Abstract
This study investigated the antioxidant and antimicrobial properties, as well as the volatile compounds, of Lactobacillus bulgaricus (L. bulgaricus) postbiotics (at concentration of 150 and 300 mg/L) and their combination with chitosan coatings (0.5% and 1%) on sausage quality (with 100 ppm nitrite) during 40 days of cold storage. The results were compared to a control group, as well as to sausages containing commercial formulation (120 ppm) and reduced (100 ppm) levels of nitrite. To further assess the antimicrobial effects, it also inoculated E. coli and Staphylococcus aureus (S. aureus) into the sausages in order to examine how the postbiotics and chitosan coatings impacted the growth of these foodborne pathogens during the 40-day cold storage period. The reults indicated that those containing 300 mg/L postbiotic and 1% chitosan generally met the desired condition for pH, moisture, fat, and total volatile base-nitrogen. These samples also showed the strongest inhibition of mesophilic and psychrophilic bacteria, mold and yeast. Notably, no E. coli or S. aureus were detected in any of the samples, indicating that the postbiotic and chitosan combination effectively inhibited the growth of these pathogens in sausages. The findings suggest that using chitosan coatings and L. bulgaricus postbiotic can enhance the quality of sausages, ultimately lowering the risk of contamination by harmful bacteria and improving overall food safety.
Similar content being viewed by others
Introduction
Perishable foods like fruits, vegetables, and meat products have a limited shelf-life post harvest or production. As consumers increasingly prioritize the nutrition, health, and natural products, the meat industry must produce high-quality and safe products1. Meat and its derviatives are a crucial part of the modern diet, but they prone to chemical deterioration due to factors like oxidation, air, light, and temperature. Microbial growth can also cause unpleasant changes. To address this, researchers are exploring the use of biocompatible coatings and packagings with functional materials to prevent or minimize these negative reactions, offering a promising way to sustainably preserve meat products2.
Sausages, being a complex and emulsified mixture of meat, seasonings, and various nutrients, are susceptible to chemical reactions and microbial spoilage. Although heat treatment can eliminate pathogens, contamination during production can still pose risks of foodborne bacteria3. In response to consumer demand for fewer chemical additives, the meat industry is searching natural preservatives from sources like bacteria, plants, and animals4,5,6,7. Additionally, due to growing environmental concerns about traditional packaging materials, there is a growing interest in biodegradable alternatives made from biopolymers, which offer a sustainable solution for sausage packaging and coating8.
Chitosan, a versatile biopolymer, is derived from chitin, the second-most abundant biopolymer in the world. Chitin is found in various natural sources, including the shells of mollusks and crustaceans, fungi cell walls, and insect cuticles9. Chitosan shares similarities with cellulose and possesses a range of physical, chemical, and biological properties, making it a valuable material with diverse and multifunctional applications. In the food industry, chitosan is widely used as a bioactive coating due to its ability to form films, and it can be used alone or combined with other ingredients and constituents10.
Lactic acid bacteria (LAB) (such as Lactobacillus, Lactococcus, Pediococcus, Leuconostoc and Streptococcus) and their by-products and metabolites have shown potential in food packaging by inhibiting and impeding the growth of pathogenic microorganisms. LAB’s antimicrobial properties come from their ability to reduce pH, prevent toxins, and produce inhibitory compounds11,12. Postbiotics, which are bioactive substances derived from probiotics, have gained attention as natural preservatives11. These inert microbial components or their beneficial by-products have antimicrobial potency. Postbiotics are easy to use in industrial settings, and are stable under various conditions. Examples of postbiotics include bacteriocins, vitamins, peptides, and organic acids13,14. However, some postbiotics have limited effectiveness against certain bacteria, requiring the use of a chelating agent to enhance their antibacterial spectrum by altering the bacterial membrane permeabilizaton15.
Chitosan can not only act as an antimicrobial agent but also as a chelating agent, enhancing the antibacterial effects of postbiotics against a broader range of bacteria16,17. Postbiotics can also serve as decontaminants. Combining chitosan and postbiotics can create a synergistic effect, improving the microbial and chemical quality of sausages. This novel approach leverages the biodegradable and antimicrobial properties of chitosan, along with the antimicrobial properties of postbiotics, to inhibit microbial growth and oxidative processes in sausages15,18. The unique physical and chemical properties of chitosan make it an ideal choice for food coatings, and integrating postbiotics can further enhance its effectiveness. This innovative combination of chitosan and postbiotics addresses the challenges of chemical deterioration and microbial spoilage in meat products, while also meeting the growing consumer demand for natural and sustainable food coating solutions. This approach promotes environmental sustainability and provides a secure and effective preservation method for meat products, aligning with the evolving requirements of the modern food industry. Totally, research on characterizing postbiotics derived from LAB is relatively scarce. To date, there is no published research on the characterization of L. bulgaricus -derived postbiotics and its combined use with chitosan as a coating for sausages with reduced nitrite content, specifically in terms of its effectiveness against E. coli and S. aureus during cold storage. This study aims to investigate this feature and compare the results to a commercial sausage formulation with 120 ppm nitrite.
Materials and methods
Preparation of postbiotic obtained from L. bulgaricus
To prepare the postbiotic, L. bulgaricus (Persian Type Culture Collection, Iran) was first cultured in De Man Rogosa & Sharp (MRS) broth at 37 °C for 24 h. The resulting bacterial suspension was then subjected to stirring and ultrasonication to break down the cells. The mixture was centrifuged at 4000 rpm for 15 min and filtered through a 0.45 μm filter to obtain the postbiotic solution. The final appropriate soulution of postbiotic was prepared in two concentrations of 150 and 300 mg/L, using physiological serum as the solvent15,18.
