Abstract
The European pond turtle (Emys orbicularis) is a wide-ranging, long-living freshwater species with low reproductive success, mainly due to high predation pressure. We studied how habitat variables and predator communities in near-natural marshes affect the survival of turtle eggs and hatchlings. We followed the survival of artificial turtle nests placed in marshes along Lake Balaton (Hungary) in May and June as well as hatchlings (dummies) exposed in September. We found that the fewest nests remained intact in the least disturbed, most extensive area with the largest turtle population without predator control. Hatchlings, compared to nests had a significantly higher probability of survival. The density of carnivore dens indicated the negative influence on the number of exposure days, while marsh vegetation coverage was unrelated. The role of carnivores, especially the red fox (Vulpes vulpes) in the predation of nests was more significant, while predation by corvids (Corvidae) and wild boar (Sus scrofa) increased for the hatchlings. Predation by mammals increased with the limited availability of dry terrestrial surfaces suitable for laying eggs and with distance to water. Our multifactorial analyses highlight the need for targeted conservation efforts to improve the reproductive success of turtles in these ecologically sensitive environments.
Similar content being viewed by others
Introduction
Most reptiles, including turtles and tortoises are seriously threatened with extinction on a global scale1,2. Predation is a well-known and significant threat to turtle and tortoise populations3,4, alongside other factors such as habitat loss and degradation5,6, invasive predator and competitor species7,8, environmental pollution9,10, disease and parasitism11,12, overexploitation2,13, and global climate change1,8,14. The impact of predation varies depending on habitat type15,16, nest density and turtle age group12,17,18,19,20. The loss of eggs is often caused by mammalian predators, which are effective in detecting buried eggs. Mammalian predators use olfactory cues to detect scents left by the female turtles21,22, turtle tracks or the visual presence of digging turtles23 and a combination of these with tactile cues4,24. Avian predators, mostly corvids (Corvidae), mainly use visual cues to search for food25,26,27,28,29, but some, e.g. common raven (Corvus corax) can also use olfactory cues to find food30. Predators can alter the demographic structure of turtle populations, for example by shifting the age structure, which can ultimately influence population survival and negatively affect reproductive success17,19,20,31,32,33. The main predators of freshwater turtle nests are mostly generalist, common, and often introduced species such as the red fox (Vulpes vulpes), raccoon (Procyon lotor), wild boar or feral pig (Sus scrofa)4,17. Locally, rodents34 and birds can also act as secondary predators3,35, consuming the remains left behind by carnivores, and ravens can exert high predation pressure36.
For freshwater turtles the survival of eggs, hatchlings and juveniles is generally low and stochastic3,12,37,38,39 while the mortality rates of eggs and hatchlings are largely unknown3,40. Survival and mortality rates cannot be estimated without knowledge of the initial population numbers41,42 (detecting nests is difficult due to the hidden lifestyle of hatchlings3,40), or data collected using the capture-mark-recapture method16,43. Alongside methods such as track surveys and camera trapping44,45,46, predation tests using artificial prey are often applied3,31,32,40,47 to estimate predation losses. While these tests come with certain limitations48, they hold considerable value due to the challenges of detecting real nests, as well as for conservation and ethical considerations49,50.
Survival of hatchlings is generally higher than that of eggs51. The survival of freshwater turtle eggs and hatched turtles can be affected by habitat characteristics16,52,53 and ecological edge effects, such as wooded and water edges54,55. In some studies, a higher rate of predation loss was found near water54,55 and proximity of fox dens56. These findings suggest that the spatial and temporal dynamics of predation mortality on freshwater turtle eggs and hatchlings would differ substantially depending on environmental variables4,57,58 and this was our hypothesis. Exploring the temporal and spatial predation patterns in natural areas can provide valuable insights into the phenomena observed in more disturbed or human-influenced environments, aiding in the selection of effective turtle protection strategies59,60,61.
The European pond turtle (Emys orbicularis) is a widespread freshwater turtle endemic to the Western Palearctic62. In the evaluation of IUCN the species is classified as Near Threatened63. Its populations declined considerably due to the reduction in suitable wetlands in recent decades64. The life history of the European pond turtle is characterized by a long lifespan, delayed sexual maturity and low reproductive output26,43,65. It can lay its eggs in different habitats, such as forest ponds, fish ponds, recreational lakes, bogs, marshes, backwaters, canals, and slow small watercourses26,64,65,66, making it an optimal model species for studying predation effects and designing nest protection solutions based on this. However, compared to other freshwater turtle species in other continents4, the European pond turtle has been under-researched in terms of exploring the causes of predation67.
We assessed predation losses of artificial turtle nests (eggs) and hatchlings using artificial nests and turtle dummies during the egg-laying and hatching periods in marshes with varying habitat features. To explore our hypothesis, we tested five predictions:
-
predation on turtle nests will increase over time4 (P1),
-
nests will be more vulnerable to predation than hatchlings51 (P2),
-
predation loss will decrease with the proportion of marsh habitats and increase with proximity to water54,55 (P3),
-
predation loss negatively relates to the presence of predator-inhabited dens56 (P4), and
-
area and prey type will influence predator composition, with generalist mammalian predators being dominant4,17 (P5).
The aims of our study were: (1) to analyze spatial and temporal effects of predation on the survival of European pond turtle nests and hatchlings, (2) to quantify the impact of key environmental factors influencing survival, and (3) to identify factors affecting predation patterns.
Results
Survival of turtle nests and hatchlings
In our study with artificial turtle nests (eggs) and plasticine hatchlings (dummies), the pattern of predation over time varied. The number of days survived varied between 2.7 and 4.9 in May, 0.9 and 5.7 in June, and 5.9 and 7.9 in September 2018 among the three study areas (Fig. 1). Within the first three days, on average (± SE) 49.5 ± 13.8% of artificial nests were predated in May, 54.5 ± 16.2% in June, and 6.1 ± 3.3% of hatchlings (dummies) in September.
Log-linear analysis of the occurrence data of intact (non-predated) artificial turtle nests and hatchlings indicated a significant effect of area (χ2 = 33.88, df = 2, P < 0.0001) and month (χ2 = 49.95, df = 2, P < 0.0001). Most artificial nests, or hatchling turtle dummies, were predated on A1 (Kis-Balaton) and remained most intact on A2 (Nagyberek). Compared to the survival of artificial nests in May, the survival of small turtles in September was higher. The interaction was not significant (χ2 = 2.74, df = 4, P = 0.603).
The survival probability of artificial nests was significantly positively influenced by the order of months (May or June) and significantly negatively influenced by the presence of fox dens and the distance to water (Table 1a). The survival of turtle hatchlings was significantly negatively affected by the presence of fox dens (Table 1b). The survival was significantly related positively to prey type (eggs or hatchlings), as turtle hatchlings had a significantly higher probability of survival, while the presence of fox dens and distance to water both had significantly negative correspondence to the probability of survival (Table 1c). The effect of the marsh vegetation coverage was not significant.
Identified predators
Predation was recorded on 79.3% and 71.8% of the artificial nests in May and June, respectively. Based on the tooth marks found on the eggs, the predators in each area were mainly red foxes and more precisely unspecified “medium-sized carnivorous mammals” (MSC). Rarely, European badgers (Meles meles), and in June wild boar were also identified. The footprints found around the nests (Table SI1) confirmed the identification of predators, primarily foxes, and occasionally badgers and wild boar.
Among the plasticine turtle dummies, we found at least one damaged in 58 nests (29.3%) (Fig. SI1). Based on the prints left on the dummies, predation losses were most often caused by foxes, followed by corvids and wild boar. Predation by brown rats (Rattus norvegicus) rarely occurred. In rare cases, the predator could not be identified.
