Abstract
Macular hole (MH) is a disease of the vitreoretinal interface that develops in relation to age and gender, and is 3.3 times more prevalent in females than in males. However, it remains inconclusive whether gender plays a role in the pathogenesis of MH. We adopted a two-sample Mendelian randomisation (MR) analysis to explore the relationship between free testosterone, bioavailable testosterone, oestradiol, menopause, smoking, alcohol consumption, type 2 diabetes, diastolic blood pressure, and systolic blood pressure and the risk of MH. We found that genetically predicted free testosterone levels in males were significantly associated with an increased risk of MH (IVW model: OR = 1.642; 95% CI, 1.162–2.322; P = 0.005), while genetically predicted oestradiol levels in females were significantly associated with a reduced risk of MH (IVW model: OR = 0.711; 95% CI, 0.517–0.978; P = 0.036). A sensitivity analysis verified the robustness of the causal relationship. MVMR results indicate that oestradiol in females is associated with MH risk using the IVW method (OR = 0.66; 95% CI, 0.47–0.88; P = 0.011). Our study demonstrates that the genetic risk of free testosterone in males and oestradiol in females may be correlated with MH risk.
Similar content being viewed by others
Background
A macular hole (MH) is a vitreoretinal interface disease characterized by a full-thickness neurosensory retinal defect at the fovea. It is a prevalent cause of visual impairment among the older population, with an annual incidence of 8.69 eyes per 100,000 people1. Previous studies have suggested its association with age and sex, highlighting that MH prevalence is 3.3 times higher in women than in men and even more pronounced among older women1,2.
A previous observational study reported the beneficial effect of oestrogen against MH3, suggesting a correlation between oestrogen and MH development through abrupt hormonal changes4. However, the biological basis for this relationship remains unclear. Current evidence on MH risk relies primarily on observational studies that are susceptible to confounding and selection biases5. It is uncertain whether oestrogen, as an observed association, is a relevant causal factor for MH risk. Randomised clinical trials (RCTs) are the most credible approach for assessing the impact of oestrogen on MH risk; however, RCTs are time-consuming and costly. Therefore, we chose Mendelian randomisation (MR), an alternative method for causal inference that can provide evidence for the role of sex hormones in relation to MH development.
At the core of MR, a form of instrumental variable analysis, genetic data can be utilised as a bridging mechanism. It employs single-nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to identify and quantify the causal relationships between exposure factors and outcomes. Similar to RCTs, which randomly assign participants to trial or control groups, MR ‘randomises’ alleles influencing risk factors to determine whether carriers of these genetic variants exhibit a distinct risk of disease development compared with non-carriers. The ability of MR to mitigate potential confounding factors and reverse causality enhances the robustness of correlation results, rendering them more reliable than those from observational studies6. MR is an important strategy for inferring causality in the absence of RCTs.
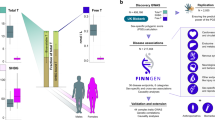
In this study, we used two-sample MR to assess the potential roles of free testosterone (FT), bioavailable testosterone (BT), oestradiol (E2), menopause, smoking, alcohol consumption, type 2 diabetes (T2D), diastolic blood pressure (DBP), and systolic blood pressure (SBP) in MH. We comprehensively evaluated these exposures for genetic associations using publicly available genome-wide association study (GWAS) summary statistics. This assessment has significant clinical importance in the early detection and prevention of visual impairment in the older population.
Methods
Study design
In this study, we used a two-sample MR analysis to avoid the risk of the winner’s curse and weak instruments, which can occur in a one-sample MR analysis7.
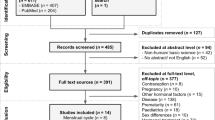
FT, BT, E2, menopause, smoking, alcohol consumption, T2D, DBP, and SBP were analysed as exposures, and MH was analysed as outcome (Fig. 1). Eligible exposure and outcome datasets were searched using publicly available GWAS databases, including OPEN GWAS, GWAS Catalog, and FinnGen (Supplementary Table S1). Considering that population mixing may lead to biased results, the study was limited to people of European ancestry.