Properties of postbioitic
Antimicrobial activity
The antimicrobial activity of the postbiotics was evaluated using the well diffusion method19. The postbiotics were applied to agar plates that had been overlaid with E. coli (ATCC 35150) at a concentration of 0.5 McFarland turbidity standard. This standard approximately corresponds to 1.5 × 108 CFU/mL. To fix the inoculum concentration, E. coli suspension compared with this standard in order to adjust at desired level. The plates were then incubated at 37 °C for 24 h. The resulting inhibition zones were measured in millimeters. Chloramphenicol served as the positive control, while MRS broth was used as the negative control to validate the assay results.
Antioxidant activity
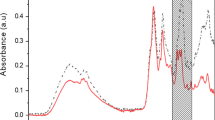
Stock solution of the postbiotic was prepared at 1 mg/mL. A DPPH (1,1-diphenyl-2-picrylhydrazyl radical) solution was prepared by dissolving 2.5 mg of this compound in 100 mL of methanol. Then, 100 µL of the postbiotic sample was mixed with 100 µL of Trolox solution (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) and 3.9 mL of DPPH solution. After 30 min in an ultrasonic bath, the mixture was incubated in the dark for 45 min, and the absorbance was measured at 517 nm using a spectrophotometer (Jenway-6505 UV/Vis, UK). The percentage radical-scavenging activity (%SA) was calculated using the Eq. 1:
where Acontrol is the absorbance of a solution with DPPH and methanol, and Asample is the absorbance of the DPPH solution in the presence of the postbiotic or the standard used, i.e. Trolox solution20.
Determination of volatile compounds
To extract the volatile compounds from the postbiotic, 30 mL of postbiotic was twice mixed with an equal amount of ethyl acetate for 15 min. The mixture of supernatant and ethyl acetate was then separated into aqueous and organic portions and these portions were combined and dried using a rotary evaporator. The dried sample was then dissolved in 500 µL of methanol and then left to sit overnight at room temperature. The sample was then filtered and analyzed using gas chromatography-mass spectrometry (GC-MS) (Agilent 7890 and MS Agilent 5975, USA) to identify the volatile compounds present in the postbiotic. The oven temperature was set to 40 °C and held for 5 min to allow for the initial vaporization of the sample. The temperature of injector was 250 °C for 1 µL of the sample solution. It was used helium as the carrier gas. The oven temperature was ramped up from 40 °C to 250 °C at a rate of 10 °C per minute, then maintained at 250 °C for 5 min in order to ensure complete elution of volaite compounds. The total run time for the GC program was approximately 30 min. Compound identification was facilitated using the NIST Library and Wiley databases21.
Preparation of chitosan solution
Low molecular weight chitosan (Sigma-Aldrich, USA) was dissolved in acetic acid to prepare 0.5% and 1% solutions. The chitosan solutions were then combined with varying amounts of postbiotics and homogenized using a magnetic stirrer for 1 h to create uniform mixtures (Table 1)18.
Preparation of pathogenic bacteria culture
E. coli (ATCC 35150) and S. aureus (ATCC 25923) were cultured in Tryptic soy broth (TSB) at 37 °C for 24 h to prepare the bacterial inoculum for sausage contamination. The cultures were then centrifuged at 4200 rpm for 10 min, and the resulting bacterial pellets were resuspended in 0.1% (w/v) peptone water. To standardize the concentration of inoculum, the pellets were resuspended and diluted to 10 mL, targeting an optical density of 0.5 at 600 nm. The optical density was measured and compared with McFarland standards to confirm an approximate concentration of 1.5 × 108 CFU/mL using a spectrophotometer (Jenway-6505 UV/Vis, UK). This calibration ensured consistent inoculum concentrations, enabling reliable contamination of the sausages22.
Production and treatment of sausage samples
The sausage formulation consisted of a mixture of beef (750 g), ice (100 g), oil (30 g), sodium chloride (15 g), starch (30 g), soy protein isolate (50 g), dry milk (20 g), sodium phosphates (3 g), and nitrite (120 ppm). These ingredients are commonly used in meat processing plants in Iran. The sausages were stored at a refrigerated temperature of 4 ± 1 °C. To simulate foodborne contamination, 350 µL of a solution containing E. coli and S. aureus was spread onto the surface of the sausages18. The products were then left at room temperature for 30 min to allow the bacteria to attach on the surface. The treatment solutions were prepared in two forms: test and control, as outlined in Table 1. These solutions were added to the heated sausage formulation before the introduction of foodborne pathogens. The treated sausages were then packaged and stored at 4 ± 1 °C. The sausages underwent various quality tests during 40 days (day of production, day 10, 20, 30, and 40), including chemical and microbial analyses as described in following sections.
Chemical analysis
The pH of the sausage samples was measured using a digital pH meter (Metrohm, Switzerland) after calibration with pH 4 and 7 buffer solutions. For each sample, 10 g was homogenized in 50 mL of distilled water and the pH was measured at 25 °C23. To determine the moisture content, 3 g of sausage sample was dried in an oven (Behdad, Iran) at 103 °C for 5 h, then cooled in a desiccator to obtain the dry weight. The moisture content was calculated by dividing the weight difference between the initial and dry samples by the initial weight24. The total fat content was analyzed according to the Iranian Standard and Industrial Research Institute guidelines (2002)25. For the Total Volatile Basic Nitrogen (TVB-N) analysis, 10 g of sausage was mixed with 2 g of MgO and 200 mL of distilled water until 125 mL of distillate was collected. The distillate was titrated with 0.1 N hydrochloric acid solution, and the TVB-N content was reported in mg/100 g of sausage26 .