Log-linear analysis of the predation events of the predated artificial turtle nests (Table 2) showed a difference depending on the period and prey type in the medium-sized carnivore (MSC) and corvid groups. The role of carnivores as predators was more significant on the predation of artificial nests (eggs), compared to on the predation of hatchlings (dummies). Corvid predation prevailed in autumn for hatched turtles (Fig. 2, Fig. SI1). In the case of wild boar, it depended on the area and period, or there were differences depending on the prey type. Wild boar predation occurred mainly in autumn and only in the two smaller areas (A2-A3). The effects of brown rats were not significant.
Red foxes were recorded with camera traps in all cases (A1; Fig. SI2). During the egg-laying period, the appearance of the foxes followed a two-peaked activity curve, the majority of recordings were made in the hours after dusk and before dawn (Fig. SI3). Foxes visited the artificial nest sites several times during a single night, and more than one fox visited the site each evening. In all cases, the foxes observed in May were mature individuals, while in June a young fox sniffing an artificial nest was also recorded (Fig. SI2). In autumn, the presence of foxes was recorded during the late evening and early morning (twilight and light) periods for the hatched turtles (Fig. SI3). In these cases, we confirmed the predation event by the fox during the subsequent checks.
Effect of habitat variables on the predation pattern
Kruskal-Wallis tests indicated the significant effects of marsh surface area (χ2 = 54.51, P < 0.0001) and distance from water (χ2 = 17.64, P = 0.0004). After the Dunn’s post hoc tests, considering mean marsh surface area (Fig. 3a), carnivores (MSC, 5.45 ha) and wild boar (7.16 ha) predated artificial nests or turtle hatchling dummies with significantly higher area compared to intact ones (3.73 ha). Predation events by carnivores (MSC, 39.6 m) and wild boar (mean 45.5 m) were detected at significantly lower distances to water compared to corvids (60.9 m) and intact artificial nests or turtle dummies (32.1 m; Fig. 3b).
Effect of marsh habitat extent (left panel) and distance from water (right panel) around study sites on nest predation, in three wetlands in Hungary. MSC – summarized medium-sized carnivores. Significant between-group differences by Dunn’s post hoc test are shown with different letters (a-b). The box spans the interquartile range, representing the middle 50% of the data, with its lower edge corresponding to the first quartile and its upper edge corresponding to the third quartile. The line inside the box marks the median. The whiskers extend from the box to the smallest and largest values.
Discussion
Our first prediction that nest predation would increase over time was only partially supported, as the temporal pattern varied by area. According to the optimal foraging theory (i.e. foragers select food items by finding a compromise between costs and benefits to maximize their net rates of energy intake68,69,70, it is expected that predators quickly discover and exploit turtle eggs as an abundant short-term food source23 with high energy content71. Predators focus their search image72 on egg-laying turtles, nests and hatchlings21,23,36, resulting in rapid nest detection24, especially at frequently used areas such as habitat edges near water or disturbed patches like roads54,55. Through learning and due to the nest site fidelity of turtles73 increasing predation rates over time are expected4,24,36,74. However, Oddie et al.24 suggest that cues used by carnivores to find nests may weaken over time, although predation can continue throughout incubation and may even peak near hatching time. For nocturnal mammalian predators, olfaction plays a critical role in detecting and identifying prey25,75. These findings suggest that both the odour and visual appearance of prey have a significant influence on the predation process by mammalian predators.
The predation pressure on the hatching turtles was much lower compared to nests, regardless of the area and location, supporting our second prediction. The average ratio of the surviving dummies was 0.70. This corresponds to the 0.65 rate estimated by Pike et al.76 for turtles based on population size data and survival rates of adult turtle populations. In the case of the European pond turtle, the first-year mortality was estimated to be 98%, but it also covers the nest (egg) predation67. Information on post-hatching survival rates is very scarce, with data available for only a few species, e.g., for the 3 cm sized northern map turtle (Graptemys geographica) hatchlings 0.22 rate77, and for Blanding’s turtles (Emydoidea blandingii) 0.71 rate was determined for the first year78. A study using 3D models of juvenile box turtles (Terrapene carolina) found 18% interaction rate among 372 fielded dummies, with 8% being attacked, which corresponds to our results in the first three days40.
The study areas are wetlands of varying composition but in near-natural conditions. We found that habitat composition, specifically marsh vegetation coverage, had less impact on the survival of turtles, contrary to our expectation (third prediction). The European pond turtle prefers small water bodies with muddy bottoms, old riverbeds, wetlands, and little waterholes65,66. The female European pond turtle has a high degree of habitat and nestsite fidelity over several seasons79,80,81,82. Gene flow between populations is driven by dispersal of older males, while juveniles and females show strong philopatry83. The European pond turtle has a complex orientation and can return to its original habitat from a great distance84. The marshland, containing linear structures (e.g. embankments), is an apparently optimal habitat for the turtle nests. At the same time, terrestrial predatory mammals with advanced sensory abilities use the embankments that pass through the marshland on their foraging trips, thus easily reaching dry areas that are also turtle nesting sites55. The problem is that the females return to sites where their nests are regularly depredated; therefore, embankments can be considered an ecological trap85.
Contrary to our expectation (third prediction54,55), predation by mammals (MSC, wild boar) increased with distance from water. In two sites, nest survival was significantly higher (61% and 68%) at those sites closer to the water (7 m and 20 m) compared to other nesting sites. This resulted in a trend contrary to the literature, but none of the environmental factors we investigated explained the higher survival. Since the artificial nests were installed next to natural nest sites at both locations, some unobserved factors86 may have influenced the presence of predators.
The presence of inhabited red fox dens close to turtle nesting sites negatively impacted both nest and hatchling survival, as expected (fourth prediction), considering that medium-sized carnivores, including mainly the red fox, were dominant in predation (fifth prediction). Lactating red fox females stay in the breeding den with their pups for the first few weeks, and then the fox pups remain nearby for 8–15 weeks56,77. Therefore, the risk of predation by foxes is likely higher around breeding dens56. In studies of the predation of nests of the green sea turtle (Chelonia mydas) and loggerhead sea turtle (Caretta caretta), the distribution of red fox den entrances and nesting sites has been shown to follow a similar pattern, with clustering of red fox den entrances inversely related to the distance to predated sea turtle nest87.
The role of corvids was recorded only in the predation of hatchling turtles, as corvids cannot generally detect olfactory signals of prey32 with some exceptions30. Predation by corvids remained subordinate in our study, although anthropogenic closure can facilitate the fecundity of this bird group, leading to the hyperpredation on the offsprings of particular populations of sea turtles, freshwater turtles and tortoises worldwide29,88,89,90. A good example is the Mediterranean spur-thighed tortoise (Testudo graeca), where the extent of common raven predation in the < 75 mm CL (carapax length) size range resulted in 100% of total mortality in unvegetated areas, whereas in vegetated areas raven predation was only 12–27% of total mortality. As a consequence, population structure, particularly the proportion of larger size classes (81–100 mm CL), decreased from 41% (covered) to 16% (uncovered)91. Similar results were obtained for Agassiz’s desert tortoises (Gopherus agassizii) in North America where also the common raven also caused hyperpredation on juvenile tortoises. The predation rate on juveniles below < 140 mm CL may have been as high as 42.1–59.5% of total mortality92. This may lead to a significant reduction in the number of juveniles under 8 years of age, which, based on modelling, may no longer be able to produce stable populations in the presence of a higher adult mortality (e.g. disease onset)88. In our study areas, the lower rate of predation loss caused by corvids may be related to the higher and dense vegetation cover of the marshland, in which hatchlings can hide effectively. Presumably, corvids did not specialize in the predation on hatchlings under these circumstances.
