Abstract
Our understanding of the basic relationships of microbiota associated with flowers is still quite limited, especially regarding parasitic plant species. The transient nature of flower parts such as pistil stigmas provides a unique opportunity for temporal investigations. This is the first report of the analysis of bacterial and fungal communities associated with the pistil stigmas of the lucerne parasite, Orobanche lutea. We compared the microorganism communities at different developmental stages and assessed the impact of pollution at the sampling sites. We also examined the plant growth properties (PGP) of bacteria in a culture-dependent analysis. The predominant colonizers of the pistil stigmas were Proteobacteria (99.25%), with Enterobacteriaceae (49.88%) and Pseudomonadaceae (48.28%) being the major families. The prevalent fungal phylum was Basidiomycota (71.64%), with Filobasidiales (33.14%) and Tremellales (27.27%) as dominant orders. Microbial populations in polluted area showed increased bacterial and fungal diversity. Mature stigmas exhibited greater microbial variety compared to immature ones. We found higher fungal than bacteria abundance at both polluted and unpolluted sites. In culture-dependent analysis, immature stigmas from unpolluted area had the least bacterial morphotypes. Identified culturable bacteria represented the Acinetobacter, Erwinia, Micrococcus, Oceanobacillus, Pantoea, Pseudomonas, Serratia, and Staphylococcus genera. The assessment of PGP traits revealed multiple strains with plant growth-promoting potential. Microbial composition varied between polluted and unpolluted sites and was influenced by the flower’s developmental stage.
Similar content being viewed by others
Introduction
Parasitic plants constitute approximately 1.6% of angiosperm species, encompassing 26 families, 292 genera and approximately 4750 species, and occur in almost all terrestrial vegetation types, with being some of them serious pests of crops. With 102 genera and 2100 species, Orobanchaceae stands as the most numerous family among parasitic plants1. Parasitic plants have the potential to act as keystone species or allogenic engineers due to their influence on the growth, reproduction, and proportions of other species, along with their broader impact on ecosystems and ecological communities through trophic interactions. They are often rare and endangered species2,3,4. Parasitic plants are capable of thriving in polluted soils and can accumulate heavy metals such as Cd, Ni, and Zn, thereby helping alleviate the adverse effects of metal stress on the host plant5,6,7,8. Orobanche nowackiana Markgr. (= Phelipanche nowackiana (Markgr.) Soják) and P. nana (Rchb. f.) Soják proved capable of accumulating high amounts of Ni5,7. O. laxissima Uhlich & Rätzel accumulated more Mg, Ca, Ni, and Zn and less Co and Fe in flowers than in stems8. Mycorrhizal colonization and arbuscular richness were reported to be significantly higher in plants colonized by parasitic plants at polluted sites than at unpolluted sites6.
Holoparasitic Orobanchaceae are a diverse group of organisms with regard to their taxonomy, with respect to the reduction of vegetative organs, and are often difficult to identify. Due to the lack of chlorophyll, these species depend entirely on their host for nutrition, facilitated by haustoria that directly link to the tissues of the host root9. Furthermore, a range of morphological reductions, such as scale-like leaves and shoots, is typically confined to a single inflorescence. Despite this, a significant variation is maintained in other traits, such as color and floral or inflorescence morphology, as well as scent. Thus, Orobanchaceae species create intensely colored inflorescences adapted to both insect pollination and self-pollination10,11,12,13. There are only a few studies on the microbiome of parasitic plants, particularly those in the tribe Orobancheae14,15,16,17,18,19,20,21,22,23,24, and even less is known about the microorganisms associated with their floral structures. The floral features are crucial for identifying these species and can also provide valuable insights into the composition and role of microorganisms inhabiting these structures. This area remains largely underexplored, leaving significant gaps in our understanding of how microbial communities interact with and influence the reproductive biology of these unique plants and their hosts. Further research is needed to shed light on these interactions.
The floral microbiome, known as the anthosphere, is a segment of plant microbiome relatively neglected in research. The role of flowers evolves throughout their lifespan, starting with the primary function of attracting pollinators to enable sexual reproduction, and later transitioning to maturity, safeguarding and aiding in seed dispersal for the next generation25,26. Due to the short period of development of individual flower elements, it is extremely interesting to study the biodiversity of microorganism populations inhabiting them. As previous research shows, flowers are characterized by a specific microbiome composition. Flowers can be further broken down into smaller, microscale landscapes, such as the petals, sepals, stigma, anthers, pollen, and nectary25,27. The stigma serves as a platform for pollen grain germination, connected to a chamber that facilitates the growth of pollen tubes. It sustains the ideal conditions of moisture and nutrients required for pollen tube development, which can also be advantageous for microorganism development28.