Microbial analysis
The mesophilic bacteria were determined by mixing 1 g of homogenized sausage sample with 9 mL of peptone water solution. Serial dilutions of the sample were then prepared and plated on Plate Count Agar (PCA). The plates were incubated at 37 °C for 48 h, and the colonies were counted on plates with 30–300 colonies. The mesophilic bacteria values were expressed as Log CFU/mL27.
To count psychrotrophic microorganisms in the sausage samples, serial dilutions were prepared and plated on Plate Count Agar (PCA) using the spread plate method. The plates were then incubated at 7 °C for 7 days, and the psychrotrophic microorganism population was reported as Log CFU/mL28.
For the enumeration of molds and yeasts, Yeast Extract Glucose Chloramphenicol (YGC) agar was used. The plates were incubated at 25 °C for 3–5 days, and the colonies were counted. Yeast colonies were distinguished from mold colonies based on their morphology29.
To enumerate E. coli, serial dilutions were prepared in buffered peptone water, and then 1 mL of each dilution was spread on Violet Red Bile Glucose Agar (VRBGA) plates. The plates were incubated at 37 °C for 48 h, and only plates with fewer than 300 colonies were considered for enumeration30.
For S. aureus enumeration, decimal serial dilutions were prepared, and 1 mL of each sample was streaked onto baird parker egg yolk tellurite agar. The plates were incubated at 37 °C for 48 h, and the resulting colonies were counted and expressed as Log CFU/mL31.
Statistical analysis
The experiments were conducted in duplicate over the 40-day cold storage period. The results were expressed as mean values ± standard deviation. Statistical analysis was performed using SPSS software version 18. Analysis of Variance (ANOVA) and Tukey’s test used to determine significant differences between treatment groups and sampling days at 95% confidence level.
Results and discussion
Antimicrobial activity of L. bulgaricus postbiotic
In te current study, it was examined L. bulgaricus postbiotics at 150 and 300 mg/L concentrations, chosen for their proven antioxidant and antibacterial effects in preliminary trial (Fig. 1). The tested concentrations effectively inhibited E. coli growth, with the highest inhibition zones observed at both levels. This indicates a promising starting point for evaluating postbiotics’ antimicrobial potential without causing adverse effects from high concentrations. Using moderate levels helps establish a dose-response relationship, balancing antibacterial protection. Focusing on 150 and 300 mg/L strikes a balance between practical application, safety, and research opportunities. Future studies can explore higher concentrations to better understand the underlying mechanisms and potential enhancements in antioxidant and antibacterial activities. Postbiotics have shown antimicrobial effects on harmful and spoilage bacteria according to Moradi et al.11. The antimicrobial activity of L. bulgaricus postbiotics at concentrations of 150 and 300 mg/L against E. coli was examined in the study using the well diffusion method. Findings demonstrated a clear link between the concentration of postbiotics and their ability to inhibit growth. The largest inhibition zone was 6.61 ± 0.30 mm for a postbiotic concentration of 150 mg/L and 9.66 ± 0.19 mm for 300 mg/L. The primary postbiotic compounds from lactobacillus species consist of ribosomally produced peptides, like bacteriocins, and metabolic by-products with various chemical compositions, such as lactic acid, organic acids, hydrogen peroxide (H2O2), diacetyl, acetoin, and phenolic compound32,33. This situation suggests that these compounds exhibit antimicrobial properties, which can be considered a valid justification. In another investigation conducted by Rasouli and colleagues (2021), meat samples wrapped in films containing postbiotics showed lower levels of pathogens compared to samples without a film coating34. The decrease in bacteria numbers shows the promise of postbiotics in averting infections and food decay35. In addition, Wang et al., (2019) employed bacteriocins derived from L. plantarum LPL-1 to combat Listeria monocytogenes. The findings indicated that bacteriocins can prevent the growth of Listeria monocytogenes by acidifying the cell membrane and forming pores in the bacterial membrane36.
Antioxidant capacity of L. bulgaricus postbiotics
Different types of bioactive metabolites produced by probiotics or postbiotics have shown antioxidant properties37. The DPPH radical-scavenging method was used to measure the antioxidant activity of L. bulgaricus postbiotics, showing antioxidant percentages of 48.50% and 45.75% at concentrations of 150 and 300 mg/L. This method relies on the ability of antioxidants to neutralize the DPPH radical, causing a visible color shift from purple to yellow. By measuring this color change, the antioxidant capacity of a sample can be quantitatively assessed, reflecting its ability to quench free radicals and donate hydrogen atoms.The antioxidant properties of postbiotics have significant implications in combating oxidative stress, a condition linked to various diseases such as cardiovascular disorders, neurodegenerative diseases, and certain cancers. These compounds effectively neutralize free radicals, which contribute to oxidative damage and disease progression. Furthermore, incorporating probiotics and their postbiotic metabolites into functional foods presents a promising approach to prevent oxidative stress-related diseases, promoting overall health through dietary intervention. Consuming probiotic-rich foods or those containing postbiotic compounds may provide protective effects against these diseases, advocating for their integration into a healthy diet38.
Chang et al. (2021) examined the antioxidant effects of postbiotics derived from six types of probiotic L. plantarum strains (specifically, RG11, RG14, RI11, RS5, TL1, and UL4). Findings showed that postbiotics are effective in decreasing protein and lipid oxidation by inhibiting radicals. The formulated media experienced an increase in hydroxyl radical scavenging activity. RI11 exhibited the greatest reducing power activity among the tested postbiotics but showed no significant distinction from RG14 and UL439. Moreover, in another study, it was examined the antioxidant capabilities of supernatants derived from different strains of lactobacillus, demonstrating their effectiveness in preventing DPPH radicals. Various factors, such as metal ions and preexisting antioxidant metabolites, were responsible for the variations in antioxidant activity among different strains40.