In the case of the two tortoise species29,88,89,90,91 hyperpredation occurs as a result of the good learning ability and specialization of the corvids on easily available alternative food. The significant effect is due to predation by breeding pairs feeding on the novel food. In our study, as individuals are not observed by camera traps, we can only infer the species group based on the traces on the plasticine models. These do not allow us to determine whether single individuals or couples caused predation. We did not find carapace remains in the vicinity of the nests presented in the tortoise studies, and the level of predation we observed was relatively low, compared to other studies mentioned above. This suggests that, at this stage, we are faced with sporadic, individual predation. However, the specialization for the European pond turtle as an alternative food source has also been described in other species in the surrounding areas. Among avian species, significant predation on the species and the presence of carapace remains around the nest, as described in tortoise cases, were observed in the case of the white-tailed sea-eagle (Haliaeetus albicilla) during the breeding season93. In non-bird species, hyperpredation on European pond turtle was found in the case of the Eurasian otter (Lutra lutra), which occurred during turtle hibernation18. In this case, similarly to corvids, the learned behaviour was later detected from otter fecal samples18 and observed to a lesser extent in offspring, in the surrounding wetlands (T. Molnár, unpublished data). In this context, corvid predation, even observed at low rates, merits further investigation and monitoring in the area. Although wild boar was present as a predator on nests and hatchlings, its importance was more pronounced on hatchlings in areas with smaller turtle populations. Our results thus basically support the fifth prediction, but draw attention to the necessity for a more subtle approach in predator management. The species detected in this study have also been shown to be dominant predators in other studies on European pond turtles26,64,94.
The difference in prey type (egg, hatching) may be because newly emerged turtles on the ground surface, may encounter predators with different activity, foraging tactics, and senses25,92. In addition to mammals with superior olfaction, corvids, a generalist family of passerines with visual perception, have also been reported as secondary predators, rarely primary, for both nests and hatchling turtles, and their predation on turtles is reported worldwide29,95,96. The published predation losses caused by corvids are more considerable in tortoises29,91,92, comparing to sea or freshwater turtles, when the birds only have a short time frame from the detection of a hatchling to the point of submersion. Red fox and badgers may regularly visit turtle nesting sites used annually, presumably on a targeted foraging basis97. Young foxes that join the mother in June, as shown in our camera trap records, and they learn the technique of getting to the turtle eggs. By September, the young foxes become mostly independent and disperse98,99, while the young corvids follow their parents and can learn the technique of killing hatchling turtles during the turtle hatching period. The special technique learned can be used in other areas in the following years, so it may be of longer-term importance21,36.
Based on the camera trap recordings, predation events in turtle nests occurred mostly at night, in the hours after dusk and before dawn, according to the known activity times of predators100. This may be related to the fact that egg-laying usually starts after 6 pm (between 4 and 5 pm in cooler weather) and usually ends by midnight65,101,102. Foxes follow turtle egg-laying activity in the late afternoon and early evening, then search for nests using their scent in the dark. In contrast, for hatchlings, camera recordings were taken in the late evening and early morning (twilight and dawn). In autumn, in the absence of intense scent trails, predatory mammals may prefer to search for hatchling turtles visually4,23 as corvids25.
A potential limitation of our study is that the relatively short, three-months study period may have influenced the results. For example, although the year of the study was wet, considerable predation was also observed in the A1 area during the previous year, which had normal climatic conditions97. Thus, while logistically challenging, expanding the study to include a greater number of areas with varied sizes and extending the research over multiple years would more accurately capture the dynamics of predation across space and time. The process of teaching by predator parents and specialization cannot be measured. Only by monitoring predation events of living European pond turtles could the impact on population structure be determined. Additionally, more nesting sites need to be located and monitored, along with the development of nest protection measures41,103,104,105. The trampling loss of hatchling turtles due to wild ungulates has been shown in some cases. Although such losses are not direct predation, they nonetheless contribute to turtle mortality106. As indicated by basic data and analyses from the National Game Management Database107, the population densities of ungulate species (mainly red deer and wild boar) are high in Hungary.
Our findings support the notion that strong territorial protection does not necessarily ensure effective conservation of turtle populations19. The high predation losses observed may also have been partly influenced by the prohibition of generalist predator population control in the strictly protected A1 area while hunting was allowed in the other two areas. However, predator control or removal17,31,41,103,105 is not always the most effective management technique19,104, especially without a detailed knowledge of predation rates and the specific predators involved. For effective conservation management, it is important not only to identify turtle nesting sites, but also to monitor predator dens. Considering the nest fidelity of female turtles, active management strategies, such as providing alternative nesting sites away from linear structures or implementing nest protection techniques could help mitigate predation losses61,103,104,107,108. We also stress that maintaining transitional habitat features of marshes110, which are among the globally111 and locally112,113 undergoing drastic degradation, should receive greater attention for the management of freshwater turtle populations. Concurrently, the maintenance, creation, or active management of larger dryland patches, may aid in predator control or protection measures, such as nest guarding103,108. Removing non-native tree species from elevated areas of small islands in the marshy habitats would also be advantageous. These implementations could improve the survival of European pond turtle nests from both hydrological and predation perspectives.
In conclusion, the results of this study can be directly applied to the planning of conservation management strategies aimed at the active protection and long-term preservation of populations at risk from predation. In addition to urgently monitoring European pond turtle survival and exploring additional environmental variables affecting their populations, we recommend long-term studies on predation and impact assessment of foxes, other mesocarnivores, and corvids during the turtle nesting and hatching season.
Materials and methods
Study area and sampling sites
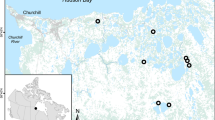
We conducted our investigation in the Pannonian region, in marshes located along Lake Balaton (Hungary), the largest lake in Central Europe (596 km2, 104 m a.s.l). We designated three marshes (wetlands) southwest and south of Lake Balaton, namely A1 – Kis-Balaton, A2 – Nagyberek (Fehérvíz), A3 – Ordacsehi-berek, and three sampling sites per area (Fig. 4). The marshes are part of the Natura 2000 network (Table SI2), all three areas are important bird habitats (nesting, wintering and migration area), A1 and A2 are Ramsar sites as well. These marshes have long been known as important European pond turtle habitats97.
The first study area (A1; center: 46°38’ N, 17°12’ E, 54 km2) is within the Kis-Balaton Phase II (Fig. 4), which is a strictly protected marshland97. The dominant species of the plant communities in the marsh are common reed (Phragmites australis), common bulrush (Typha latifolia), lesser pond-sedge (Carex acutiformis), at higher spatial levels, patches of groves are formed by willows (Salix alba and S. cinerea), poplars (Populus sp.), and common alder (Alnus glutinosa) (Database of the Balaton Uplands National Park Directorate, Table SI2). In some places, near the embankments, there are sand hills covered with grasslands that have been used for a long time by turtles for laying their eggs97. Human activity within the study area is very low; mostly limited to conservation management and water protection.
The second study area (A2; center: 46°38’ N, 17°30’ E, 15.50 km2) is the Fehérvíz Nature Reserve, which is part of the Nagyberek marshland area. The area is dominated by mesotrophic grasslands marshes and fens (Table SI2). Reeds and sedges are the predominant vegetation in the deepest marsh areas; the higher, dry levels are covered by grove forests and shrubs113,114. The grasslands are grazed by beef cattle.
