NHHR and CAP levels, with a threshold effect inflection point at 3.398. This helps identify individuals with NAFLD due to lipid metabolism disorders. Maintaining NHHR within an appropriate range through lifestyle interventions may reduce the prevalence of NAFLD. This research offers robust evidence for the primary prevention of NAFLD.
We investigated the relationship between NHHR, NAFLD, and CAP in the general population based on the NHANES study. NHHR has frequently been utilized in prior research as an indicator of cardiovascular diseases21, specifically myocardial infarction and stroke22. NHHR, emerging as a new lipid marker, can precisely predict atherosclerosis risk, a critical factor in cardiovascular disease23. Additionally, cardiovascular disease is a major cause of mortality among NAFLD patients24. With further exploration of NHHR indicators, numerous studies have confirmed the significant correlation with DM25, cardiovascular disease26, chronic kidney disease27, metabolic syndrome28, depression29, and periodontitis30. Furthermore, NHHR may have an impact on mental health as a study found a nonlinear connection between NHHR levels and suicidal thoughts19. Maintaining NHHR below 3.100 in patients with impaired fasting glucose significantly increased the likelihood of returning to normoglycemia, indicating that NHHR dysregulation may affect glucose and lipid metabolism31. However, the connection between NHHR and NAFLD remains ambiguous.
NHHR’s impact on NAFLD may stem from various pathophysiological mechanisms. The pathogenesis of NAFLD is related to lipid metabolism disorders32, oxidative stress33, inflammation34, and insulin resistance35. As a composite indicator, NHHR is potentially linked to NAFLD pathogenesis. An elevated NHHR suggests an increase in non-HDLC and/or a decrease in HDLC, indicating a relative rise in harmful lipid particles and a decline in protective ones that can lead to liver lipid deposition and metabolic disorders36. HDL-C facilitates lipid transport and has roles in reducing inflammation, oxidative stress, and thrombosis, essential for the health of vascular endothelial cells. When the NHHR value is elevated and HDL-C decreases, the body’s ability to scavenge oxidative substances and maintain the vascular endothelium decreases, thereby inducing oxidative stress, which can further activate macrophages and Kupffer cells to release pro-inflammatory cytokines, attracting immune cells to the liver and exacerbating inflammation37. High NON-HDL-C levels indicate an increase in LDL-C, VLDL-C, and IDL-C, leading to excess fatty acids and lipid accumulation in the liver, disrupting the insulin signaling pathway and causing insulin resistance38. HDL-C can independently predict NAFLD39. The ratio of TC to HDL-C is a crucial indicator for assessing the risk of NAFLD40. The present study further complements and confirms the detrimental impact of NHHR on NAFLD, suggesting its potential utility as a predictive marker for NAFLD progression.
Strengths and limitations
Strengths: First, it investigates the association between the NHHR, CAP, and NAFLD from the NHANES, a representative sample database of the U.S. population making the findings robust and generalizable. Second, by exploring the relationship between NHHR and CAP, our study provides greater insight into the grading of hepatic steatosis and the potential for targeted interventions, enhancing macro-risk assessment and micro-clinical guidance for NAFLD progression. Third, the study employed three analytical models to adjust for a wide range of covariates, thereby improving the reliability and validity of the results. Lastly, the study highlights NHHR as a potential indicator for further research into NAFLD risk assessment, contributing valuable insights into lipid metabolism and liver health.
Limitations: First, the present study, being cross-sectional, can only explore the association of risk between NHHR and NAFLD without establishing causality; therefore, future research should focus on larger prospective cohort studies to confirm these findings. Second, the data used in this study were derived from the NHANES, which is limited to the American population and may not be generalizable to other populations or ethnic groups. Third, the study period (2017–2020) may not fully capture long-term trends or changes over time in NHHR and NAFLD prevalence. Lastly, despite adjusting for multiple covariates, potential unmeasured confounding factors still exist.
Conclusions
NHHR is strongly associated with the prevalence of NAFLD, which may serve as a potential marker for predicting NAFLD. Maintaining NHHR within an appropriate range may reduce the prevalence rate of NAFLD.
Data availability
The datasets generated and analyzed during the current study are available in the NHANES repository, https://www.cdc.gov/nchs/nhanes/index.htm.
References
Targher, G., Tilg, H. & Byrne, C. D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 6(7), 578–588. https://doi.org/10.1016/S2468-1253(21)00020-0 (2021).
Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 7(9), 851–861. https://doi.org/10.1016/S2468-1253(22)00165-0 (2022).
Quek, J. et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 8(1), 20–30. https://doi.org/10.1016/S2468-1253(22)00317-X (2023).
Younossi, Z. M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 70(3), 531–544. https://doi.org/10.1016/j.jhep.2018.10.033 (2019).
Lekakis, V. & Papatheodoridis, G. V. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. https://doi.org/10.1016/j.ejim.2023.11.005 (2023).
Sheka, A. C. et al. Nonalcoholic steatohepatitis: A review. Jama 323(12), 1175–1183. https://doi.org/10.14744/hf.2023.2023.0042 (2020).
Sheng, G. et al. Utility of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio in evaluating incident diabetes risk. Diabetes Metab. Syndr. Obes. 15, 1677–1686. https://doi.org/10.2147/DMSO.S355980 (2022).
Lin, W. et al. Association between the non-HDL-cholesterol to HDL-cholesterol ratio and abdominal aortic aneurysm from a Chinese screening program. Lipids Health Dis. 22(1), 187. https://doi.org/10.1186/s12944-023-01939-4 (2023).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 92, 82–97. https://doi.org/10.1016/j.metabol.2018.11.014 (2019).
García, A. G. et al. Diagnostic accuracy of triglyceride/glucose and triglyceride/HDL index as predictors for insulin resistance in children with and without obesity. Diabetes Metab. Syndr. 13(4), 2329–2334. https://doi.org/10.1016/j.dsx.2019.05.020 (2019).
Flowers, E. et al. Use of plasma triglyceride/high-density lipoprotein cholesterol ratio to identify increased cardio-metabolic risk in young, healthy South Asians. Indian J. Med. Res. 141(1), 68–74. https://doi.org/10.4103/0971-5916.154506 (2015).
Buzzetti, E., Pinzani, M. & Tsochatzis, E. A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65(8), 1038–1048. https://doi.org/10.1016/j.metabol.2015.12.012 (2016).
Wang, S. et al. Hyperoside attenuates non-alcoholic fatty liver disease in rats via cholesterol metabolism and bile acid metabolism. J. Adv. Res. 34, 109–122. https://doi.org/10.1016/j.jare.2021.06.001 (2021).
Cheng, T. D. et al. Trends in dietary vitamin A intake among US adults by race and ethnicity, 2003–2018. Jama 329(12), 1026–1029. https://doi.org/10.1001/jama.2023.0636 (2023).
Zhang, K. et al. The association between serum vitamin A and NAFLD among US adults varied in different BMI groups: A cross-sectional study. Food Funct. 14(2), 836–844. https://doi.org/10.1039/d2fo02204d (2023).
Eddowes, P. J. et al. Accuracy of fibroscan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with Nonalcoholic fatty liver disease. Gastroenterology 156(6), 1717–1730. https://doi.org/10.1053/j.gastro.2019.01.042 (2019).
Xie, R. et al. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front Immunol. 13, 925690. https://doi.org/10.3389/fimmu.2022.925690 (2022).
Sheng, G. et al. Evaluation of the value of conventional and unconventional lipid parameters for predicting the risk of diabetes in a non-diabetic population. J. Transl. Med. 20(1), 266. https://doi.org/10.1186/s12967-022-03470-z (2022).
Qing, G. et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and suicidal ideation in adults: A population-based study in the United States. Lipids Health Dis. 23(1), 17. https://doi.org/10.1186/s12944-024-02012-4 (2024).
Peng, H. et al. Prediction of MAFLD and NAFLD using different screening indexes: A cross-sectional study in US adults. Front Endocrinol (Lausanne) 14, 1083032. https://doi.org/10.3389/fendo.2023.1083032 (2023).
Voight, B. F. et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 380(9841), 572–580. https://doi.org/10.1016/S0140-6736(12)60312-2 (2012).
Holmes, M. V. et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J. Am. Coll. Cardiol. 71(6), 620–632. https://doi.org/10.1016/j.jacc.2017.12.006 (2018).
Liu, Y. et al. Relationship between the non-HDLc-to-HDLc ratio and carotid plaques in a high stroke risk population: A cross-sectional study in China. Lipids Health Dis. 19(1), 168. https://doi.org/10.1186/s12944-020-01344-1 (2020).
Meng, H., Ruan, J., Yan, Z., et al. New progress in early diagnosis of atherosclerosis. Int. J. Mol. Sci. 23(16) (2022). https://doi.org/10.3390/ijms23168939.