Volatile compounds in L. bulgaricus postbiotics
GC-MS was employed to detect volatile components in L. bulgaricus postbiotics. GC efficiently segregated elements in the mixture, while MS identified those elements. The retention time and peak area of eleven identified compounds showed variability, as illustrated in Table 2. Various studies have identified a variety of components and volatile profiles in postbiotics15,39. The volatile compound profiles of postbiotics of six strains of probiotic L. plantarum isolated from Malaysian foods were examined using GC-MS. Different organic substances, such as esters, acids, and pyrrole compounds were recognized. Postbiotics’ functional traits was different and it depended on the strains. RG11, RI11 and RS5 showed greater levels of inhibition and antioxidant properties due to their increased levels of acetic acid, caproic acid, and lactic acid. The highest variety of volatile compounds was observed in Postbiotic RI11, which was generated in a specially designed culture medium, in comparison to other versions. On the other hand, postbiotic UL4 produced with MRS control culture medium had 10 volatile compounds, whereas postbiotics RI11 and RS5 each had 9 volatile compounds. Furthermore, postbiotics RG11 and RG14 contained 8 volatile compounds, while postbiotic TL1 had 7 volatile compounds according to Chang et al.39.
The pH, moisute, total fat, and TVB-N values of sausage samples during the cold storage
Measuring pH in meat and its products is essential for evaluating quality characteristics. Different factors like animal traits, breeding methods, processing techniques, and storage conditions impact the quality of meat and meat derivatives. During production and storage of meat, pH is a crucial indicator of quality41. Table 3 shows the changes in pH of sausage samples during the 40-day refrigeration period. The pH levels gradually dropped from samples S2 to S10 as they were stored. At first, there were noticeable variations in pH levels. Later on, there were observations of pH fluctuations by day 30, varying from 5.77 to 6.31. The samples with 300 mg/L postbiotic and 0.5% chitosan (S9) had the lowest, while the S1 sample with 120 ppm nitrite had highest pH values. By the end of storage, the pH continued to decrease, with the 300 mg/L postbiotic sample (S6) measuring 5.53 on day 40.
While stored at cold temperatures, sausages may experience a drop in pH caused by different factors, like the conversion of carbohydrates into lactic acid and acetic acid by certain LAB such as Lactobacillus, Streptococcus, Pediococcus, etc42,43. The quick drop in pH from the acidulants added causes proteins to release water. This phenomenon results in a condition that is not appropriate for the proliferation of harmful microorganisms44.
The application of different techniques for processing and storing can cause changes in the physical and chemical properties of meat products. Physical changes refer to alterations in the texture and structure that impact sensory qualities such as volume, appearance, color, texture, aroma, and taste. These alterations, like decreasing surface moisture through dehydration and preserving fats, boost protein performance and enhance functional characteristics as a result of various compound interactions45.
Modern meat technology is founded on the muscle tissue’s capacity to either retain or release water. Comprehending how water is absorbed and retained in raw meat allows for proper manipulation of its functional and technological traits to reach specific goals. The presence of water in meat and meat-based products has a noticeable effect on the sensory, structural, and mechanical characteristics of the raw materials, as well as on the quality of the products and their shelf life46. Table 4 illustrates the variations in moisture levels in sausage samples throughout the period of cold storage. Findings showed a decrease in moisture levels in all samples as time passed, with noticeable differences between the moisture levels at the beginning and end of the study. A decrease in moisture content happens in sausage when stored in cold conditions due to moisture vapor moving from the sausage surfaces to the surrounding cold air because of a difference in water vapor pressure47.
Fat is a highly diverse raw ingredient and a vital quality attribute in processed meat products like sausages. It is important in creating meat emulsions alongside other ingredients, and impacts the sensory and textural qualities, such as flavor, dryness, and tenderness, of meat-based products. Therefore, it is important to comprehend the impact of alterations in fat quality on the overall quality of sausages48. Changes in fat composition can have adverse effects on taste, appearance, moisture retention, health benefits, and safety of meat, impacting consumer choices. Glycerolipids are made up of monoglycerides, diglycerides, and triglycerides according to Tagrida et al. (2022), with triglycerides being the most important category. Glycerophospholipids are molecules consisting of fatty acids linked to a glycerol molecule, with a phosphatidyl ester located at the end carbon49. The results of examining the sausage samples’ fat content during cold storage, as shown in Table 5, revealed a total fat content range of 22.32% (S10, day of production) to 22.66% (S2, day 40). A small rise in overall fat content was noticed in all samples during the storage period. In the same way, variations in the fat levels at each stage of sausage manufacturing process were examined. The fat percentage in raw ground meat from prototype and two traditionally made sausages was 19.3, 17.9, and 18.1, respectively. After that, the level of fat gradually went down during the technological process, especially following roasting and cooking50.