Genetic predictors for MHs
Genetic association data for MH risk were obtained from a publicly available GWAS dataset (https://www.finngen.fi/en). The study included 315,134 Europeans, comprising 1,092 cases and 314,042 controls, totalling 20,168,812 SNPs. The 1118 patients were diagnosed with MH out of a total of 356,077 individuals, including 663 females with a prevalence of 0.33% and 455 males with a prevalence of 0.29%, and the overall prevalence was 0.31%. The mean age of onset was 68.69 years for females and 72.78 years for males, with an overall mean age of onset of 70.36 years. After filtering based on genotype quality control, 1092 cases were used for analysis.
GWAS datasets for sex hormones and behavioural and clinical risk factors
In our study, sex hormone-related SNPs were extracted from the databases of FT, BT, E2, and menopause (Table 1 and Supplementary Table S2). Considering the significant difference in the prevalence of MH between males and females, we performed sex-stratified MR analysis of sex hormone-related risk factors using sex-stratified GWAS datasets. Behavioural risk factor-related SNPs were extracted from the smoking and alcohol consumption databases (Table 1 and Supplementary Table S3). Clinical risk factors related to SNPs were extracted from the T2D, DBP, and SBP databases (Table 1 and Supplementary Table S4).
IVs selection and assumption
The SNPs from the GWAS database were used as instrumental variables (IVs), the selection of genetic variations as IVs was based on three main assumptions of MR: (1) strong correlation between IVs and risk factors of interest, (2) genetic variants are not associated with confounders, (3) genetic variants can only influence the outcomes through risk factors.
We started by searching for SNPs as IV in the GWAS database, with the threshold set at P < 5 × 10− 8 (genome-wide significance). Our choice of P < 1 × 10− 6 as a threshold only for E2 (females) was to obtain a higher statistical power for IV; this threshold is acceptable according to previous studies8. To ensure the independence of genetic variation, genetic instruments were grouped using a 10-Mb window and a maximum linkage disequilibrium of r2 = 0.001 between instruments. Palindromic SNPs were excluded.
Statistical analysis
Two-sample MR (version 0.5.8) and MR-PRESSO packages (version 1.0) in R software (version 4.3.2, R Foundation for Statistical Computing, Vienna, Austria) were used for the analysis. The primary MR analysis used the inverse-variance weighted (IVW) method. The IVW method provided a relatively stable and accurate causal assessment by combining Wald ratios (SNP-outcome estimate/SNP-exposure estimate) for each IV using a meta-analytic approach9. In addition, we performed MR analyses using the MR-weighted median, weighted mode, MR-Egger, and leave-one-out-SNP-exclusion analyses (Supplementary Tables S5–S7).
F statistics < 10 is considered a weak IV (F = R2(N − K−1)/K(1 − R2), N represents the sample size of the GWAS database, K represents the number of included IVs, and R2 is the GM taxa variance, which is explained by SNPs10. Statistical significance was defined as P < 0.05.
Sensitivity analysis
The MR-PRESSO test was used to detect pleiotropy, remove outlier SNPs, estimate the corrected results, and test for significant distortion between the results before and after correction11. We also explored horizontal pleiotropy using MR-Egger, which indicates an imbalance in directional horizontal pleiotropy if the Egger intercept is insignificant at zero12.
Heterogeneity was tested in the MR analysis and was considered absent when the P > 0.05. If P < 0.05, a random-effects model was used; otherwise, a fixed-effects model was used. The stability of the MR results was determined by eliminating SNPs individually using leave-one-out sensitivity tests13.