The third study area (A3; center 46°45’ N, 17°37’ E, 7.49 km2) is the Ordacsehi-berek. Most of the area is covered by marsh and bog vegetation (Table SI1), reeds, sedges and euhydrophyte vegetation of naturally eutrophic and mesotrophic still waters are the predominant vegetation in the wetter areas, marshes and fens have formed on the higher ground levels113,114. The grasslands are unmanaged.
Coverage of the main habitat types differ significantly in the three areas (Table SI2). Human impacts (game management, crop cultivation, animal husbandry, level of protection) also differ (Table SI2). The Balaton-Uplands National Park Directorate (BUNPD) is the nature conservation manager of the areas.
The test sites were selected from the BUNPD database at known locations where nest predation was known in previous years97 (BUNPD rangers’ and own observations). These were permanently dry patches along artificial embankments covered with short grass (Site 1–9, Fig. 5) that were slightly higher than the surrounding marsh habitats and were used by turtles for nesting. The parts of the embankments suitable for egg laying were the sides of sown grass with loose soil (S2, S4, S5) and sandhills (S1, S3, S6-S9) covered with natural grass (mainly tall fescue Festuca arundinacea). These linear infrastructures offer passage between habitat patches at a higher spatial level for terrestrial carnivores. There were shallow open waters, marsh sedge and reed habitats in the surroundings of the study sites. The habitats were grouped into five main types (Fig. 5).
The location and habitat type composition of the study sites (S1-S9) in three wetlands (A1-A3) along Lake Balaton. Areas: A1 – Kis-Balaton (Phase II), A2 – Nagyberek (Fehérvíz), A3 – Ordacsehi. In the investigated area, the following habitat types were distinguished: Grasslands (mesic grasslands, dry and semi-dry grasslands, stands of invasive forbs). Marshes (calcareous fens with Cladium mariscus, marshes with Solidago, eutrophic and mesotrophic reed and bulrush beds, wet and mesic pioneer scrub, non-tussock tall-sedge beds, Glyceria, Sparganium and Schoenoplectus beds, fine scale mosaic or zonation of marsh communities, willow carrs). Euhydrophyte vegetation of naturaly eutrophic and mesotrophic still waters. Forests and woody habitats (scattered native trees or narrow tree lines, pioneer softwood forests, hardwood forests, Populus × euramericana plantations, spontaneous stands of non-native tree species). Artificial structures (roads, dirt roads). Source of maps: satellite image from Google Maps and habitat maps from Balaton Uplands National Park Database (Table SI2).
The study areas are located in the continental climatic region, but there are some Mediterranean features. The study conducted in 2018 took place during a warmer (annual mean temperature 11.9 °C) and wetter year (841 mm annual precipitation amount; Hungarian Meteorological Service), compared to the long-term average of the microregion (10.1–10.4 °C and 620–670 mm, respectively114).
European pond turtle and its potential predators
The estimated turtle population was a minimum of 1,000 individuals in A1, minimum of 100 individuals in A2 and in A3 (Table SI2). In the previous year (2017), more than 400 nests were destroyed in A197, 38–104 nests in 2014–2016 in A2 and a growing number of depredated turtle nests were seen in A3 (BUNPD database).
Frequent potential predators of European pond turtles on the wetlands were the red fox, European badger and wild boar (Table SI2). We did not find inhabited (breeding or active115) fox dens around test sites on A2 and A3, just on A1. Other less common potential nest predators in all three areas were pine marten (Martes martes), European polecat (Mustela putorius), Eurasian otter, stoat (Mustela erminea), golden jackal (Canis aureus) and European wildcat (Felis silvestris). Common predatory birds in the area were hooded crow (Corvus cornix) and Eurasian jay (Garrulus glandarius), while Eurasian magpie (Pica pica), common raven (Corvus corax) and white-tailed sea-eagle were rare species. Corvid nests were scattered throughout the areas, crows mainly in forest patches and jays in trees along embankments. As white-tailed eagles have a fairly distinctive beak mark compared to the corvid species, and we did not record its predation in our study, hereafter we will refer to predation by corvids.
Experiment with artificial turtle nests
When designing our nest predation study, we relied on the methods previously used on freshwater turtles3,47,97. During the European pond turtle’s main nesting period, we performed our experiments with artificial turtle nests one month apart (first period: May 15–23, 2018; second period: June 11–19, 2018). Following the methods described in our previous study97 we selected three egg-laying sites in all three study areas where at least 22 artificial nests can be created simultaneously without significant disturbance. On A1, the test locations were the same as the previous year97, and the locations of the artificial nests in May and June were the same in all three areas.
Before the beginning of the study, we formed artificial eggs similar to turtle eggs by using natural plasticine for each of them (Fig. SI4), the role of which was to preserve the predator’s tooth or beak mark. We fixed the plasticine eggs using string and then sprayed them with liquid rubber spray (PlastiDip®) to make them odorless. In all three study areas and sites, we dug 22 artificial nests (10–13 cm deep, 5 cm in diameter, in total n = 396 nests) without significant disturbance in May and June. At each sampling site, we created artificial nests along two parallel lines at 2 m apart, about 1 m from each other along that line. We placed a quail egg, which came from breeders and was used as bait for the predators, at the bottom of the hole. Next to this we placed one plasticine egg and clean and dry turtle eggshells collected from previously looted turtle nests nearby. Then we covered the artificial nest with soil, and finally, we sprinkled the surface of each nest with turtle urine diluted with water. We used sterile rubber gloves during the work to minimize human odors.
Experiment with turtle hatchling dummies
We used plastic turtle dummies to estimate the potential survival of the young turtles that hatched from the nest and left the nest. The test locations were identical to the locations of the artificial nest tests. The turtle hatchling dummy test (September 11–19) was timed for the turtle hatching period experienced in the A1. The turtle dummies were made from non-toxic plastic described above, based on the dimensions and photos of turtles hatched from eggs26,65,116. We tied strong twine to a button with a diameter of 1 cm inside the turtle dummies. Then, we painted the turtle dummies olive green in several layers with the previously described odorless spray. The turtle dummies, 2–2 per nest, were placed close to each other (at a distance of 10–15 cm), next to the artificial nests (Fig. SI4), and fixed into the ground with a nail. We placed a total of 396 turtle dummies at the 198 nests (3 areas × 3 locations × 22 nests × 2 turtles).
In all cases, we checked the nests and turtle dummy’s predation on the 1st, 2nd, 3rd, and 8th days after placement in the early morning hours. In the tests, we initially used frequent (daily) monitoring because turtle eggs are most affected by predation in the days after nesting and young specimens after hatching47,89,117,118.
Predator identification
The predators were primarily identified based on the tooth and beak marks (Fig. SI4) left on the plasticine eggs and plasticine turtle dummies48,49,119. Furthermore, footprints left near the nests and fresh predator droppings23,44,97 were also used for identification. We also placed camera traps at the study sites to identify predators (species, age group, number of individuals) and collect additional information, e.g., the time of predation45,46. The camera traps (types: Minox DTC 450, Bushnell Trophy Cam and Dörr Snap Shot Mini) were used on the first three days of the tests, hidden according to the terrain120. These IR cameras were set up to record three images per trigger, without delay between triggers, medium sensitivity, 8–16 MP photo resolution and 24 h target capture time. In the nest predation test, there were six camera traps on A1, one on A2, and two on A3 (a total of nine); in the plasticine turtle dummies test, there were six on A1, two on A2 and three on A3 (a total of 11 camera traps) were used.