TVB-N analysis is a popular way to evaluate the quality of meat and meat-based products. In general, the decomposition of nitrogen-containing proteins from spoilage processes such as microbial activity leads to the build-up of organic amines, referred to as TVB-N. These include unstable and harmful nitrogen compounds, like primary, secondary, and tertiary amines. Compounds like methylamines, which are biogenic amines, have the ability to change the color and taste of meat products like sausages, impacting how well they are received by consumers. The TVB-N levels in meat products typically rise with storage duration, showing comparable patterns to spoilage markers such as microbial growth and sensory alterations51,52. Table 6 demonstrates the variations in TVB-N values of the sausage samples over the duration of cold storage. During the forty-day storage period, there was a significant rise in TVB-N values. During the day of production, all samples had TVBN values of 21%. By the tenth day, there was a noticeable rise with values reaching a peak of 25.86 ± 0.11% in S1 to S10. On the twentieth day, there were no notable differences (p < 0.05) between the treatment groups, with rates ranging from 27.43 ± 0.2% (S1) to 29.7 ± 0.72% (S2). On the following day (thirthiest day), TVB-N levels in sausage samples varied from 31.40 ± 0.17% (S1) to 33.26 ± 0.11% (S4), decreasing compared to the control group (41.23 ± 0.07). Ultimately, at the end of the cold storage period, there was a significant rise in TVB-N levels, reaching 36.35 ± 0.17% (S2). These results are consistent with research conducted by Hua et al. (2022) on fish fillets, which found a notable increase in TVB-N levels during cold storage53. Covering fish fillets with sodium alginate, probiotics and postbiotics led to decreased TVB-N levels, keeping them under the 25 mg/100 g limit during the entire 9-day storage period. Sun et al., (2019) examined how the TVB-N levels of Harbin dry sausages changed during storage when a mixture of Staphylococcus xylosus and L. plantarum was used as a starter culture along with vacuum packaging. The findings indicated that the TVB-N levels in non-inoculated sausages were higher than those in inoculated sausages, possibly due to the starter culture in dry sausages hindering the growth of certain spoilage microorganisms through competitive inhibition or bacteriocin production, leading to a decrease in TVB-N formation. The TVB-N values consistently rose during storage in all samples, possibly due to the bacterial enzymes’ activity51.
Microbial assessment including mesophilic bacteria, psychrotrophic bacteria, mold, yeast, E. coli and S. aureus of sausage samples during the cold storage
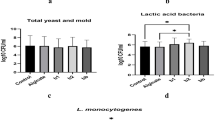
Sausages are highly perishable and require refrigeration or freezing to maintain their quality. The shelf life of sausages largely depends on the initial balance of microorganisms and how they develop during storage, which is heavily influenced by temperature. Refrigeration conditions, in particular, can stimulate the growth of certain microorganisms, especially those that thrive in cold temperatures54. Figure 2 depicts the changes in the mesophilic bacteria of sausage samples over 40 days of refrigerated storage. The mesophilic bacteria values increased steadily over time, ranging from 2.23 to 9.93 Log CFU/mL (Control sample). Initially, all samples had similar mesophilic bacteria values, but by day 10, the sample treated with 1% chitosan and 300 mg/L post-biotic (S10) had the lowest mesophilic bacteria (3.85 Log CFU/mL), while the control sample had the highest mesophilic bacteria (5.89 Log CFU/mL). By day 20, the sample treated with 1% chitosan and 300 mg/L postbiotic (S10) had the lowest mesophilic bacteria (4.86 Log CFU/mL), while the control sample had the highest (6.85 Log CFU/mL). This trend continued on day 30, with S10 having the lowest mesophilic bacteria (5.01 Log CFU/mL) and the control having the highest (8.10 Log CFU/mL). By the end of the 40-day storage period, S10 still had the lowest mesophilic bacteria (6.12 Log CFU/mL), while the control had the highest (9.93 Log CFU/mL). Overall, the combination of 1% chitosan and 300 mg/L postbiotic was found to be the most effective in inhibiting microbial growth, suggesting that this combination can be used to reduce the population of pathogenic bacteria in sausages. Dalvandi et al. (2020) studied the effect of vacuum packaging and edible coatings containing black pepper seeds and turmeric extract on the shelf life of chicken breast fillets during refrigerated storage. The results indicated a gradual increase in aerobic thermophilic bacteria across all samples over time. However, vacuum-sealed samples had significantly lower bacterial counts (around 0.8 Log CFU/mL) compared to air-packed control samples, which exceeded 6 Log CFU/mL by the 4th day. The edible coatings had no significant impact on microbial growth during the 12-day storage period, suggesting that vacuum packaging was the primary factor in inhibiting bacterial growth55.
Psychrotrophic bacteria, such as Pseudomonas, Aeromonas, Schwanella, and Flavobacterium, are major contributors to food spoilage at refrigerated temperatures. Figure 2 shows the growth of psychroterophilic microorganisms in sausage samples during cold storage. The results indicate a significant increase in psychrotrophic bacteria over time, with counts ranging from 2.11 Log CFU/mL (control sample, day production) to 7.53 Log CFU/mL (control sample, day 40). Notably, the treatment with 300 mg/L postbiotic and 1% chitosan (S10) reduced psychrotrophic bacterial growth by 2.27 log CFU/mL compared to the control sample (day 40), suggesting its potential as a spoilage inhibitor. Shahrampour and Razavi (2023) investigated the effect of a lemon root gum coating with rosemary essential oil nanoemulsions on the shelf life of chicken meat. It was found that psychrotrophic bacteria counts on chicken fillet surfaces increased significantly, particularly in the control sample, during refrigerated storage. By day 8, the control sample had reached 7 Log CFU/mL of psychrotrophic bacteria, whereas samples coated with lemon root gum maintained lower bacterial counts, remaining below the threshold even after 12 days of storage56.
Molds and yeasts are aerobic microorganisms that can grow on the surface of sausages, with mold populations sometimes reaching high densities of 105 to 107 CFU/mL. Yeast populations in raw sausages are typically lower, ranging from 103 to 105 CFU/mL. While molds and yeasts can contribute to the flavor and preservation of sausages, they also pose risks to food safety, spoilage, and product consistency. Therefore, sausage manufacturers must carefully manage these microorganisms to produce high-quality, safe, and appealing products57. Figure 2 demonstrates the changes in mold and yeast populations in sausage samples during the 40-day refrigerated storage period. The study found that treating sausage samples with chitosan and postbiotics significantly reduced fungal growth. On the production day, the sample treated with 300 mg/L postbiotic (S6) had the lowest fungal population (1.01 Log CFU/mL), while the sample treated with 0.5% chitosan and 150 mg/L postbiotic (S7) had the highest (1.42 Log CFU/mL). By day 10 of storage, fungal populations ranged from 1.4 Log CFU/mL (S9) to 3.02 Log CFU/mL (control sample). On day 20, the sample treated with 1% chitosan (S4) had the lowest fungal population (2.01 Log CFU/mL), while the control sample had the highest (4.69 Log CFU/mL). By day 30, the sample treated with 1% chitosan (S4) still had the lowest mold and yeast population (2.86 Log CFU/mL), while the control sample had the highest (5.52 Log CFU/mL). At the end of the 40-day storage period, fungal populations ranged from 3.89 Log CFU/mL (sample treated with 1% chitosan and 300 mg/L postbiotic: S10) to 6.86 Log CFU/mL (control sample).