There are a few studies on the diversity of microorganisms inhabiting the stigmas of plant species of economically important agricultural crops. The dominant bacterial group linked to the stigmas of these plants consists primarily of Proteobacteria, with Pseudomonadaceae particularly represented by the Pseudomonas genus, and the Enterobacteriaceae family. Less prevalent bacteria are Actinobacteria, Bacteroidetes, and Firmicutes29,30,31,32,33. In recent studies, it has been shown that bacteria and fungi are common inhabitants of the pistil stigmas of rare species of parasitic plants23,24. Stigmas are habitats abundant in Actinobacteria and Proteobacteria, mainly Enterobacteriaceae, Cellulosimicrobium, Pantoea and Pseudomonas which comprise up to 75% of operational taxonomic units (OTU) detected in this ecological niche. Fungi such as Aureobasidium, Cladosporium, Malassezia, Mycosphaerella and Pleosporales comprise 53% of OTUs detected23. These communities can be transmitted vertically during the development and life cycle of parasitic plants (especially from the host plant), and can be shared horizontally through environmental transmission, facilitated by pollinators, atmospheric factors, or soil34. Therefore, it is assumed that microorganisms inhabiting stigmas may constitute a part of seed microbiota and are transmitted throughout the plant’s life cycle, and the stigma’s microbiome may affect the initial phases of seed conditioning and germination.
Heavy metals in soil can be absorbed by plant roots and transported to above-ground tissues, including the anthosphere. Exposure to heavy metals can affect reproductive processes in plants in species-specific ways. While some plants may maintain their reproductive success, others may experience challenges such as reduced seed production, impaired germination, or alterations in reproductive structures35. Moreover, heavy metal contamination can significantly influence bee pollinator behaviors, including visitation frequency, foraging duration, and navigation abilities. Pollinators, such as bees, ingest contaminated nectar and pollen, leading to the bioaccumulation of heavy metals in their bodies over their lifespan36,37. Studies on the flower nectar microbiome showed that it changes with the urbanization gradient38. Nectar microbiome composition was reported to be strongly affected by the local species composition of bacteria initially colonizing plants, and by environmental conditions39,40. It can therefore be expected that the influence of environmental conditions will largely shape the communities of microorganisms inhabiting the pistil stigmas.
The study focuses on addressing the following questions related to analysis of the microbiome of the holoparasitic plant O. lutea:
(I) How does environmental pollution affect the microbiome composition and diversity of O. lutea stigmas?
(II) What are the similarities and differences in the microbial composition between immature stigmas from closed flower buds (unexposed to the external environment) and mature stigmas from opened flowers (exposed to the external environment)?
(III) Which culturable bacterial strains inhabiting the stigmas of O. lutea exhibit plant growth-promoting properties, and what are their biochemical performances?
Materials and methods
Plant material and collecting samples
Orobanche lutea Baumg. (Orobanchaceae) is an oligophagous, obligatory, non-photosynthetic, holoparasite that primarily affects plant roots, mainly those of the Fabaceae family, especially species like wild and cultivated Medicago. O. lutea populations are most abundant, among other representatives of the genus, in Europe10,41. O. lutea occurs from Western Europe (France) to Eastern Europe (Ukraine, European Russia). In Central Europe, it is present northward to the Netherlands, Germany, Poland, the Baltic states, and in northern parts of southern Europe, i.e. Italy and northern Greece. O. lutea occurrence also extends in an eastern direction encompassing the Caucasus, Asia Minor, and Central Asia10,42. O. lutea is one of the most abundant representatives of the Orobanche genus in Poland, where it mostly occurs in the belt of the Polish Uplands, i.e., the Silesia-Cracow, Małopolska and Lublin Uplands, rarely in the lower Vistula and Oder valleys and in the Carpathian Foothills41. The plant prefers sunny places like xerothermic communities, grasslands and shrubs located on the slopes of hills, and river valleys. It was also recorded on the margins of forests and fields, in former quarries, baulks, alfalfa fields, on alkaline soils, and in ruderal habitats43,44. O. lutea height ranges from 10 to 60 cm. This species blooms relatively early in the year and the flowering time begins from the end of May to July10,41. The flowers remain opened at night, with the flowering period ranging from a few to several days. The plant develops a single, scaly stem that ends in yellow–brown flowers which are usually purplish towards the margin, and which are bisexual, zygomorphic, and insect pollinated (Fig. 1B, C, F, G). The stigma of this species consists of two lobes and is yellowish, rarely orange (Fig. 1C, D, G, H)10,13,41.
Studied holoparasitic Orobanche lutea. General habit from polluted and unpolluted areas (a, b, e, f); closed and opened flowers (c, g); ZOOM micrographs of two-lobed mature stigmas of O. lutea with numerous papillae covered with a viscous secretion from polluted and unpolluted areas (d1, d2, h1, h2). Scale bars: 1000 µm. Phot. Karolina Wiśniewska.