Multivariate MR analysis
Considering that testosterone and E2 are strongly correlated, we performed multivariate MR to analyse the effects of FT, BT, and E2 on MH risk by sex. The databases used for the multivariate MR analysis were the same as before. After clumping, 79 genetic instruments were used for multivariate MR analysis in males, and 136 for multivariate MR analysis in females. Multivariate MR analysis was performed using the MR and multivariate MR packages.
Results
Two-sample MR analysis of sex hormones in relation to the MH
To investigate whether sex hormones increased the risk of developing MHs, we performed a two-sample MR analysis. Elevated FT levels in males were strongly correlated with an elevated risk of MH (IVW model: odd ratio [OR] = 1.642; 95% confidence interval [CI], 1.162–2.322; P = 0.005) (Table 2; Fig. 2, Supplementary Table S5). However, FT levels in females were not associated with MH risk (IVW model: OR = 0.896; 95% CI, 0.680–1.182; P = 0.438) (Table 2, Supplementary Table S5).
The X-axis represents 75 FT gene tools, their effect size estimates (ORs) for FT, and the Y-axis represents the association of the same variants with macular hole risk. No abnormal genetic variation was detected in the MR-PRESSO test.
We also found that E2 levels in females were strongly correlated with a reduced risk of MHs (IVW model: OR = 0.711; 95% CI, 0.517–0.978; P = 0.036) (Table 2; Fig. 3, Supplementary Table S5). We did not find that E2 levels in males were associated with MH risk (IVW model: OR = 0.844; 95% CI, 0.663–1.074; P = 0.168) (Table 2, Supplementary Table S5). We did not detect a trend toward a causal relationship with the risk of MH for BT or menopause (Table 2, Supplementary Table S5).
The X-axis represents 19 oestradiol gene tools, their effect size estimates (ORs) for E2, and the Y-axis represents the association of the same variants with macular hole risk. No abnormal genetic variation was detected in the MR-PRESSO test.
Two-sample MR analysis of behavioural and clinical risk factors associated with MH
Using two-sample MR analysis, we found no indication of a trend toward a causal relationship between smoking behaviour (cigarettes smoked per day), alcohol intake frequency, and the risk of MH (Table 2, Supplementary Tables S6). In addition, no associations were observed between genetically determined T2D, DBP, SBP, and MH risk (Table 2, Supplementary Table S7).
Sensitivity analysis
The results of horizontal pleiotropy using MR-Egger are presented in the supplementary material ( Supplementary Table S8). We found horizontal pleiotropy for FT (females) in the MR-Egger analysis (P = 0.028) but not in the MR-PRESSO test (P = 0.089). No other exposures exhibited horizontal pleiotropy using either the MR-PRESSO or MR-Egger tests. The results of the heterogeneity analysis of the MR analysis of the risk factors on MHs are presented in the supplementary material ( Supplementary Table S9). There was no heterogeneity in all exposures except for BT (males); therefore, BT (males) was used in the random-effects model.
Multivariate MR analysis of sex hormones in relation to the MH.
Multivariate MR results suggest that E2 in females is associated with the risk of MH formation using the IVW method (OR = 0.66; 95% CI, 0.47–0.88; P = 0.011), consistent with univariate results. The P-value for the heterogeneity test was 0.5572. The multivariate MR results in males did not show an association between FT, BT, or E2 levels and the risk of MHs (Supplementary Table S10).
Discussion
In this study, we investigated the association of sex hormone levels and clinical and behavioural factors with MH risk using an MR framework. We found genetic evidence for the potential causal effect of FT in males and E2 in females for MH risk. There was no horizontal pleiotropy or heterogeneity in these associations in the sensitivity analyses.
