Abstract
Toluene sulfonic acid remimazolam is a novel benzodiazepine that differs from traditional benzodiazepines (BZDs) due to its rapid onset, swift metabolism, and lack of hepatic or renal metabolism, as well as its reduced effects on cardiac and cerebral functions. Despite its potential advantages, clinical experience with this agent remains limited. This study investigated the effect of remizolam on postoperative delirium in elderly patients undergoing painless bronchoscopy. Among elderly patients undergoing routine painless bronchoscopy examinations, 50 cases were included in the remimazolam group, while the other 50 cases were selected as the propofol group. The primary outcome measured was the incidence of delirium on the first, second, third, fifth, and seventh postoperative days. The secondary outcome was the occurrence of adverse events, including hypotension, hypoxia, physical activity, agitation during recovery, dizziness, and nausea/vomiting during the procedure.This study confirmed that no cases of delirium in the remimazolam group, which also demonstrated a significantly lower incidence of adverse events compared to the propofol group (20% vs. 50%, P < 0.05). The results indicated remimazolam provides effective sedation with minimal adverse reactions and is associated with a lack of complications such as postoperative delirium in elderly patients undergoing painless bronchoscopy.
Similar content being viewed by others
Postoperative delirium (POD) is a significant complication following surgery, characterized by disturbances in consciousness and cognitive function. POD typically occurs 2–5 days postoperatively, with hospital stays extending by 2–3 days and increase the one-month postoperative mortality rate increasing by 7–10%1. The prevalence of painless bronchoscopy among elderly patients is rising due to demographic changes and advancements in medical practices. Elderly patients are at an increased risk of anesthesia-related complications due to age-related declines in organ function, reduced compensatory capacity, and comorbidities. Therefore, the careful selection of anesthetic agents is crucial for this population.
Remimazolam, a novel benzodiazepine, is noted for its rapid onset, non-hepatic and non-renal metabolism, and minimal effects on cardiac and cerebral functions compared to traditional BZDs2. As such, it is particularly suitable for short procedures like painless bronchoscopy in elderly patients. Not only advanced age is a recognized risk factor for POD3, but the cognitive effects of BZDs remain contentious in the literature, with some studies suggesting a risk for cognitive dysfunction while others do not4,5. Notably, there is a lack of clinical studies examining the impact of remimazolam on POD in elderly patients undergoing bronchoscopy.
This study aims to investigate the effects of toluenesulfonic acid remimazolam on POD in this demographic. We hope to provide insights for the safer and more effective use of remimazolam in geriatric anesthesia.Therefore, we performed this retrospective study to investigate the effects of remimazolam on postoperative delirium in elderly patients undergoing painless bronchoscopy.
Materials and methods
Patients and research protocols
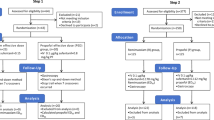
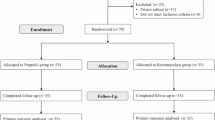
A retrospective analysis was performed on clinical records of patients who underwent painless bronchoscopy between October 2023 and March 2024, according to the inclusion and exclusion criteria, 50 cases were included in the remimazolam group, and 50 cases were selected as the propofol group during the same period. The primary focus was on the incidence of delirium on the first, second, third, fifth, and seventh postoperative days. Inclusion criteria included individuals aged 65 to 80 years, classified as American Society of Anesthesiologists (ASA) physical status II or III, with a body mass index (BMI) between 18.5 and 23.9 kg/m2. Exclusion criteria included prior diagnoses of dementia or preoperative conditions such as schizophrenia, epilepsy, or Parkinson’s disease; severe hypertension, diabetes, or organ failure; severe chronic obstructive pulmonary disease (COPD) with a forced expiratory volume in one second (FEV1) < 60% of the predicted value or requiring home oxygen therapy; history of allergic reactions to propofol, benzodiazepines, or other anesthetics; and substance misuse. Additionally, patients who experienced adverse events during the bronchoscopy that necessitated procedure discontinuation were excluded (Fig. 1). All research methods followed the Declaration of Helsinki. This study was approved by Zaozhuang City Hospital’s ethics committee (approval number: zzslyykyll2023083001). Due to the retrospective nature of the study, Zaozhuang City Hospital’s ethics committee waived the need of obtaining informed consent.