In May 2021, stigma samples were collected from localities in Poland. A detailed analysis of toxic metal and nutrient concentrations in the polluted soil from Silesia-Cracow Upland and control – unpolluted from the Ponidzie regions can be found in Turnau et al.6. The most important results from this work proved that soil analysis showed large differences in toxic metal concentrations between the polluted and unpolluted localities, especially in Cd and Zn concentrations6. The first location was a heavy metal-polluted site (marked as PO), 330 m above sea level. (a.s.l), in Dąbrowa Górnicza, Ząbkowice district (Silesia-Cracow Upland, Silesian Voivodeship) at the edge a forest by a path (Fig. 1A). This location emerged as a result of calamine mining, and is abundant in heavy metals (soils rich in zinc and cadmium). In Europe, the Silesia-Cracow Upland is recorded as a region with one of the most abundant populations of Orobanchaceae. This region was home to an intensive shallow exploitation of calamine (rich in zinc, lead and silver). Changes in the environment have led to the creation of areas rich in heavy metals, covered by resistant plants forming specific associations, especially favorable for O. lutea41. High levels of nutrients and heavy metal concentrations in soil and in plants from this site have been confirmed in research by Turnau et al.6. The Silesian Voivodeship is characterized by a high level of industrialization and high levels of air pollution (dust and gas pollution). The air pollution is much higher than in any other voivodeship in the country45,46,47. Furthermore, this region has been known for centuries as one of the largest zinc and lead deposits in Europe48,49. The second sampling location was the unpolluted (UPO) legally protected and ecologically valuable area of Ostra Góra, south of Pęczelice (Małopolska Upland) situated on xerothermic grasslands, surrounded by south-facing wastelands and field margins, 240 m a.s.l., with alkaline soils41 (Fig. 1E). Ostra Góra is located within the Ponidzie region, in the mesoregion of the Pińczowski Garb (Świętokrzyskie Voivodeship). It is a gypsum hill and is not situated near industrial areas. Furthermore, in the Świętokrzyskie Voivodeship, no exceedances of the permissible values for cadmium, zinc and other heavy metals (chromium, cobalt, copper, nickel, lead, mercury) have been recorded. Additionally, among the analyzed trace elements, no trend of their accumulation in the surface layer of agricultural soils has been observed over the past 15 years. The content of heavy metals in the soil did not change significantly during the individual years of the study50. Moreover, detailed soil analyses for toxic metals were performed in this region of Ponidzie, from a location 16 km in a straight line from where we took samples for our research, and they also showed low concentrations of toxic metals6. The sampling sites are situated at a distance of 110 km from each other. In these localities, O. lutea parasitizes Medicago falcata L. (Pęczelice) and M. sativa L. (Dąbrowa Górnicza), from which it was sampled.
To describe the climate conditions, monthly mean values of weather parameters (2010–2021) for each location were analyzed, including monthly minimum and maximum temperatures (°C) as well as precipitation (mm). The historical monthly weather data were obtained from WorldClim 2.151, which was downscaled from CRU-TS 4.0652. Detailed data are added in Supplemental file 1: Table S1. The polluted site is characterized by higher annual minimum temperature and higher rainfall compared to the unpolluted site. Over the analyzed period, the average annual minimum temperature ranged from 3.6 to 5.6 °C for the polluted site, whereas for the unpolluted site, it ranged from 3.7 to 5.3 °C. Conversely, the average annual maximum temperature at the unpolluted site was higher than that of the polluted site, ranging from 12.9 to 15.4 °C, while the latter ranged from 12.3 to 15.1 °C. Additionally, the average annual precipitation was greater at the polluted site, varying from 49.6 to 79.5 mm, whereas at the unpolluted site, it ranged from 46.7 to 74.0 mm.
Plant material sampling was conducted with permission no. WPN.I.6400.3.1.2021.AD (Ostra Góra) and WPN.6400.4.2021.MS1.1 (Dąbrowa Górnicza) from the Regional Directors for Environmental Protection in Poland. Experimental research and field studies on plants, including the collection of plant material complied with relevant institutional, national, and international guidelines and legislation, and necessary permits were obtained. O. lutea is partially protected in Poland, and only a limited number of individuals have been authorized to conduct research in this project. In our study, we selected populations from Dąbrowa Górnicza and Ostra Góra, which are among the largest in terms of numbers in Poland. The voucher specimen was stored in the Herbarium of Jan Kochanowski University in Kielce (KTC), Poland (acronym according to Thiers53). The collection is not numbered, but the herbarium sheets are stored in a separate section under the name “Parasitic plants”, and the species are sorted alphabetically, in boxes labeled with the species name. Renata Piwowarczyk performed the plant material identification, while both Renata Piwowarczyk and Karolina Wiśniewska were involved in the plant collection. The plant names were updated based on The International Plant Names Index (IPNI)54.