Our findings align with those of previous observational studies consistently indicating that oestrogen is a protective factor against MH development3,14,15. First, there have been repeated reports on the neuroprotective effects of oestrogen on the retina16,17. The antioxidant effect is attributed to the cellular-level protection of the retina18. A previous study demonstrated the protective effect of E2 and oestrogen analogues against 5-mM glutamate damage in 661 W cells derived from mouse retinal cone cells16. Moreover, oestrogen can regulate tissue perfusion by regulating blood flow in the retina and choroid. Previous studies have shown that reduced blood flow contributes to ocular diseases (such as age-related macular degeneration, diabetic retinopathy)19,20. Several studies comparing haemodynamic differences between premenopausal and postmenopausal women have found that sex hormones affect ophthalmic perfusion and are correlated with disease progression. A previous study demonstrated higher blood flow velocity and lower vascular resistance index in premenopausal women21. Retinal blood flow is higher in women who received hormone replacement therapy (HRT) than in those who did not receive HRT22. Based on these studies, we hypothesised that oestrogen has protective functions in the retina by promoting vasodilation, decreasing vascular resistance, and regulating ocular perfusion. Furthermore, E2 has been shown to inhibit the retinal pigment epithelium cell-mediated contraction of collagen gels23. The sudden drop in oestrogen levels in women during menopause leads to the loss of vitreous collagen and further contraction of vitreous collagen, leading to the development of posterior vitreous detachment and a more pronounced MH occurrence in postmenopausal women2,24.
In recent years, attention has been paid to the effects of anti-oestrogenic drugs on MH. Two case reports revealed that five women undergoing tamoxifen therapy exhibited cystic changes in the central concave region, focal destruction of photoreceptors, and retinal atrophy, indicative of an initial stage of MH14,25. In another retrospective study that analysed 300 cases of MH, patients treated with tamoxifen showed an increased incidence of binocular MH; however, the difference was not remarkable26. Cronin et al. hypothesised that plasma oestrogen levels in patients chronically treated with anti-oestrogenic medications are in a state of depletion, with elevated serum fibrinogen levels, which ultimately predispose the foveal tissue to vitreous traction, which correlates with the development of MH precursor lesions3,26. Feng et al. found a statistically significant correlation between women treated with anastrozole and exemestane and the development of MH, with an increase in vitreous macular temporal traction in women treated with anastrozole27.
Despite the correlation between oestrogen and the pathogenesis of MH, as suggested by the above studies, there is a scarcity of epidemiological or biological evidence supporting this theory, with some even presenting contradictory findings. A previous report evaluated oestrogen levels in the vitreous of patients with MH and observed that mean E2 levels were substantially higher in MH vitreous samples than those in control vitreous samples28. The sample size of this study was limited, and we believe that vitreous oestrogen levels should be considered separately from systemic oestrogen levels.
Importantly, we found that FT in men increases the risk of MH. There are three different forms of testosterone in the circulation: inactive SHBG-bound testosterone, mildly active albumin-bound testosterone, and active free testosterone (FT)29. FT and albumin-bound testosterone are collectively referred to as bioavailable testosterone (BT), which is the portion of total testosterone that can move to tissues and function30. Several epidemiologic studies have found that BT and FT are more strongly associated with androgen-dependent outcomes than total testosterone31. Therefore, we investigated the relationship between FT and BT with MH risk. Dedania et al. used data from a large national insurance database of US for a retrospective matched cohort study. Comparing 35,784 testosterone users with 178,860 matched controls, 93 (0.3%) retinal artery occlusion (RAO)s were found in the testosterone group and 316 (0.2%) RAOs in the control group, and testosterone supplementation significantly increased the risk of RAOs32. Çiloğlu et al. collected 30 patients with central serous chorioretinopathy (CSC) and 32 healthy volunteers, measured and compared total testosterone levels between the two groups, and found that CSC was associated with increased total testosterone levels33. Nudleman et al. reviewed male patients receiving exogenous testosterone therapy for low testosterone in two tertiary vitreoretinal care centers from 2011 to 2013, nine of these patients developed CSCR after initiation of testosterone therapy, symptoms and subretinal fluid resolved after discontinuation of therapy in two patients, and exogenous testosterone may be an independent risk factor for CSCR34.