Prior to the procedure, patients were informed to refrained from using anesthetics. Upon arrival in the operating room, monitoring procedures were performed, including electrocardiogram, pulse oximetry, bispectral index (BIS, Mindray), and continuous non-invasive blood pressure monitoring. Intravenous access was established, and patients received supplemental oxygen at 3 L/min via a mask. Aftern the preparation of resuscitation equipment and medications, anesthesia induction began. The Remimazolam group received intravenous remimazolam tosilate at a dosage of 0.15 mg/kg, with an additional 2.5 mg administered after one minute if sedation was insufficient (MOAA/S score ≤ 3). The Propofol group received intravenous propofol at a dosage of 1–2 mg/kg, with all infusions completed within one minute. During anesthesia maintenance, the Remimazolam group received continuous infusions of remifentanil at 2–4 µg/kg.h and remimazolam at 0.2–0.3 mg/kg.h, while the Propofol group received remifentanil at 2–4 µg/kg.h and propofol at 2–3 mg/kg.h. Bronchoscopy commenced when the patient’s modified alertness/sedation score (MOAA/S) was ≤ 1. Following the procedure, medications were discontinued, and patients were transferred to the recovery room for routine oxygen administration and continuous vital signs monitoring, before being moved to the ward once stabilized and fully conscious.
Delirium was assessed using the Family Confusion Assessment Method (FAM-CAM) questionnaire (Fig. 2), with evaluations conducted through telephone interviews with caregivers. Secondary outcomes included the incidence of adverse events such as hypotension, hypoxemia, body movement, dizziness, and nausea/vomiting. Hypotension was defined as a decrease in blood pressure exceeding 20% from baseline, while hypoxemia was identified as blood oxygen saturation below 90% persisting for 10 s without resolution.
Statistical analysis
A post hoc power analysis was performed on the FAM-CAM questionnaire to determine the minimum number of subjects needed. Based on this study’s data, the inclusion of 100 patients with an allocation ratio of 50/50 would provide 80% power and a 2-sided level of 0.05 to detect the clinical relevant differences between the two groups. Data analysis was performed using Statistical Package for the Social Sciences (SPSS, Version 25.0, https://www.ibm.com/spss)statistical software. Normally distributed measurement data were expressed as mean ± standard deviation (x ± s), and group comparisons were conducted using the independent samples t-test. Categorical data were presented as cases (%) and analyzed using the χ2 test or Fisher’s exact test. A P value of less than 0.05 was considered statistically significant.
Results
The study included a cohort of 100 patients, and the data from all participants were collected for statistical analysis. Comparisons between the Remimazolam and Propofol groups revealed no significant differences in demographic variables such as gender, age, BMI, and ASA physical status. However, a significant difference in anesthesia duration was noted between the two groups (Table 1).
The incidence of delirium on the first, second, third, fifth, and seventh postoperative days indicated no cases of POD in the remimazolam group, while one case was reported in the propofol group on the first postoperative day. This difference did not reach statistical significance (P > 0.05) (Table 2).
A total of 100 patients were included in the study, with 35 adverse events reported. The Remimazolam group experienced 10 adverse events, while the Propofol group reported 25 adverse events. The Remimazolam group demonstrated a significantly lower overall incidence of adverse events compared to the Propofol group (20% vs. 50%, P < 0.05) (Table 3).
Discussion
Postoperative delirium (POD) is characterized by the onset of delirium within one week following surgery, typically manifesting within 24 to 72 h postoperatively. The presence of POD adversely affects both short-term and long-term patient outcomes, with research indicating a prevalence of postoperative cognitive dysfunction in elderly individuals ranging from 25–40%6.
As medical practices continue to evolve and patient-friendly examination methods gain traction, there is an increasing trend of elderly patients opting for painless bronchoscopy. This demographic often faces challenges related to declining organ function decline and multiple comorbidities, which can lead to reduced anesthesia tolerance and increased risks. Therefore, the careful selection of anesthetic agents is critical for ensuring the safe and effective performance of painless bronchoscopy in elderly patients.
Remimazolam, an ultra-short-acting benzodiazepine, is characterized by its specific antagonism by flumazenil, rapid onset, and swift metabolism. It is metabolized by non-specific plasma esterases, resulting in pharmacologically inactive metabolites and no drug accumulation in the body7,8,9,10. Due to its minimal adverse effects and unique pharmacokinetic properties, remimazolam shows promise for use in geriatric anesthesia.
The findings of this study indicate that remimazolam is more effective than propofol in reducing the incidence of adverse events. Furthermore, remimazolam has been associated with protective effects on cognitive function in elderly patients. Previous research has shown that remimazolam administration can mitigate postoperative stress responses and inflammatory reactions, thereby enhancing cognitive outcomes post-surgery11. In elderly patients undergoing upper gastrointestinal endoscopy, remimazolam at a dosage of 0.15 mg/kg demonstrated favorable anesthetic outcomes, reduced adverse reactions, and minimal impact on postoperative cognitive function12. Consistent with previous studies, no instances of delirium were observed in the remimazolam group during the postoperative assessment period. Additionally, animal studies suggest that remimazolam has negligible effects on cognitive function in healthy elderly rats, potentially due to the upregulation of apolipoprotein E expression in the hippocampus, which may influence Tau protein phosphorylation and neuronal cell integrity13.