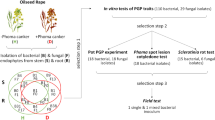
The flowers of O. lutea were carefully placed inside sterile plastic tubes. Two types of samples were collected, samples with closed petal flowers (immature stigmas, IM) and fully opened (mature stigmas, MA). These samples were stored at a temperature of 4 ± 0.5 °C until the analysis, within 24 h. Each sample was approximately 1 g and contained 250 stigmas from fully opened flowers (mature stigmas, MA) and 250 stigmas from closed petal flowers (immature stigmas, IM). The stigmas were dissected using sterile tools, immediately placed into plastic tubes, and stored at -80 ± 0.5 °C for culture independent microbial analysis, using Next Generation Sequencing (NGS). The pistils were removed as close to the stigma’s base as possible (Fig. 1D, H). For the purpose of NGS analysis, to assess microbiome composition a total of nine samples was analyzed: three samples containing O. lutea immature stigmas were collected from the polluted area (marked since as PO IM1-3), three samples containing mature stigmas from the polluted area (marked since as PO MA1-3), and three samples from the unpolluted area, one containing immature stigmas (UPO IM1), and two samples containing mature stigmas (UPO MA1-2). The nine samples were submitted for molecular analyses by NGS. At the same time, to assess the diversity and species composition of culturable bacteria inhabiting stigmas of O. lutea, four separate samples of stigmas were collected from the same sampling site. The four samples comprised: immature stigmas from closed flowers from the polluted area (PO IM), mature stigmas from opened flowers from the polluted area (PO MA), immature stigmas from closed flowers from the unpolluted area (UPO IM), and mature stigmas from opened flowers from the unpolluted area (UPO MA). Each sample comprised 1 g of stigmas (Fig. 1D, H).
Obtaining culturable strains of bacteria and their characterization
The collected samples of stigmas of O. lutea, both mature and immature, were homogenized in a sterile physiological buffer (0.97% NaCl). Next, aliquots of 0.5 mL of homogenate of each sample were streaked on the Tryptic Soy Agar medium (TSA) (Sigma Aldrich, Poland). The grown colonies were submitted to a series of reductional streaks until pure cultures were obtained, which was confirmed under an optical microscope, after Gram staining. Each pure culture, from now on referred to as a strain, was identified biochemically and molecularly. Biochemical identification was done using the BIOLOG® Gen III system (v. 2.8 database) (BIOLOG®, Hayward, USA) following the manufacturer’s instructions. Molecular identification was performed based on the analysis of 16S rDNA partial nucleotide sequences of a length of 1500 bp, using the following primers: 16SA1 (AGAGTTTGATCMTGGCTCAG) and 16SB1 (TACGGYTACCTTGTTACGACTT) based on the method described55. The pure bacterial cultures were streaked on tryptic soy agar medium (TSA) (Caso Broth 30 g (Sigma Aldrich, Merck), agar 15 g (Difco)). After 24 h of cultivation, single colonies were selected, resuspended in 100 µl of sterile distilled water, and incubate at 90 ℃ for 10 min. After cooling, the solution was centrifuged for 10 min at 10,000 rcf. One microliter of the supernatant was used as the template in the PCR reaction, utilizing the GoTaq® Green Master Mix (Promega, Madison, Wisconsin, USA). The proper size PCR products (1500 bp) were sequenced and the nucleotide sequence of 16S rRNA region of each identified strain was submitted to the GenBank database, where accession numbers were assigned.
Plant growth promoting characteristics of culturable bacteria
The tested strains were examined for their plant growth promoting activity. The occurrence of biochemical traits considered to be plant growth promoting were assessed. The following biochemical tests were conducted: the production of hydrogen cyanide, cellulase, auxin, lipase, protease, siderophores, and ammonia, as well as motility, phosphate solubilization, and salt tolerance. Each test was repeated twice. The methodology of each test is described below.
Hydrogen cyanide production
The production of hydrogen cyanide (HCN) was assessed using a colorimetric method involving filter paper. A 100 µl aliquot of bacterial suspension, cultivated on Tryptic Soy Agar, was inoculated onto nutrient agar medium (Difco, Poland) supplemented with 4.4 gL-1 glycine. Filter papers were impregnated with a reagent solution (2% sodium carbonate, 0.5% picric acid) and affixed to the inner lid of Petri dishes. To prevent volatilization, plates were sealed with Parafilm and then incubated at 28 ºC for 4 days. A control plate, without bacterial inoculation, was included for comparison. HCN production was determined by observing color changes in the filter papers, transitioning from yellow to cream, light brown, dark brown, or reddish brown. To semi-quantitatively assess HCN production among bacterial isolates, scores ranging from 0 to 4 were assigned based on the observed color changes. Isolates displaying yellow, cream, light brown, and dark brown colors were assigned scores of 0 (no ability), 1 (low ability), 2 (medium ability), 3 (high ability), and 4 (very high ability), respectively56.