According to the Iranian national standard, sausage samples must be negative for Salmonella and E. coli58. In this study, all tested sausage samples were negative for E. coli, meeting the standard. Additionally, the microbiological test results showed that the sausage samples were also negative for S. aureus. A similar study found that the postbiotic L. paracasei Postbio-P6 exhibited antimicrobial activity against various bacteria and fungi, including strong inhibition against S. aureus, Y. enteritis, and E. coli. However, common probiotics like L. plantarum, L. rhamnosus, and L. paracasei did not show inhibitory activity against certain bacteria59.
Conclusion
There is a growing trend towards using natural antioxidants and antimicrobial agents in food products, including meat and meat-based products, due to increasing consumer demand. One area of focus is reducing the use of nitrites in sausages, as they can form compounds that have negative health effects. To address this, it is required to improve the quality and safety of sausages. This study investigated the effects of chitosan and L. bulgaricus postbiotic on the quality attributes of sausages during storage. Results confirmed that L. bulgaricus postbiotic has antioxidant and antimicrobial properties, effective against pathogens like E. coli and S. aureus. During cold storage, the combination of 300 mg/L postbiotic and 1% chitosan was the most effective treatment in inhibiting microbial growth. The results suggest that postbiotics, with their antioxidant and antimicrobial capabilities, can be used as a coating to prevent biological contamination in food products, offering a promising strategy for improving food safety with the reduction of the amount of nitrite. The results obtained from current study showed that treating sausage samples with chitosan and postbiotic significantly improved their quality characteristics. This approach not only enhances the quality of perishable foods like meat products but also improves food safety by reducing the presence of pathogenic bacteria, ultimately extending the product’s shelf life with reduced nitrite.
Data availability
The datasets generated and analyzed during the current study were available from the corresponding author (Z.E) on reasonable request.
Change history
04 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-92136-z
References
Pateiro, M., Domínguez, R. & Lorenzo, J. M. Recent research advances in meat products. Foods 10 (6), 1303 (2021).
Li, C., Bassey, A. P. & Zhou, G. Molecular changes of meat proteins during processing and their impact on quality and nutritional values. Annual Rev. Food Sci. Technol. 14 (1), 85–111 (2023).
Zeraat Pisheh, F. et al. The effect of plasma-activated water combined with rosemary extract (Rosmarinus officinalis L.) on the physicochemical properties of Frankfurter sausage during storage. Foods 12 (21), 4022 (2023).
Hugo, C. J. & Hugo, A. Current trends in natural preservatives for fresh sausage products. Trends Food Sci. Technol. 45(1), 12–23 (2015).
Novais, C. et al. Natural Food Colorants and preservatives: A review, a demand, and a challenge. J. Agricut. Food Chem. 70, 2789–2805 (2022).
Hojati, N., Amiri, S., Abedi, E. & Radi, M. Effect of cinnamaldehyde-nanoemulsion and nanostructured lipid carriers on physicochemical attributes of reduced-nitrite sausages. Food Chem. 444, 138658 (2024).
Amiri, S., Sepahvand, S., Radi, M. & Abedi, E. A comparative study between the performance of thymol-nanoemulsion and thymol-loaded nanostructured lipid carriers on the textural, microbial, and sensory characteristics of sausage. Curr. Res. Food Sci. 8, 100704 (2024).
Cunningham, M. F. et al. Future green chemistry and sustainability needs in polymeric coatings. Green Chem. 18 (2019).
Iber, B. T., Kasan, N. A., Torsabo, D. & Omuwa, J. W. A review of various sources of chitin and chitosan in nature. J. Renew. Mater. 10 (4), 1097 (2022).
Rouhi, A. et al. Exploring the potential of melittin peptide: Expression, purification, anti-pathogenic properties, and promising applications as a bio-preservative for beef slices. LWT 199, 116083 (2024).
Moradi, M. et al. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 19 (6), 3390–3415 (2020).
Kaveh, S., Hashemi, S. M. B., Abedi, E., Amiri, M. J. & Conte, F. L. Bio-preservation of meat and fermented meat products by lactic acid bacteria strains and their antibacterial metabolites. Sustainability 15 (13), 10154 (2023).
Salminen, S. et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Reviews Gastroenterol. Hepatol. 18 (9), 649–667 (2021).
Vinderola, G., Sanders, M. E. & Salminen, S. The concept of postbiotics. Foods 11 (8), 1077 (2022).
İncili, G. K. et al. Characterization of Pediococcus acidilactici postbiotic and impact of postbiotic-fortified chitosan coating on the microbial and chemical quality of chicken breast fillets. Int. J. Biol. Macromol. 184, 429–437 (2021).
Tsai, T. et al. Chitosan augments photodynamic inactivation of Gram-positive and Gram-negative Bacteria. Antimicrobail Agents Chemother. 55(5), 1883–1890 (2011).
İncili, G. K. et al. Whole-cell postbiotics: An innovative approach for extending the shelf life and controlling major foodborne pathogens in chicken breast fillets. Food Bioprocess. Technol. 16, 1502–1524 (2023).