Despite the initial experience identified, this study also has limitations. A notable constraint is that the relatively small sample size may be not large enough to identify a true difference. Secondely, The assessment of postoperative delirium relied on caregiver questionnaires, which may introduce bias. Thirdly, this study was a retrospective study which may be influenced by unmeasured, unnoticed and unaccounted bias and confounding, and then, may make it underpowered.
Conclusion
In summary, remimazolam is a novel ultra-short-acting sedative that offers effective sedation, minimal adverse reactions, and a low incidence of postoperative delirium in elderly patients. Its application in this population is considered safe, effective, and well-tolerated. The widespread use of remimazolam in clinical practice has advanced medical protocols, addressed the need for patient comfort during procedures, and diversified treatment options, thereby enhancing healthcare standards and broadening the scope of eligible patients. Consequently, remimazolam is anticipated to become the preferred sedative agent in this context.
Data availability
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
References
Jin, Z., Hu, J. & Ma, D. Postoperative delirium: Perioperative assessment, risk reduction, and management. Br. J. Anaesth. 125 (4), 492–504. https://doi.org/10.1016/j.bja.2020.06.063 (2020).
Shi, M. et al. Protective effects of Remimazolam on Cerebral Ischemia/Reperfusion Injury in rats by inhibiting of NLRP3 inflammasome-dependent pyroptosis. Drug Des. Devel Ther. 16, 413–423. https://doi.org/10.2147/DDDT.S344240 (2022).
Méndez-Martínez, C. et al. Related factors and treatment of postoperative delirium in Old Adult patients: an integrative review. Healthcare 9 (9), 1103. https://doi.org/10.3390/healthcare9091103 (2021).
Picton, J. D., Marino, A. B. & Nealy, K. L. Benzodiazepine use and cognitive decline in the elderly. Am. J. Health Syst. Pharm. 75 (1), e6–e12. https://doi.org/10.2146/ajhp160381 (2018).
Helmes, E. & Østbye, T. Associations between Benzodiazepine Use and Neuropsychological Test scores in older adults. Can. J. Aging. 34 (2), 207–214. https://doi.org/10.1017/S0714980815000082 (2015).
Weiss, Y. et al. Preoperative cognitive impairment and postoperative delirium in Elderly Surgical patients: a Retrospective large cohort study (the CIPOD Study). Ann. Surg. 278 (1), 59–64. https://doi.org/10.1097/SLA.0000000000005657 (2023).
Kilpatrick, G. J. Remimazolam: Non-Clinical and Clinical Profile of a New Sedative/Anesthetic Agent. Front Pharmacol. 12:690875. https://doi.org/10.3389/fphar.2021.690875 (2021).
Kim, K. M. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth. Pain Med. (Seoul). 17 (1), 1–11. https://doi.org/10.17085/apm.21115 (2022).
Sneyd, J. R. & Rigby-Jones, A. E. Remimazolam for anaesthesia or sedation. Curr. Opin. Anaesthesiol. 33 (4), 506–511. https://doi.org/10.1097/ACO.0000000000000877 (2020).
Goudra, B., Gouda, G. & Mohinder, P. Recent Developments in Drugs for GI Endoscopy Sedation [published correction appears in Dig Dis Sci. 65(11):3407. doi: 10.1007/s10620-020-06143-3]. Dig Dis Sci. ;65(10):2781–2788. doi: (2020). https://doi.org/10.1007/s10620-020-06044-5 (2020).
Liao, Y. Q., Min, J., Wu, Z. X. & Hu, Z. Comparison of the effects of remimazolam and dexmedetomidine on early postoperative cognitive function in elderly patients with gastric cancer. Front Aging Neurosci. 15:1123089. Published. Jun 5. doi: (2023). https://doi.org/10.3389/fnagi.2023.1123089 (2023).
Tan, Y. et al. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J. Gastroenterol. Hepatol. 37 (3), 576–583. https://doi.org/10.1111/jgh.15761 (2022).
Liu, X., Guo, L., Duan, B., Wu, J. & Wang, E. Novel benzodiazepine remimazolam tosylate delays neurodegeneration of aged mice via decreasing tau phosphorylation. Neurotoxicology 92, 156–165. https://doi.org/10.1016/j.neuro.2022.08.003 (2020).
Author information
Authors and Affiliations
Contributions
YS contributed to the conception and design of the study. YS, LZ, YD, and MZ drafed the manuscript. LZ, YD, and MZ collected and analyzed the data. YS and MZ revised the manuscript. All authors contributed to the manuscript revision and read and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, Y., Zhang, L., Diao, Y. et al. The effect of toluene sulfonic acid remimazolam on postoperative delirium in elderly patients undergoing painless bronchoscopy. Sci Rep 14, 31239 (2024). https://doi.org/10.1038/s41598-024-82582-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82582-6