Cellulase production
For the evaluation of growth and cellulase production on solid media, the following procedure was implemented. After 48 h of incubation, the dimensions of colonies grown on Tryptic Soy Agar medium were measured. Subsequently, the surface of the medium was flooded with a 1% (w/v) aqueous solution of hexadecyltrimethylammonium bromide57. This reagent facilitated the precipitation of undegraded carboxymethyl cellulose (CMC) resulting in the formation of a transparent zone where CMC had undergone degradation, occasionally accompanied by a region of concentrated precipitation. The dimensions of this zone were measured, and the ratio of the zone’s size to the colony diameter was calculated57.
Auxin production
The assessment of auxin production by PGPR strains followed the methodology outlined by Patten and Glick58. Each bacterium was cultured in nutrient broth medium (beef extract 1 g, peptone 5 g, yeast extract 2 g, and sodium chloride 5 g), adjusted to a pH of 6.8, and then incubated at 28 °C for 48 h in a shaker incubator. Subsequently, 50 μL of each bacterial suspension was transferred to a nutrient broth containing 50 μg/mL tryptophan. After an additional 48 h of incubation, the suspensions were centrifuged at 10,000 rpm for 10 min. Following centrifugation, 1 mL of the supernatant was mixed with 4 mL of Salkowski reagent (comprising 2 mL of 0.5 mol L-1 FeCl3 and 98 mL of 35% HClO4). After a 20-min incubation period, samples exhibiting a red color were considered positive, and the absorbance of the mixture was measured at 535 nm using a spectrophotometer56.
Motility
The motility test was conducted by preparing motility agar in Petri dishes. The preparation procedure involved dissolving 10 g of tryptone, 5 g of sodium chloride, and 5 g of agar in 1 L of water, followed by adjustment of the pH to 6.8–7.0. The resulting sterile semi-solid medium was inoculated by inserting a stab into the center of each plate. Incubation was performed, and observations were recorded at 24 and 48 h post-inoculation. The presence of motility was indicated by the formation of a diffuse zone of growth emanating from the line of inoculation. The spread of growth could encompass the entire medium or originate from one or two specific points59.
Phosphate solubilization
The Phosphate-Solubilizing Microorganisms (PSM) were characterized on Pikovskaya’s agar medium (PVK). For each strain 50 µl of bacterial suspension (1.3 × 106 cfu/mL) was carefully transferred onto circular filter papers positioned on Pikovskaya’s agar medium (PVK), comprising glucose (10 g), yeast extract (0.5 g), (NH4)2SO4 (0.5 g), MgSO4·7H2O (0.1 g), Ca3(PO4)2 (5 g), NaCl (0.2 g), KCl (0.2 g), MnSO4·2H2O (0.002 g), FeSO4·7H2O (0.002 g), and agar (15 g), with a pH adjusted to 7.0. The inoculated plates were then incubated at 27–30 °C for a duration of 7 days. Positive phosphate solubilization was indicated by the presence of colonies surrounded by halo zones60.
Lipase production
The bacteria were cultured on a medium composed of peptone (10 g), CaCl2 (0.1 g), NaCl (5 g), agar (15 g), and sterile Tween 20 (10 mL), with the pH adjusted to 7.0, and incubated at 27 °C for 48 h. The presence of depositions encircling the bacterial colonies indicated lipase enzyme activity. Subsequently, scores ranging from 0 to 4 were assigned to evaluate the level of lipase production, with 0 representing no ability, 1 denoting low ability, 2 indicating medium ability, 3 representing high ability, and 4 signifying very high ability56.
Protease activity
The evaluation of protease enzyme activity was performed according to the protocol described by Maurhofer et al.61 cited by Ghodsalavi et al.56. Bacterial samples were inoculated onto plates containing SMA medium (skim milk 15 g, yeast extract 0.5 g, agar 9 g, pH 7.0) and subsequently incubated at 27 °C for 48 h. The presence of colorless halo zones surrounding the bacterial colonies served as an indicator of protease production ability56.
Fluorescent siderophore production
The investigation of siderophore production was conducted using succinate medium composed of succinic acid (4 g), K2HPO4 (6 g), KH2PO4 (3 g), (NH4)2SO4 (1 g), MgSO4 (0.2 g), and agar (15 g), adjusted to a pH of 7.0. Siderophore production in succinate media can be confirmed by the structural features of pyoverdines, wherein the three amino moieties of the chromophore are substituted with various acyl groups derived from succinate, malate, and α-ketoglutarate. After 48 h of incubation at 28 °C, bacterial colonies grown on the media plates were transferred under a UV lamp to observe fluorescence. This fluorescence is indicative of siderophore production62.
Salt tolerance
Salt tolerance was assessed using a 1% proteose peptone solution supplemented with varying concentrations of sodium chloride. A 20 μL aliquot of an overnight test culture was inoculated into the medium containing 1% proteose peptone and different salt concentrations (0.0%, 5%, 10%). After incubation for 24 h, the absorbance of the culture was measured at 600 nm, with uninoculated medium serving as a blank63.