İncili, G. K. et al. Impact of chitosan embedded with postbiotics from Pediococcus acidilactici against emerging foodborne pathogens in vacuum-packaged frankfurters during refrigerated storage. Meat Sci. 188, 108786 (2022).
Sevin, S. et al. Postbiotics secreted by Lactobacillus sakei EIR/CM-1 isolated from cow milk microbiota, display antibacterial and antibiofilm activity against ruminant mastitis-causing pathogens. Italian J. Anim. Sci. 20 (1), 1302–1316 (2021).
Kocabey, N. İ., Yilmaztekin, M. U. & Hayaloglu, A. A. Effect of maceration duration on physicochemical characteristics, organic acid, phenolic compounds and antioxidant activity of red wine from Vitis vinifera L. Karaoglan. J. Food Sci. Technol. 53 (9), 3557–3565 (2016).
Chang, H. M., Foo, H. L., Loh, T. C., Lim, E. T. C. & Abdul Mutalib, N. E. Comparative studies of inhibitory and antioxidant activities, and organic acids compositions of postbiotics produced by probiotic lactiplantibacillus plantarum strains isolated from Malaysian foods. Front. Veterinary Sci. 7, 602280 (2021).
Tong, Y. et al. Optimizing postbiotic production through solid-state fermentation with Bacillus amyloliquefaciens J and lactiplantibacillus plantarum SN4 enhances antibacterial, antioxidant, and anti-inflammatory activities. Front. Microbiol. 14, 1229952 (2023).
Institute of Standards and Industrial Research of Iran. Meat and its products - determination of PH reference test method. No. 1028 [Online]. [cited 2007]; Available from: https://standard.inso.gov.ir [In Persian].
Institute of Standards and Industrial Research of Iran. Meat and meat products - Determination of moisture by the reference method-test methods. No. 745 [Online]. [cited 2003]; Available from: https://standard.inso.gov.ir [In Persian].
Institute of Standards and Industrial Research of Iran. Meat and meat products - determination of total fat - test method. No. 742 [Online]. [cited 2002]; Available from: https://standard.inso.gov.ir [In Persian].
Yousefi, M., Farshidi, M. & Ehsani, A. Effects of lactoperoxidase system-alginate coating on chemical, microbial, and sensory properties of chicken breast fillets during cold storage. J. Food Saf. 38(3), e12449 (2018).
Khanjani, M., Ariaii, P., Najafian, L. & Esmaeili, M. Investigating the effect of polylactic acid-nanocellulose composite film along with Lactobacillus casei on the quality and shelf life of beluga sturgeon (Huso huso) fillet. J. Food Meas. Charact. 17(4), 4161–4174 (2023).
Kopuz, S., Akman, P. K., Tekin-Cakmak, Z. H. & Karasu, S. Development and characterization of antimicrobial films from gums obtained from cold-pressed flaxseed oil by-product. Polym. Bull. 81(2), 1767–1787 (2024).
Ehrampoush, M. H. et al. Microbiological quality of sausage during slicing at food retail stores in Shiraz, Iran. Int. J. Nutr. Sci. 2(1), 21–26 (2017).
Sukumaran, A. T. et al. Effect of deboning time on the growth of Salmonella, E. Coli, aerobic, and lactic acid bacteria during beef sausage processing and storage. Meat Sci. 139, 49–55 (2018).
Kaban, G. & Kaya, M. Effect of starter culture on growth of Staphylococcus aureus in sucuk. Food Control. 17(10), 797–801 (2006).
Rocchetti, M. T. et al. Bioprospecting antimicrobials from lactiplantibacillus plantarum: Key factors underlying its probiotic action. Int. J. Mol. Sci. 22(21), 12076 (2021).
Hosseini, S., Homayouni-Rad, A., Kafil, H. S. & Dorud, N. Evaluating the antimicrobial effect of postbiotic extract from Lactobacillus casei on Escherichia coli in commercial sterilized milk. J. Food Saf. Hygiene. 8(1), 42–52 (2022).
Rasouli, Y., Moradi, M., Tajik, H. & Molaei, R. Fabrication of anti-listeria film based on bacterial cellulose and Lactobacillus sakei-derived bioactive metabolites; application in meat packaging. Food Biosci. 42, 101218 (2021).
Homayouni Rad, A. et al. Postbiotics, as dynamic biomolecules, and their promising role in promoting food safety. Biointerface Res. Appl. Chem. 11(6), 14529–14544 (2021).
Wang, Y., Qin, Y., Zhang, Y., Wu, R. & Li, P. Antibacterial mechanism of plantaricin LPL-1, a novel class IIa bacteriocin against Listeria monocytogenes. Food Control. 97, 87–93 (2019).
Noori, S. M. A. et al. Antimicrobial and antioxidant properties of natural postbiotics derived from five lactic acid bacteria. Jundishapur J. Nat. Pharm. Prod., 18(1), (2023).
Singh, R. P., Sharad, S. & Kapur, S. Free radicals and oxidative stress in neurodegenerative diseases: Relevance of dietary antioxidants. J. Indian Acad. Clin. Med. 5 (3), 218–225 (2004).
Chang, H. M., Foo, H. L., Loh, T. C., Lim, E. T. C. & Abdul Mutalib, N. E. Comparative studies of inhibitory and antioxidant activities, and organic acids compositions of postbiotics produced by probiotic lactiplantibacillus plantarum strains isolated from Malaysian foods. Front. Veterinary Sci. 7, 602280 (2021).
Sornsenee, P. et al. Lyophilized cell-free supernatants of Lactobacillus isolates exhibited antibiofilm, antioxidant, and reduces nitric oxide activity in lipopolysaccharide-stimulated RAW 264.7 cells. PeerJ. 9, e12586 (2021).