Malan et al. showed a positive correlation between serum FT levels and the central retinal artery vascular diameter in men, suggesting that FT has a prominent vasodilatory response to increased retinal microvascular aperture in men, and this study suggests that a larger vascular calibre is a marker of severe microvascular damage35. Several studies have shown that testosterone impairs microvascular function36,37. A previous study in women with polycystic ovary syndrome showed that higher FT levels were associated with microvascular endothelial dysfunction and impaired microvascular dilation38. Nevertheless, the mechanism underlying the effect of FT on the vasculature is currently unclear. Burgos-Blasco et al. observed in a retrospective study that 10 of 14 patients (71.4%) with “macular abnormalities of unknown origin” had received 5α-reductase inhibitor (5-ARI), an antiandrogen drug and that as the disease progressed, optical coherence tomography in 2 of these patients showed “enlarged foveal lesions resembling lamellar holes and outer foveal defects resembling impending MHs”, which suggested that the use of antiandrogen drugs may lead to macular abnormality39. Serum-FT levels are reportedly reduced after treatment with 5-ARI40.
Based on our current results, we concluded that FT levels significantly associated with increased risk of MH in men (IVW model: OR = 1.642; 95% CI, 1.162–2.322; P = 0.005); and due to pleiotropy issues, we ended up using the database with ID number ieu-b-4868 and found no association between BT in males and MH. Given the discrepancy between our findings and those of previous studies on the correlation between testosterone and MH, we reran the two-sample MR analysis using MH data from the OPEN GWAS as the endpoint. Both BT (BT, id: ieu-b-4868, IVW model: OR = 2.074; 95% CI, 1.254–3.432; P = 0.004), (BT, id: ebi-a-GCST90012103, IVW model: OR = 2.056; 95% CI, 1.275–3.315; P = 0.003) and FT (FT, PMID: 36653534, IVW model: OR = 2.014; 95% CI, 1.290–3.144; P = 0.002) were positively associated with MH risk. Although E2 and testosterone may play different roles between the sexes, we cannot exclude the possibility that correlations could not be detected because of the relatively small variance explained by the IVs. Therefore, future exploration of GWAS data based on larger sample sizes is necessary to obtain more accurate estimates. In addition to sex, age is an important determinant of the effect of sex hormones on MH. Therefore, further exploration of GWAS data for patients of different age groups is necessary. This result suggests that we should pay attention to fundus diseases in elderly people with imbalanced hormone levels, which is important for early detection and prevention of visual impairment in elderly people.
This study used MR to analyse the risk factors for MH. Our study has some limitations. First, due to the limitations of the current MH GWAS database, we were unable to obtain cases and data with age and gender information, and therefore were unable to analyze subgroups based on age. We could not conduct MR analysis on the sex-stratified GWAS database for MH. The number of MH cases used in our MR studies is limited compared with other outcomes. When more cases with gender and age information are available, it is hoped that age - and sex-stratified analysis will be performed. Second, although our sensitivity analysis combining the MR-PRESSO and MR-Egger intercept tests did not identify horizontal pleiotropy, there may still be vertical pleiotropy41. Third, because all GWAS databases in this study originated from European populations, the findings may not apply to other ethnic groups. Finally, despite revealing an association between FT levels in males and E2 levels in females with MH risk, further research is required to elucidate the underlying mechanisms.
Conclusions.
In conclusion, this is an MR study that reveals the relationship between sex hormones and MH risk. We found that E2 levels in females were negatively associated with MH risk, and FT levels in males were positively associated with MH risk.
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information. The dataset(s) supporting the conclusions of this article are available in the [IEU Open GWAS] [https://gwas.mrcieu.ac.uk/], [GWAS Catalog] [GWAS Catalog (ebi.ac.uk)], [FinnGen] [https://www.finngen.fi/en].
References
McCannel, C. A., Ensminger, J. L., Diehl, N. N. & Hodge, D. N. Population-based incidence of macular holes. Ophthalmology 116(7), 1366–1369 (2009).