Ammonia production
The evaluation of ammonia production was performed as previously described64. A 20 μL aliquot of an overnight-grown test culture was inoculated into 5 mL of 1% proteose peptone broth and then incubated at 30 °C in a shaking water bath. Following 24 h of incubation, 0.5 mL of Nesseler’s reagent was added to the culture, and any observed color change was recorded. A positive result was indicated by the appearance of a yellow coloration, with the intensity of the color correlating with the quantity of ammonia produced by the test strain.
Sequencing
The composition and temporal dynamics of microbiome of stigma of O. lutea was assessed by using a high throughput Next Generation Sequencing (NGS) technique. The molecular analyses were determined according to previous work23,24. DNA extraction and PCR amplification were carried out by A&A Biotechnology (Poland), while Macrogen (Netherlands) handled NGS library preparation and performed Illumina and Sanger sequencing for the 16S rRNA and ITS products. The microbial populations inhabiting the studied samples were investigated by sequencing the V3-V4 region of the 16S rRNA gene and the ITS region. The gene segments were amplified using PCR primers designed for the Illumina methodology as suggested. Primers ITS3F (GCA TCG ATG AAG AAC GCA GC) and ITS4R (TCC TCC GCT TAT TGA TAT GC) for the fungal ITS library, 341F (CCT ACG GGNGGC WGC AG) and 805R (GAC TAC HVGGG TAT CTA ATC C) for bacterial 16S rRNA libraries were employed. The quality of the library was checked according to the Illumina qPCR Quantification Protocol Guide using the 2200 TapeStation System (Agilent, United States). Finally, sequencing was performed on an Illumina MiSeq PE300 platform. Each file underwent quality control (QC), which included quality filtering (removing sequences with ≥ 5 ambiguous base pairs) and length filtering (removing sequences with a length ≥ 2 standard deviations from the mean). Sequences below three reads (singletons) or with abundance less than 0.0005% were removed after generating the ASV table. Illumina metagenomic datasets were submitted to NCBI’s Sequence Read Archive (SRA) under the project number PRJNA1092550. The results were obtained using QIIME65 and Pipeline based on the SILVA66 or UNITE67 databases.
Statistical calculation and data analysis
Domination classes were established based on prior research in Ruraż et al.23,24. Microorganism diversity in samples was determined with the use of the Shannon diversity index, Pielou’s evenness index, and Simpson’s dominance index. Agglomerative Hierarchical Clustering (AHC) was performed using Bray–Curtis distance and the Wards agglomeration method. The accuracy of the non-metric multidimensional scaling (nMDS) representation was evaluated through the Kruskal stress. The tests for microbial community composition dissimilarity between groups (localization, the stage of stigma development and both of them) were performed using a nonparametric permutational multivariate ANOVA (PERMANOVA) with the adonis function (Adonis). These calculations were conducted using XLSTAT and XLSTAT-R Vegan68. The PCA was performed with PAST 4.0369.
Results
Identification and plant growth promoting potential of culturable strains of bacteria obtained from stigmas of Orobanche lutea
The culturable strains of bacteria obtained from stigmas of O. lutea samples were identified using both biochemical and molecular methods. Among the 25 tested strains, the following bacterial species were distinguished: Acinetobacter guillouiae, A. septicus, Erwinia persicina, E. rhapontici, Micrococcus aloeverae, M. luteus, Oceanobacillus sojae, Pantoea agglomerans, Pseudomonas canadensis, P. fluorescens, P. helmanticensis, P. trivialis, Serratia liquefaciens, S. myotis, Staphylococcus caprae, S. epidermidis, and S. warneri (Table 1).
The plant growth promoting potential of tested culturable strains was assessed by determining the occurrence of biochemical traits reported as plant growth promoting. The sample containing the immature stigmas of the unpolluted area (UPO IM) was the least abundant in bacterial morphotypes involving only two strains (3A1D, 3B-1D). The other three samples were more abundant on bacterial morphotypes whose number was between 7 to 9 (Table 1). Of the 25 strains tested, two strains (2A-1D and 2A4D1) were reported to produce cellulase (Table 2, Additional file 2: S3). No strain was able to produce auxin (Table 2, Additional file 2: S4). Six strains were reported as motile (2A-1D, 2A4D1, M3, M4, M8, M9-1) (Table 2, Additional file 2: S5). Eight strains were able to phosphate solubilization (3A1D, 4A-1D, 4A-2D, M1, M2, M3, M4, M5) (Table 2, Additional file 2: S6). Six strains expressed an ability for protease production (2A-1D, 4A3D, M3, M4, M8, M9-1) (Table 2, Additional file 2: S7). Two strains (4A3D2, M4) produced fluorescent siderophore (Table 2, Additional file 2: S8). Two strains (2A4D1, M9-1) were able to produce ammonia (Table 2, Additional file 2: S9). Three strains expressed a high or very high ability to produce lipase (2A-1D, 2A4D1, M3) (Table 2, Additional file 2: S10). Three strains (M2, M3, M4) were reported to be of high hydrogen cyanide (HCN) production ability (Table 2, Additional file 2: S11). And six strains (3A1D, M1, M4, M8, M9-1, M12) showed high tolerance for salinity (10% NaCl) (Table 2, Additional file 2: S12).