Artificial turtle nests were only considered predated if the nest cavity was opened, the quail egg or plasticine egg was damaged or disappeared from the nest. Plasticine turtle dummies were considered predated if at least one of the two turtle dummies in the same location was damaged or missing. In cases when we could not exactly determine the carnivore that robbed the turtle’s nest or damaged the turtle dummies, we classified it in an “undetermined medium-sized carnivore (UMSC) category. In the analyses, losses caused by fox, badger and UMSC were treated together “medium-sized carnivore” (MSC).
During inspections, we spent as short a time as possible at the sites and collected all materials from the depredated nests and their surroundings. Then, the artificially placed materials were fully collected at the last inspection.
The research permit for the carnivore monitoring program carried out on Kis-Balaton was granted by the competent authorities of Zala and Somogy counties (permit numbers: ZA/KTF/01115-7/2017 and SO-04Z/TO/1356-6/2017), and the other two freely accessible areas based on consultation with the Balaton Uplands National Park Directorate.
Data analysis
We used Mayfield’s121 method to calculate the exposure days of nests or turtles for the survival days of artificial turtle nests (eggs) and hatchling turtle dummies. For this, we used the exposure time of artificial nests and turtle dummies (the number of cumulative days) and the number of known predation events.
The environmental variable used in our analysis was also the presence (yes or no) of inhabited red fox dens within 1 km from the test site. We measured the distance from the center of the test site to the water edge (in meters), considering that the distance from the water edge has a strong effect on nest predation3,54.
We computed generalized linear mixed models (GLMM) with the glmmTMB function of package glmmTMB version 1.1.8122 in R version 4.2.2123 in three sets, the first one was used on the database of artificial turtle nests, the second was on turtle hatchlings (dummies) and the third was run on the whole database. We made binomial distribution models with logit link function, using the probability of survival as response variables derived from the exposure time of nests, hatchlings or both, by dividing the value of each nest by the number of experimental days (eight days in particular). We used the following fixed effects in all three models: the presence of inhabited fox dens in a 1 km buffer as a factor, the log-transformed value of the cover of marsh and the distance from water in the formula of log (1 + x). In the artificial nest model, we also used month as a factor and for the whole database, we also used prey type (egg or hatchling) as a factor. We included site identity (S1-S9) as a random factor throughout our models.
We applied general log-linear analysis first on occurrence data of artificial turtle nests or turtle hatchlings to test for predation differences (predation has occurred or not) within areas (A1-A3), and months (May, June, September). The unit of analysis was the number of intact nests or turtles from each study area and month, and the response variable was the presence of intact or predated nests and hatchlings. The model was fitted using study area and month as categories (independent variables) and P < 0.05 was accepted. We used general log-linear analysis second on occurrence data of predator-destroyed artificial nests or turtle hatchlings (dummies) to test differences in predation (predator) composition within areas (A1-A3) and “prey type” (turtle eggs or hatchlings). The unit of analysis was the number of turtle nests or turtle hatchlings destroyed by a predator from each study area, and the response variable was the presence or absence of predation by the given predator. We separated the following four predator categories: medium-sized carnivores (MSC), corvids, brown rats, and wild boar. We excluded trampling caused by wild ungulates (mean ± SE, 0.7 ± 0.4%) and unknown causes of turtle loss (3.4 ± 1.1%) from this analysis. The model was fitted using study area and prey type as categories (independent variables). Owing to the large number of comparisons (four predator), we adjusted the level of significance to 0.0125 (P = 0.05/4) with a Bonferroni correction.
For the analysis of the habitat composition around the nine study sites we used the surface of a circle with a radius of 200 m73,82 (12.56 ha) calculated from the centre of the test site based on European pond turtle radio telemetry tracking, on typical home range area sizes (12.5–22.3 ha)124,125 and egg-laying migration distances (10–800 m)102,124,126. We used the habitat map databases of the BUNPD (Table SI2) in Quantum GIS program v 3.16127 to determine the surface of the five main habitat types, namely, grasslands, marshes, euhydrophyte vegetation, forests (and woody habitats), and artificial structures (dirt roads) within the 200 m diameter areas (Fig. 5). Due to the autocorrelation of the surface area (ha) of the two most important habitat types (marsh and grassland, mean, 39.3% and 34.8%; Spearman correlation, rS = − 0.717, P = 0.030), we included the marshes with a larger surface as a variable in the model.
We compared the three types of predators (MSC, corvids and wild boar) with the intact artificial turtle nests or turtle hatchlings in terms of marsh surface area and distance from water by using the Kruskal-Wallis test for overall differences with Dunn’s post hoc test with Bonferroni correction. P values were considered significant under 0.05. These particular analyses were computed in Past v.2.17128.
Data availability
Data are included in the paper or in the online version as supplementary information. For any other reasonable request, contact the corresponding author.
References
Gibbons, J. W. et al. The global decline of reptiles, déjà vu amphibians. BioScience 50, 653–666 (2000).
Stanford, C. B. et al. Turtles and tortoises are in trouble. Curr. Biol. 30, R721–R735 (2020).
Marchand, M. N., Litvaitis, J. A., Maier, T. J. & DeGraaf, R. M. Use of artificial nests to investigate predation on freshwater turtle nests. Wildl. Soc. Bull. 30, 1092–1098 (2002).
Geller, G. A. & Parker, S. L. What are the primary cues used by mammalian predators to locate freshwater turtle nests? A critical review of the evidence. Front. Ecol. Evol. 9, 784786 (2022).
Kenneth, D. C. Effects of habitat fragmentation on a stream-dwelling species, the flattened musk turtle Sternotherus depressus. Biol. Conserv. 54, 33–45 (1990).
Kingsford, R. T., Basset, A. & Jackson, L. Wetlands: conservation’s poor cousins. Aquat. Conserv. Mar. Freshw. Ecos. 26, 892–916 (2016).
Cadi, A. & Joly, P. Competition for basking places between the endangered European pond turtle (Emys orbicularis galloitalica) and the introduced red-eared slider (Trachemys scripta elegans). Can. J. Zool. 81, 1392–1398 (2003).
Cerasoli, F., Iannella, M. & Biondi, M. Between the hammer and the anvil: how the combined effect of global warming and the non-native common slider could threaten the European pond turtle. Manag. Biol. Invasions 10, 428–448 (2019).
Semlitsch, R. D. & Bodie, J. R. Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv. Biol. 17, 1219–1228 (2003).
Bergeron, C. M., Husak, J. F., Unrine, J. M., Romanek, C. S. & Hopkins, W. A. Infuence of feeding ecology on blood mercury concentrations in four species of turtles. Environ. Toxicol. Chem. 26, 1733–1741 (2007).
Verneau, O. et al. Invasive species threat: parasite phylogenetics reveals patterns and processes of host-switching between non-native and native captive freshwater turtles. Parasitology 138, 1778–1792 (2011).
Mullin, D. I. et al. Predation and disease limit population recovery following 15 years of headstarting an endangered freshwater turtle. Biol. Conserv. 245, 108496 (2020).
Colteaux, B. C. & Johnson, D. M. Commercial harvest and export of snapping turtles (Chelydra serpentina) in the United States: trends and the efcacy of size limits at reducing harvest. J. Nat. Conserv. 35, 13–19 (2017).
Schwanz, L. E. & Janzen, F. J. Climate change and temperature-dependent sex determination: can individual plasticity in nesting phenology prevent extreme sex ratios?. Physiol. Biochem. Zool. 81, 826–834 (2008).