Geletu, U. S., Usmael, M. A., Mummed, Y. Y. & Ibrahim, A. M. Quality of cattle meat and its compositional constituents. Veterinary Med. Int.1, 7340495 (2021).
Lestari, R. B., Permadi, E. & Harahap, R. P. Decrease quality during storage packaged beef sausage edible coating by durian seeds starch-chitosan with the addition of kesum leaf extract. In IOP Conference Series: Earth and Environmental Science. IOP Publishing. 478(1). 012036 (2020).
Luan, X., Feng, M. & Sun, J. Effect of Lactobacillus plantarum on antioxidant activity in fermented sausage. Food Res. Int. 144, 110351 (2021).
Leroy, F. & Vuyst, L. D. Fermentation and acidification ingredients. In: (ed Tarté, R.) Ingredients in Meat Products (Springer, New York, NY, 2009).
Gómez, I., Janardhanan, R., Ibañez, F. C. & Beriain, M. J. The effects of processing and preservation technologies on meat quality: Sensory and nutritional aspects. Foods 9(10), 1416 (2020).
Kudryashov, L. S. & Kudryashova, O. A. Water-holding and water-holding capacity of meat and methods of its determination. Theory Pract. Meat Process., 8(1), 62–70 (2023).
El-Nashi, H. B., Fattah, A. F. A. K. A., Rahman, N. R. A., El-Razik, A. & M. M Quality characteristics of beef sausage containing pomegranate peels during refrigerated storage. Annals Agricult. Sci. 60(2), 403–412 (2015).
Carballo, J. Sausages: Nutrition, safety, processing and quality improvement. Foods 10(4), 890 (2021).
Tagrida, M. et al. Polylactic acid film coated with electrospun gelatin/chitosan nanofibers containing betel leaf ethanolic extract: Properties, bioactivities, and use for shelf-life extension of Tilapia slices. Molecules 27(18), 5877 (2022).
Safar, G. N., Abdul, A. N., Amirkhan, B. A. & Natiq, S. Y. Development of new types of combined meat products and dynamic changes depending of their indicators on various technological stages of production. Food Sci. Technol. 42, 59220 (2021).
Sun, Q., Sun, F., Zheng, D., Kong, B. & Liu, Q. Complex starter culture combined with vacuum packaging reduces biogenic amine formation and delays the quality deterioration of dry sausage during storage. Food Control. 100, 58–66 (2019).
Bekhit, A. E. D. A., Holman, B. W., Giteru, S. G. & Hopkins, D. L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 109, 280–302 (2021).
Hua, Q., Wong, C. H. & Li, D. Postbiotics enhance the functionality of a probiotic edible coating for salmon fillets and the probiotic stability during simulated digestion. Food Packag. Shelf Life. 34, 100954 (2022).
Raimondi, S. et al. Bacterial community of industrial raw sausage packaged in modified atmosphere throughout the shelf life. Int. J. Food Microbiol. 280, 78–86 (2018).
Dalvandi, F., Almasi, H., Ghanbarzadeh, B., Hosseini, H. & Karimian Khosroshahi, N. Effect of vacuum packaging and edible coating containing black pepper seeds and turmeric extracts on shelf life extension of chicken breast fillets. J. Food Bioprocess. Eng. 3(1), 69–78 (2020).
Shahrampour, D. & Razavi, S. M. Novel antimicrobial/antioxidant Eremurus luteus root gum coating containing rosemary essential oil nanoemulsions for extension of chicken meat shelf life. Food Sci. Nutr. 11(6), 3131–3140 (2023).
Stagnitta, P. V., Micalizzi, B., De Guzmán, A. M. S. & de Guzmán, A. M. S. Prevalence of some bacteria yeasts and molds in meat foods in San Luis, Argentina. Cent. Eur. J. Public Health, 14(3), (2006).
Institute of Standards and Industrial Research of Iran. Sausages- Specifications and test methods. No. 2303 [Online]. [cited 2021]; Available from: https://standard.inso.gov.ir [In Persian].
Dong, H., Ren, X., Song, Y., Zhang, J., Zhuang, H., Shen, J., et al. Assessment of multifunctional activity of a postbiotic preparation derived from lactobacillus paracasei Postbio-P6. Foods, 1–16 (2024).
Acknowledgements
This work was approved by the Vice-Chancellor of Research and Technology of Isfahan University of Medical Sciences under Grant number 3401686 with ethics code IR.MUI.RESEARCH.REC.1401.350.
Funding
This work was supported by the Vice-Chancellor of Research and Technology of Isfahan University of Medical Sciences, Isfahan, Iran.
Author information
Authors and Affiliations
Contributions
S.S.: conceptualization, provided resources, supervised the study, and contributed to the writing by reviewing and editing the manuscript. Z. E.: Contributed to the investigation, methodology, and writing of the original draft. H. R.: Provided resources, contributed to the methodology, and participated in writing the original draft. S.M.A.N.: Contributed to the conceptualization, provided resources, supervised the study. S.S.: Provided resources, contributed to the methodology, and participated in writing the original draft. M. S.: Provided resources, contributed to the methodology, and participated in reviewing and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the spelling of the author Mehdi Shiri Nasab which was incorrectly given as Mahdi shirinasab. The original version of this Article also contained affiliation errors. Full information regarding the corrections made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sheikhi, S., Esfandiari, Z., Rostamabadi, H. et al. Microbial safety and chemical characteristics of sausage coated by chitosan and postbiotics obtained from Lactobacillus bulgaricus during cold storage. Sci Rep 15, 358 (2025). https://doi.org/10.1038/s41598-024-82810-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-82810-z