Zhen, R. et al. The relationship between non-HDL-C /HDL-C ratio (NHHR) and vitamin D in type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 16, 2661–2673. https://doi.org/10.2147/DMSO.S414673 (2023).
Che, B. et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: An analysis of UK biobank data. Cardiovasc. Diabetol. 22(1), 34. https://doi.org/10.1186/s12933-023-01762-2 (2023).
Zuo, P. Y. et al. Non-HDL-cholesterol to HDL-cholesterol ratio as an independent risk factor for the development of chronic kidney disease. Nutr. Metab. Cardiovasc. Dis. 25(6), 582–587. https://doi.org/10.1016/j.numecd.2015.03.003 (2015).
Shin, H. G. et al. The relationship between the triglyceride to high-density lipoprotein cholesterol ratio and metabolic syndrome. Korean J. Fam. Med. 38(6), 352–357. https://doi.org/10.4082/kjfm.2017.38.6.352 (2017).
Qi, X. et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and risk of depression among US adults: A cross-sectional NHANES study. J. Affect. Disord. 344, 451–457. https://doi.org/10.1016/j.jad.2023.10.064 (2024).
Hou, K. et al. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and prevalence of periodontitis among US adults: A cross-sectional NHANES study. Sci. Rep. 14(1), 5558. https://doi.org/10.1038/s41598-024-56276-y (2024).
Mo, Z. et al. Association between non-high-density lipoprotein to high-density lipoprotein ratio and reversion to normoglycemia in people with impaired fasting glucose: A 5-year retrospective cohort study. Diabetol Metab. Syndr. 15(1), 259. https://doi.org/10.1186/s13098-023-01237-0 (2023).
Li, Z. et al. Genetic association of lipids and lipid-lowering drug target genes with non-alcoholic fatty liver disease. EBioMedicine 90, 104543. https://doi.org/10.1016/j.ebiom.2023.104543 (2023).
Chen, Z. et al. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 152, 116–141. https://doi.org/10.1016/j.freeradbiomed.2020.02.025 (2020).
Tilg, H. et al. Non-alcoholic fatty liver disease: The interplay between metabolism, microbes and immunity. Nat. Metab. 3(12), 1596–1607. https://doi.org/10.1038/s42255-021-00501-9 (2021).
Stefan, N. et al. The role of hepatokines in NAFLD. Cell Metab. 35(2), 236–252. https://doi.org/10.1016/j.cmet.2023.01.006 (2023).
Zhang, Q., Ke, Y. & Hong, H. HDL and lipid metabolism. Adv. Exp. Med. Biol. 1377, 49–61. https://doi.org/10.1007/978-981-19-1592-5_4 (2022).
Manzoor, M. F. et al. Oxidative stress and metabolic diseases: Relevance and therapeutic strategies. Front Nutr. 9, 994309. https://doi.org/10.3389/fnut.2022.994309 (2022).
Busatto, S. et al. Lipoprotein-based drug delivery. Adv. Drug Deliv. Rev. 159, 377–390. https://doi.org/10.1016/j.addr.2020.08.003 (2020).
Zelber-Sagi, S. et al. Non-high-density lipoprotein cholesterol independently predicts new onset of non-alcoholic fatty liver disease. Liver Int. 34(6), e128–e135. https://doi.org/10.1111/liv.12318 (2014).
Ren, X. Y. et al. Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: Jinchang cohort study. Lipids Health Dis. 18(1), 47. https://doi.org/10.1186/s12944-019-0984-9 (2019).
Funding
National Natural Science Foundation of China Grant (NO.81673806); Research Topic supported by the China Pharmaceutical Education Association (2020KTY001).
Author information
Authors and Affiliations
Contributions
Yajie Liu was responsible for the writing, statistical analysis, and chart drawing of the paper; Professor Ruilin Wang is responsible for formulating writing ideas, guiding writing, data curation, and data review.
Corresponding author
Ethics declarations
Ethics statement
The NHANES Research Project was approved by the Research Ethics Review Board (ERB) of the National Center for Health Statistics (NCHS) (NCHS IRB/ERB Agreement No. Continuation of Protocol#2011-17; Protocol #2018-01), in which all respondents provided written informed consent.
Competing interests
There is no conflict of interest in this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Y., Wang, R. The non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a predictor of NAFLD prevalence and steatosis severity. Sci Rep 15, 12990 (2025). https://doi.org/10.1038/s41598-024-82012-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82012-7