Zuffi, M. A. L. & Rovina, L. Habitat characteristics of nesting areas and of predated nests in a Mediterranean population of the European pond turtle. Emys orbicularis galloitalica. Acta Herpetol. 1, 37–51 (2006).
Van Dyke, J. U. et al. Conservation implications of turtle declines in Australia’s Murray River system. Sci. Rep. 9, 1998 (2019).
Spencer, R. J. & Thompson, M. B. Experimental analysis of the impact of foxes on freshwater turtle populations. Conserv. Biol. 19, 845–854 (2005).
Lanszki, J., Molnár, M. & Molnár, T. Factors affecting the predation of otter (Lutra lutra) on European pond turtle (Emys orbicularis). J. Zool. 270, 219–226 (2006).
Browne, C. L. & Hecnar, S. J. Species loss and shifting population structure of freshwater turtles despite habitat protection. Biol. Conserv. 138, 421–429 (2007).
Segura, A., Rodriguez-Caro, R. C., Graciá, E. & Acevedo, P. Differences in reproductive success in young and old females of a long-lived species. Animals 11, 467 (2021).
Burke, R. L., Schneider, C. M. & Dolinger, M. T. Cues used by raccoons to find turtle nests: Effects of flags, human scent, and diamond-backed terrapin sign. J. Herpetol. 39, 312–315 (2005).
Schindler, M. et al. Nest protection during a long-term conservation project as a tool to increase the autochthonous population of Emys orbicularis (L., 1758) in Austria. Acta Zool. Bulg. 69 Suppl. S10(10), 147–154 (2017).
Brown, L. & Macdonald, D. W. Predation on green turtle Chelonia mydas nests by wild canids at Akyatan Beach. Turkey. Biol. Conserv. 71, 55–60 (1995).
Oddie, M. A., Coombes, S. M. & Davy, C. M. Investigation of cues used by predators to detect snapping turtle (Chelydra serpentina) nests. Can. J. Zool. 93, 299–305 (2015).
Rangen, S. A., Clarc, R. G. & Hobson, K. A. Visual and olfactory attributes of artificial nests. Auk 117, 136–146 (2000).
Zuffi, M. A. L. Conservation biology of the European pond turtle, Emys orbicularis, in Italy: review of systematics and reproductive ecology patterns (Reptilia, Emydidae). Ital. J. Zool. 71, 103–105 (2004).
Rollinson, N. & Brooks, R. J. Marking nests increases the frequency of nest depredation in a northern population of Painted Turtles (Chrysemys picta). J. Herpetol. 41, 174–176 (2007).
Drobenkov, S. M. Current state, anthropogenic threats and conservation of the European pond turtle (Emys orbiclularis) in Belarus. Acta Biologica Universitatis Daugavpiliensis 14, 29–36 (2014).
Moldowan, P. D. Hyperpredation of freshwater turtles and tortoises by subsidized corvids. Herpetol. Monog. 37, 70–94 (2023).
Harriman, A. E. & Berger, R. H. Olfactory acuity in the common raven (Corvus corax). Physiol. Behav. 36, 257–262 (1986).
Spencer, R. J. Experimentally testing nest site selection: Fitness trade-offs and predation risk in turtles. Ecology 83, 2136–2144 (2002).
Strickland, J., Colbert, P. & Janzen, F. J. Experimental analysis of effects of markers and habitat structure on predation of turtle nests. J. Herpetol. 44, 467–470 (2010).
Canessa, S. et al. Challenges of monitoring reintroduction outcomes: Insights from the conservation breeding program of an endangered turtle in Italy. Biol. Conserv. 204, 128 (2016).
Caut, S., Angulo, E. & Courchamp, F. Dietary shift of an invasive predator: Rats, seabirds and sea turtles. J. Appl. Ecol. 45, 428–437 (2008).
Byer, N. W., Reid, B. N., Seigel, R. A. & Peery, M. Z. Applying lessons from avian ecology to herpetological research: Techniques for analyzing nest survival due to predation. Herpetol. Conserv. Biol. 13, 517–532 (2018).
Segura, A. & Acevedo, P. Influence of habitat and food resource availability on Common raven nest site selection and reproductive success in Mediterranean forests. Birds 2, 302–313 (2021).
Congdon, J. D., Dunham, A. E. & van Loben Sels, R. C. Delayed sexual maturity and demographics of Blanding’s turtles (Emydea blandingii): Implications for conservation and management of long-lived organisms. Conserv. Biol. 7, 826–833 (1993).
Keevil, M. G., Armstrong, D. P., Brooks, R. J. & Litzgus, J. D. A model of seasonal variation in somatic growth rates applied to two temperate turtle species. Ecol. Modell. 443, 109454 (2021).
Spencer, R. J. How much long-term data are required to effectively manage a wide-spread freshwater turtle?. Aust. Zool. 39, 568–575 (2018).
Tetzlaff, S. J., Estrada, A., DeGregorio, B. A. & Sperry, J. H. Identification of factors affecting predation risk for juvenile turtles using 3D printed models. Animals 10, 275 (2020).
Campbell, M. A. et al. The efficacy of protecting turtle nests as a conservation strategy to reverse population decline. Biol. Conserv. 251, 108769 (2020).
Altobelli, J. T., Laarman, P. B. & Moor, J. A. First year survival of hatchling Eastern box turtles (Terrapene carolina carolina) at their northern range limit in Michigan’s Lower Peninsula. J. Herpetol. 55, 432–441 (2021).
Escoriza, D., Franch, M., Ramos, S., Sunyer-Sala, P. & Boix, D. Demographics and survivorship in the European pond turtle (Emys orbicularis): A 31–Year Study. Herpetol. Conserv. Biol. 15, 41–48 (2020).
Macdonald, D. W., Brown, L., Yerli, S. & Canbolat, A. F. Behavior of red foxes, Vulpes vulpes, caching eggs of loggerhead turtles, Caretta caretta. J. Mammal. 75, 985–988 (1994).
Maier, T. J., Marchand, M. N., DeGraaf, R. M. & Litvaitis, J. A. A subterranean camera trigger for identifying predators excavating turtle nests. Herpetol. Rev. 33, 284–287 (2002).
Dawson, S. J., Crawford, H. M., Huston, R. M., Adams, P. J. & Fleming, P. A. How to catch red foxes red handed: Identifying predation of freshwater turtles and nests. Wildl. Res. 43, 615–622 (2016).
Hamilton, A. M., Freedman, A. H. & Franz, R. Effects of deer feeders, habitat and sensory cues on predation rates on artificial turtle nests. Am. Midl. Nat. 147, 123–134 (2002).
Bateman, P. W., Fleming, P. A. & Wolfe, A. K. A different kind of ecological modelling: The use of clay model organisms to explore predator–prey interactions in vertebrates. J. Zool. 301, 251–262 (2017).
Major, R. E. & Kendal, C. E. The contribution of artificial nest experiments to understanding avian reproductive success: A review of methods and conclusions. Ibis 138, 298–307 (1996).
King, D. I., DeGraaf, R. M., Griffin, C. R. & Maier, T. J. Do predation rates on artificial nests accurately reflect predation rates on natural bird nests?. J. Field Ornithol. 70, 257–262 (1999).
Sinclair, A. R. E. et al. Predicting effects of predation on conservation of endangered prey. Conserv. Biol. 12, 564–575 (1998).
Ficetola, G. F. et al. The importance of aquatic and terrestrial habitat for the European pond turtle (Emys orbicularis): Implications for conservation planning and management. Can. J. Zool. 82, 1704–1712 (2004).