Five out of the 25 tested strains (19%) (2A-1D, 2A4D1, M3, M4, M9-1) exhibited 3 or 4 biochemical traits considered to be plant growth promoting. Nine of the 25 tested strains (35%) (3A1D, 4A-1D, 4A-2D, 4A-3D1, 4A3D2, M1, M2, M5, M8) exhibited 1 or 2 plant growth promoting biochemical traits and the remaining 12 strains (46%) (1A-1D, 1B-1D, 2A-2D, 2A3B, 3B-1D, M6, M7, M-9–2, M10, M12, M14, M16) exhibited no plant growth promoting biochemical traits (Table 2, Additional file 2: S3-S12).
The characteristics of microbial communities of Orobanche lutea pistil stigmas
Based on molecular analysis, it was found that the tested stigmas of O. lutea were colonized by one dominating bacterial type, Proteobacteria (99.25%). Within Proteobacteria, the most abundant representatives were the families Enterobacteriaceae (49.88%) and Pseudomonadaceae (48.28%). The remaining groups constituted less than 0.5% for each, i.e. Firmicutes, Bacteroidetes and Actinobacteria. A detailed examination of sequences of all obtained reads revealed the presence of 11 operational taxonomic units (OTUs). Within the obtained OTUs, the presence of Pseudomonas spp. (47.54%) and Pantoea spp. (31.76%) was most abundant on O. lutea stigmas (Table 3).
The dominant fungal phylum was Basidiomycota (71.64%) followed by Ascomycota (27.53%). Basidiomycota was dominated by the Filobasidiales (33.14%) and Tremellales (27.27%) orders. In Ascomycota, Dothideales predominated (24.01%). Among the 16 OTUs recorded, the most abundant fungal taxonomical units were Filobasidium spp. (33.13%), followed by Vishniacozyma spp. (25.34%), and Aureobasidium spp. (23.94%), which were recorded as a eudominant or dominant. These microorganisms together with the recorded Mrakiella spp. (6.01%), Cystofilobasidium spp. (3.92%), and Bullera spp. (1.35%) constituted 93.69% of the entire composition (Table 4).
The microbiological composition of pistil stigmas at polluted and unpolluted sites
The occurrence of the two most abundant bacteria inhabiting the stigmas of O. lutea at the polluted and unpolluted sites was record different. Namely, Pseudomonas spp. at the polluted site was 54.52 vs. 35.02% at the unpolluted site, while Pantoea spp. was 19.50 vs. 56.18%, respectively. The abundance of other recorded OTUs, namely Enterobacteriaceae (18.01 vs. 5.32%) and Pseudomonadaceae (4.76 vs. 2.27%), at the polluted site was three or two times higher than in unpolluted area. In the polluted location, microorganisms were noted, which did not occur at the unpolluted site, or occurred very rarely, such as Moraxellaceae (1.28 vs. 0.01%), Paenibacillus spp. (0.67 vs. 0.00%), and Pedobacter spp. (0.48 vs. 0.00%). At the unpolluted site, OTUs like e.g. Plesiomonas spp. were reported to occur more frequently (0.09 vs. 0.64%) (Table 3).
The diversity of fungal communities inhabiting the stigmas of O. lutea obtained from the tested polluted and unpolluted sites was more diverse in comparison with the bacterial profile. The abundance of the two most numerous fungi, i.e. Filobasidium spp. (from 30.91 to 38.29%) and Aureobasidium spp. (from 15.91 to 38.99%), at the unpolluted site increased compared to Vishniacozyma spp., the amount of which at the unpolluted site decreased more than four times (from 34.65 to 8.21%). In relation to the next two identified OTUs, the frequency of Mrakiella spp. at the unpolluted site was higher (5.38 vs. 6.84%), and the amount of Cystofilobasidium spp. noticeably decreased (from 5.14 to 1.37%). It is also interesting that the amount of Bullera spp. and Alternaria spp. at the studied sites was at a similar level, but also opposite, 1.93 vs. 0.15% and 0.13 vs. 1.93%, respectively. The polluted site was characterized by the presence of higher amounts of Itersonilia spp. (1.20 vs. 0.17%), Cladosporium spp. (0.80 vs. 0.44%), Dioszegia spp. (0.55 vs. 0.02%), and Starmerella spp. (0.48 vs. 0.00%). However, in the case of Mycosphaerella spp. (0.53 vs. 0.89%) and Didymella spp. (0.05 vs. 0.41%), a higher frequency was recorded at the unpolluted site (Table 4).