Ali, F. S., Stein, J. D., Blachley, T. S., Ackley, S. & Stewart, J. M. Incidence of and risk factors for developing idiopathic macular hole among a diverse group of patients throughout the United States. JAMA Ophthalmol. 135(4), 299–305 (2017).
Risk factors for idiopathic macular holes. The eye disease case-control study group. Am. J. Ophthalmol. 118(6), 754–761 (1994).
Evans, J. R. et al. Systemic risk factors for idiopathic macular holes: a case-control study. Eye (Lond). 12(Pt 2), 256–259 (1998).
Davey Smith, G. & Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23(R1), R89–98 (2014).
Davey Smith, G., Paternoster, L. & Relton, C. When will Mendelian randomization become relevant for clinical practice and public health. JAMA 317(6), 589–591 (2017).
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N. & Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27(8), 1133–1163 (2008).
Sanna, S. et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51(4), 600–605 (2019).
Burgess, S., Dudbridge, F. & Thompson, S. G. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat. Med. 35(11), 1880–1906 (2016).
Burgess, S., Davies, N. M. & Thompson, S. G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 40(7), 597–608 (2016).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50(5), 693–698 (2018).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44(2), 512–525 (2015).
Zheng, J. et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep. 4(4), 330–345 (2017).
Chung, S. E., Kim, S. W., Chung, H. W. & Kang, S. W. Estrogen antagonist and development of macular hole. Korean J. Ophthalmol. 24(5), 306–309 (2010).
Nuzzi, R., Scalabrin, S., Becco, A. & Panzica, G. Gonadal hormones and retinal disorders: A review. Front. Endocrinol. (Lausanne). 9, 66 (2018).
Nixon, E. & Simpkins, J. W. Neuroprotective effects of nonfeminizing estrogens in retinal photoreceptor neurons. Invest. Ophthalmol. Vis. Sci. 53(8), 4739–4747 (2012).
Nakazawa, T., Takahashi, H. & Shimura, M. Estrogen has a neuroprotective effect on axotomized RGCs through ERK signal transduction pathway. Brain Res. 1093(1), 141–149 (2006).
Zhu, C. et al. 17β-Estradiol up-regulates Nrf2 via PI3K/AKT and estrogen receptor signaling pathways to suppress light-induced degeneration in rat retina. Neuroscience 304, 328–339 (2015).
Pemp, B. et al. Retinal blood flow in type 1 diabetic patients with no or mild diabetic retinopathy during euglycemic clamp. Diabetes Care. 33(9), 2038–2042 (2010).
Pemp, B. et al. Retinal blood flow in type 1 diabetic patients with no or mild diabetic retinopathy during euglycemic clamp. Diabetes Care. 33, 2038–2042 (2010).
Toker, E., Yenice, O., Akpinar, I., Aribal, E. & Kazokoglu, H. The influence of sex hormones on ocular blood flow in women. Acta Ophthalmol. Scand. 81(6), 617–624 (2003).
Deschênes, M. C. et al. Postmenopausal hormone therapy increases retinal blood flow and protects the retinal nerve fiber layer. Invest. Ophthalmol. Vis. Sci. 51(5), 2587–2600 (2010).
Kimura, K. et al. Inhibition by female sex hormones of collagen gel contraction mediated by retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 55(4), 2621–2630 (2014).
Gray, R. H., Gregor, Z. J. & Marsh, M. Oestrogens and macular holes: a postal questionnaire. Eye (Lond) 8(Pt 3), 368–369 (1994).
Gualino, V., Cohen, S. Y., Delyfer, M. N., Sahel, J. A. & Gaudric, A. Optical coherence tomography findings in tamoxifen retinopathy. Am. J. Ophthalmol. 140(4), 757–758 (2005).