Dawson, S. J., Adams, P., Huston, R. M. & Fleming, P. A. Environmental factors influence nest excavation by foxes. J. Zool. 294, 104–113 (2014).
Kolbe, J. J. & Janzen, F. J. Spatial and temporal dynamics of turtle nest predation: Edge effects. Oikos 99, 538–544 (2002).
Marchand, M. N. & Litvaitis, J. A. Effects of landscape composition, habitat features, and nest distribution on predation rates of simulated turtle nests. Biol. Conserv. 117, 243–251 (2004).
Carter, A., Luck, G. W. & Wilson, B. P. Ecology of the red fox (Vulpes vulpes) in an agricultural landscape. 1. Den-site selection. Aust. Mammal. 34, 145–154 (2012).
Jones, M. T. & Sievert, P. R. Elevated mortality of hatchling Blanding’s turtles (Emydoidea blandingii) in Residential Landscapes. Herpetol. Conserv. Bio. 7, 89–94 (2012).
Refsnider, J. M., Reedy, A. M., Warner, D. A. & Janzen, F. J. Do trade-offs between predation pressures on females versus nests drive nest-site choice in painted turtles?. Biol. J. Linn. Soc. 116, 847–855 (2015).
Burger, J. & Garber, S. D. Risk assessment, life history strategies, and turtles: Could declines be prevented or predicted?. J. Toxicol. Environ. Health. 46, 483–500 (1995).
Fordham, D. A., Georges, A. & Brook, B. W. Indigenous harvest, exotic pig predation and local persistence of a long-lived vertebrate: Managing a tropical freshwater turtle for sustainability and conservation. J. Appl. Ecol. 45, 52–62 (2008).
Spencer, R. J., Van Dyke, J. U. & Thompson, M. B. Critically evaluating best management practices for preventing freshwater turtle extinctions. Conserv. Biol. 31, 1340–1349 (2017).
Fritz, U. Die Europäische sumpfschildkröte (Emys orbicularis) (Laurenti Verlag, 2003).
Cox, N. A. & Temple, H. J. European Red List of Reptiles (Office for Official Publications of the European Communities, 2009).
Fritz, U. & Chiari, Y. Conservation actions for European pond turtles—A summary of current efforts in distinct European countries. Herpetol. Notes 6, 105 (2013).
Mitrus, S. & Zemanek, M. Distribution and biology of Emys orbicularis (L) in Poland. Stapfia 69, 107–118 (2000).
Puky, M., Gémesi, D. & Schád, P. Distribution of Emys orbicularis in Hungary with notes on related conservational and environmental education activities. Biologia 59, 55–60 (2004).
Horváth, E., Kaňuch, P. & Uhrin, M. Predation on nests of the European pond turtle (Emys orbicularis): Remarks from failed field experiments. Herpetol. Notes 14, 1067–1072 (2021).
MacArthur, R. H. & Pianka, E. R. On optimal use of a patchy environment. Am. Nat. 100, 603–609 (1966).
Pyke, G. H., Pulliam, H. R. & Charnov, E. L. Optimal foraging: A selective review of theory and tests. Q. Rev. Biol. 52, 137–154 (1977).
Stephens, D. W. & Krebs, J. R. Foraging Theory. Monographs in Behavior and Ecology 1st ed (Princeton University Press, 1986).
Booth, D. T. Composition and energy density of eggs from two species of freshwater turtle with twofold ranges in egg size. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 134, 129–137 (2003).
Tinbergen, L. The natural control of insects in pinewoods. Arch. Neerl. Zool. 13, 265–343 (1960).
Freedberg, S., Ewert, M. A., Ridenhour, B. J., Neiman, M. & Nelson, C. E. Nesting fidelity and molecular evidence for natal homing in the freshwater turtle, Graptemys kohnii. Proc. R. Soc. Lond. Ser. B Biol. Sci. 272, 1345–1350 (2005).
Riley, J. L. & Litzgus, J. D. Cues used by predators to detect freshwater turtle nests may persist late into incubation. Can. Field-Nat. 128, 179–188 (2014).
Bayne, E. M., Hobson, K. A. & Fargey, P. Predation on artificial nests in relation to forest type: Contrasting the use of quail and plasticine eggs. Ecography 20, 233–239 (1997).
Pike, D. A., Pizzatto, L., Pike, B. A. & Shine, R. Estimating survival rates of uncatchable animals: The myth of high juvenile mortality in reptiles. Ecology 89, 607–611 (2008).
Otten, J. G. & Refsnider, J. M. Bigger is better: Age class-specific survival rates in long-lived turtles increase with size. J. Wildl. Manag. 88, e22544 (2024).
Golba, C., Glowacki, G. & King, R. B. Growth and survival of wild and head-started Blanding’s turtles (Emydoidea blandingii). Ichthyol. Herpetol. 110, 378–387 (2022).
Mitrus, S. Fidelity to nesting area of the European pond turtle, Emys orbicularis (Linnaeus, 1758). Belg. J. Zool. 136, 25–30 (2006).
Najbar, B. & Szuszkiewicz, E. Nest-site fidelity of the European pond turtle Emys orbicularis (Linnaeus, 1758) (Testudines: Emydidae) in western Poland. Acta Zool. Cracov. 50A, 1–8 (2007).
Purger, J. J. & Molnár, T. G. An unexpected recapture of European pond turtle Emys orbicularis (Linnaeus, 1758) in the Barcs juniper woodland (Hungary). Nat. Somogy. 39, 5–10 (2022).
Kiss, I., Erdélyi, G. & Szabó, B. Nest site selection and fidelity of European pond turtle (Emys orbicularis) population of Babat Valley (Gödöllő, Hungary). Front Zool. 21, 20 (2024).
Fay, R. et al. Direct and indirect estimates of dispersal support strong juvenile philopatry and male-biased dispersal in a freshwater turtle species (Emys orbicularis). Freshw. Biol. 68, 2042–2053 (2023).
Ficetola, G. F. & Bernardi, F. Is the European “pond” turtle Emys orbicularis strictly aquatic and carnivorous?. Amphibia-Reptilia 27, 445–447 (2006).
Kokko, H. & Sutherland, W. J. Ecological traps in changing environments: Ecological and evolutionary consequences of a behaviourally mediated Allee effect. Evol. Ecol. Res. 3, 603–610 (2001).
Congdon, J. D., Tinkle, D. W., Breitenbach, G. L. & van Loben Sels, R. C. Nesting ecology and hatching success in the turtle Emydea blandingi. Herpetologica 39, 417–429 (1983).
Halls, J. N., Hill, J. M., Urbanek, R. E. & Sutton, H. Distribution pattern of red fox (Vulpes vulpes) dens and spatial relationships with sea turtle nests, recreation, and environmental characteristics. ISPRS Int. J. Geo-Inf. 7, 247 (2018).
Boarman, W. I. Managing a subsidized predator population: Reducing common raven predation on desert tortoises. Environ. Manag. 32, 205–217 (2003).
Currylow, A. F. et al. A decision tool to identify population management strategies for common ravens and other avian predators. Hum.-Wildl. Interact. 15, 556–574 (2021).
Holcomb, K. L., Coates, P. S., Prochazka, B. G., Shields, T. A. & Boarman, W. I. A desert tortoise–common raven viable conflict threshold. Hum.-Wildl. Interact. 15, 405–421 (2021).
Segura, A., Jimenez, J. & Acevedo, P. Predation of young tortoises by ravens: The effect of habitat structure on tortoise detectability and abundance. Sci. Rep. 10, 1874 (2020).
Berry, K. H., Yee, J. L., Shields, T. A. & Stockton, L. The catastrophic decline of tortoises at a fenced natural area. Wildl. Monogr. 205, 1–53 (2020).
Probst, R. & Gaborik, A. Action Plan for the conservation of the White-tailed Sea Eagle (Haliaeetus albicilla) along the Danube. Convention on the Conservation of European Wildlife and Natural Habitats (Bern Convention). Nature and Environment 163 (2011).
Kovács, T., Anthony, B. P., Farkas, B. & Bera, M. Preliminary results of a long-term conservation project on Emys orbicularis in an urban lake of Budapest, Hungary. Turtle and Tortoise Newsletter 7, 14–17 (2004).
Ricklefs, R. E. Nest predation and the species diversity of birds. Trends Ecol. Evol. 4, 184–186 (1989).
Fincham, J. E. & Lambrechts, N. How many tortoises do a pair of pied crows Corvus alba need to kill to feed their chicks?. Ornithol. Observ. 5, 135–138 (2014).
Purger, J. J., Molnár, T. G., Lanszki, Z. & Lanszki, J. European pond turtle (Emys orbicularis) nest predation: A study with artificial nests. Biology 12, 342 (2023).
Lanszki, J., Bende, Z., Nagyapáti, N., Lanszki, Z. & Pongrácz, P. Optimal prey for red fox cubs—An example of dual optimizing foraging strategy in foxes from a dynamic wetland habitat. Ecol. Evol. 13, e10033 (2023).
Lloyd, H. G. The Red Fox (Batsford Ltd, 1980).
Bennie, J. J., Duffy, J. P., Inger, R. & Gaston, K. J. Biogeography of time partitioning in mammals. Proc. Natl. Acad. Sci. 111, 13727–13732 (2014).
Meeske, M. Nesting ecology of European pond turtle (Emys orbicularis) in South Lithuania. Acta Zool. Litu. 7, 143–150 (1997).
Novotný, M., Danko, S. & Havas, P. Activity cycle and reproductive characteristics of the European pond turtle (Emys orbicularis) in the Tajba National Nature Reserve, Slovakia. Biologia 59(Suppl. 14), 113–121 (2004).
Engeman, R. M. et al. Monitoring predators to optimize their management for marine turtle nest protection. Biol. Conserv. 113, 171–178 (2003).
O’Connor, J. M., Limpus, C. J., Hofmeister, K. M., Allen, B. L. & Burnett, S. E. Anti-predator meshing may provide greater protection for sea turtle nests than predator removal. PLoS ONE 12, e0171831 (2017).
Nordberg, E. J. et al. An evaluation of nest predator impacts and the efficacy of plastic meshing on marine turtle nests on the western Cape York Peninsula, Australia. Biol. Conserv. 238, 108201 (2019).
Zuffi, M. A. L. Conservation biology of the European pond turtle Emys orbicularis (L.) in Italy. Stapfia 69, 219–228 (2000).
Csányi, S., Márton, M., Bőti, S. & Schally, G. Hungarian Game Management Database: 2023/2024 hunting year (Hungarian University of Agriculture and Life Sciences, 2024).
Kiss, I., Erdélyi, G. & Szabó, B. Nesting activity and reproductive success of Emys orbicularis in Babat Valley (Gödöllő, Hungary). Herpetol. Conserv. Biol. 16, 624–638 (2021).
Streeting, L. M. et al. A shocking result—Electric fences protect western saw-shelled turtle (Myuchelys bellii) nests from depredation by foxes. Austral. Ecol. 48, 1571–1587 (2023).
Mitsch, W. J. & Gosselink, J. G. Wetlands (Van Nostrand Reinhold, 1986).
Jantke, K. & Schneider, U. A. Multiple-species conservation planning for European wetlands with different degrees of coordination. Biol. Conserv. 143, 1812–1821 (2010).
Németh, G., Lóczy, D. & Gyenizse, P. Long-term land use and landscape pattern changes in a marshland of Hungary. Sustainability 13, 12664 (2021).
Petrovszki, J., Szilassi, P. & Erős, T. Mass tourism generated urban land expansion in the catchment of Lake Balaton, Hungary—Analysis of long-term changes in characteristic socio-political periods. Land Use Policy 142, 107185 (2024).
Dövényi, Z. et al. Inventory of Microregions in Hungary (MTA Geographical Research Institute, 2010)
Sadlier, L. M. J., Webbon, C. C., Baker, P. & Harris, S. Methods of monitoring red foxes Vulpes vulpes and badgers Meles meles: Are field signs the answer?. Mammal Rev. 34, 75–98 (2004).
Farkas, B. The European pond turtle Emys orbicularis (L) in Hungary. Stapfia 149, 127–132 (2000).
Tinkle, D. W., Congdon, J. D. & Rosen, P. C. Nesting frequency and success: implications for the demography of painted turtles (Chrysemys picta). Ecology 62, 1426–1432 (1981).
Christens, E. & Roger, B. J. Nesting activity and hatching success of the painted turtle (Chrysemys picta marginata) in southwestern Quebec. Herpetologica 43, 55–65 (1987).
Lyver, P. O. B. Identifying mammalian predators from bite marks: a tool for focusing wildlife protection. Mammal Rev. 30, 31–44 (2000).
Caravaggi, A. et al. A review of camera trapping for conservation behaviour research. Remote Sens. Ecol. Conserv. 3, 109–122 (2017).
Mayfield, H. F. Suggestions for calculating nest success. Wilson Bull. 87, 456–466 (1975).
Brooks, M. E. et al. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 9, 378–400 (2017).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2022). https://www.R-project.org/).
Cadi, A., Nemoz, M., Thienpont, S. & Joly, P. Home range, movements, and habitat use of the European pond turtle (Emys orbicularis) in the Rhône-Alpes region, France. Biologia 59, 89–94 (2004).
Drechsler, R. M., Fernandez, L. M., Lassalle, M. & Monros, J. S. Movement patterns and home range sizes of translocated European pond turtles (Emys orbicularis, Linnaeus, 1758) in eastern Spain. Herpetol. Conserv. Biol. 13, 1–9 (2018).
Rovero, F. & Chelazzi, G. Nesting migrations in a population of the European pond turtle Emys orbicularis (L.)(Chelonia Emydidae) from central Italy. Ethol. Ecol. Evol. 8, 297–304 (1996).
QGIS Development Team. WWW Document. n.d. URL http://qgis.osgeo.org. Accessed 10.11.23 (2019).
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: Palaeontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 1–9 (2001).
Acknowledgements
Surveys were performed with the support of the Balaton Uplands National Park Directorate (Csopak, Hungary). Thanks to Grace Yoxon for proofreading the manuscript, Zoltán Botta-Dukát for advice in statistical analysis, Gabriella Lanszki-Széles for participating in the fieldwork. This work was supported by the Hungarian National Multidisciplinary Laboratory for Climate Change (grant no. RRF-2.3.1-21-2022-00014 to J.L. and E.T.).
Author information
Authors and Affiliations
Contributions
J.L. J.J.P. T.M. and T.E.: conceptualization of the study; J.L.: supervision; J.L. J.J.P., Z.L. and T.M.: field surveys; G.Ó. and J.L.: data analysis; all authors wrote the manuscript, contributed critically to the drafts and approved its publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lanszki, J., Molnár, T.G., Erős, T. et al. Testing how environmental variables affect the survival of freshwater turtle nests and hatchlings using artificial nests and dummy hatchlings. Sci Rep 14, 31713 (2024). https://doi.org/10.1038/s41598-024-82032-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82032-3