Cronin, B. G., Lekich, C. K. & Bourke, R. D. Tamoxifen therapy conveys increased risk of developing a macular hole. Int. Ophthalmol. 26(3), 101–105 (2005).
Feng, Z. X. et al. Risk of ocular adverse events with aromatase inhibitors. Can. J. Ophthalmol. (2023).
Inokuchi, N. et al. Vitreous estrogen levels in patients with an idiopathic macular hole. Clin. Ophthalmol. 9, 549–552 (2015).
Rosner, W., Auchus, R. J., Azziz, R., Sluss, P. M. & Raff, H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: An Endocrine Society position statement. J. Clin. Endocrinol. Metab. 92, 405–413 (2007).
Manni, A. et al. Bioavailability of albumin-bound testosterone. J. Clin. Endocrinol. Metab. 61, 705–710 (1985).
Goldman, A. L. et al. A reappraisal of testosterone’s binding in circulation: Physiological and clinical implications. Endocr. Rev. 38, 302–324 (2017).
Dedania, V. S., Zacks, D. N., Pan, W. & VanderBeek, B. L. Testosterone supplementation and retinal vascular disease. Retina 38, 2247–2252 (2018).
Çiloğlu, E., Unal, F. & Dogan, N. C. The relationship between the central serous chorioretinopathy, choroidal thickness, and serum hormone levels. Graefes Arch. Clin. Exp. Ophthalmol. 256, 1111–1116 (2018).
Nudleman, E., Witmer, M. T., Kiss, S., Williams, G. A. & Wolfe, J. D. Central serous chorioretinopathy in patients receiving exogenous testosterone therapy. Retina 34, 2128–2132 (2014).
Malan, N. T. et al. Low serum testosterone and increased diastolic ocular perfusion pressure: a risk for retinal microvasculature. Vasa 44(6), 435–443 (2015).
Aribas, E. et al. Sex steroids and markers of micro- and macrovascular damage among women and men from the general population. Eur. J. Prev. Cardiol. 29(9), 1322–1330 (2022).
Bernini, G. et al. Vascular reactivity in congenital hypogonadal men before and after testosterone replacement therapy. J. Clin. Endocrinol. Metab. 91(5), 1691–1697 (2006).
Leão, L. M. et al. Nonobese young females with polycystic ovary syndrome have nutritive microvascular dysfunction: A pilot study. Endocr. Pract. 20(12), 1281–1289 (2014).
Shin, Y. K., Lee, G. W., Kang, S. W., Kim, S. J. & Kim, A. Y. Macular abnormalities associated with 5α-reductase inhibitor. JAMA Ophthalmol. 138(7), 732–739 (2020).
Zhang, Y., Xu, J., Jing, J., Wu, X. & Lv, Z. Serum levels of androgen-associated hormones are correlated with curative effect in androgenic alopecia in young men. Med. Sci. Monit. 24, 7770–7777 (2018).
Bowden, J. et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36(11), 1783–1802 (2017).
Acknowledgements
We acknowledge OPEN GWAS, GWAS Catalog and FinnGen database for providing their platforms and contributors for uploading their meaningful datasets.
Funding
Supported by Tianjin Binhai New Area Health Commission Project (No. 2023BWKZ006), The Science&Technology Development Fund of Tianjin Education Commission for Higher Education (No.2022ZD058) and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-037 A).
Author information
Authors and Affiliations
Contributions
Designed the study: BH and WL. Performed the study: ZN and ND. Managed and analysed the data: ZN, ND and SB. Wrote the manuscript: ZN and XZ. Revised the manuscript: BL and XL. ZN and ND contributed equally to this paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Our research is based on open-source data, so there are no ethical issues or other conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nie, Z., Duan, N., Zhang, X. et al. A two sample Mendelian randomized study of the association of sex hormones and behavioral and clinical risk factors with macular hole. Sci Rep 15, 10212 (2025). https://doi.org/10.1038/s41598-024-83469-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83469-2