The microbiological composition of pistil stigmas at different stages of flower development
The examination of microbial composition on different stages of development of stigmas from polluted and unpolluted locations revealed similarity in the amount of Pseudomonas spp. (46.25% for immature vs. 43.28% for mature stigmas) and Pantoea spp. (36.89 vs. 38.79%). In immature stigmas, the frequency of other OTUs recorded Pseudomonadaceae (from 1.30 to 5.73%), Moraxellaceae (from 0.06 to 1.23%), and Paenibacillus spp. (from 0.12 to 0.55%) was higher as opposed to Enterobacteriaceae (from 14.96 to 8.38%), the abundance of which decreased by half. The mature stigmas were characterized by a higher number of identified OTUs, as reflected by the fact that their share in the four most numerous bacteria was 96.18% compared to 99.40% in immature stigmas. Plesiomonas (0.73%) and Pedobacter (0.49%) were recorded only in mature stigmas (Table 3).
Analysis of the fungal microbiome at different stages of pistil stigma development showed a higher abundance of Filobasidium spp. (40.13 vs. 29.07%) and Vishniacozyma spp. (24.41 vs. 18.44%) in immature stigmas. However, in mature stigmas, higher amounts of Aureobasidium spp. were recorded (23.22 vs. 31.68%), also in Mrakiella spp. (4.25 vs. 7.97%), as well as Cystofilobasidium spp. (2.96 vs. 3.55%). The abundance of Bullera spp. (1.11 vs. 0.97%) and Cladosporium spp. (0.64 vs. 0.60%) remained comparable. For the remaining OTUs, Alternaria spp., Itersonilia spp., Dioszegia spp., and Mycosphaerella spp., their abundance was reported to have increased in mature stigmas. Single OTUs were recorded only in mature stigmas, e.g. Starmerella spp. and in immature stigmas, Didymella spp. (0.41 vs. 0.05%) (Table 4).
The microbiological composition of pistil stigmas at polluted and unpolluted sites at different stages of flower development
In immature stigmas, the content of the three dominant OTUs recorded for bacteria varied depending on the pollution occurrence at the sampling site. In the polluted area, the abundance of Pseudomonas spp. (53.22 vs. 39.28%) and Enterobacteriaceae (27.37 vs. 2.54%) was higher compared to Pantoea spp. (17.86 vs. 55.93%). However, the value of Pseudomonadaceae was at a quite similar level (1.10 vs. 1.51%). In the polluted area, the immature pistil stigmas were also characterized by the occurrence of the low abundance of microorganisms not recorded in the unpolluted area, i.e. Moraxellaceae (0.13%) and Paenibacillus spp. (0.24%). In turn, the value of Gammaproteobacteria (0.05 vs. 0.73%) increased in stigmas from the unpolluted area (Table 3).
In mature stigmas sampled at the polluted site, we observe a higher abundance of Pseudomonas spp. (55.82 vs. 30.75%) and Pantoea spp. (21.15 vs. 56.44%) in comparison to the unpolluted site. A similar value for both locations was found for OTU Enterobacteriaceae (8.65 vs. 8.11%). The value of the fourth most abundant OTU, Pseudomonadaceae, in mature stigmas from the unpolluted area, was three times lower (8.43 vs. 3.03%). For bacteria such as Moraxellaceae, Paenibacillus spp., and Pedobacter spp., we observed their occurrence only in the polluted area or negligible amounts also in the unpolluted area. However, at the unpolluted site, a higher frequency of Plesiomonas spp. (0.17 vs. 1.28%) was recorded (Table 3).
In fungal microbiome analysis, a similar abundance of Filobasidium spp. was recorded in immature stigmas sampled at both the polluted and unpolluted sites (39.82 vs. 40.44%). The next two most abundant fungal OTUs were recorded in an inverse relationship. Aureobasidium spp. (10.48 vs. 35.96%) was recorded as more abundant in the unpolluted area and Vishniacozyma spp. (36.13 vs. 12.69%) as more abundant in the polluted area. In immature stigmas from the polluted area, higher abundance was also recorded for Cystofilobasidium spp. (4.83 vs. 1.09%), Bullera spp. (2.22 vs. 0.01%), Itersonilia spp. (0.53 vs. 0.00%), as well as Cladosporium spp. (1.19 vs. 0.09%). Abundance of Mrakiella spp. (2.96 vs. 5.53%), Alternaria spp. (0.10 vs. 1.29%), Mycosphaerella spp., (0.21 vs. 0.71%) and Didymella spp. (0.01 vs. 0.81%) was higher in the unpolluted area (Table 4).
Mature stigmas from the unpolluted area were characterized by a higher abundance of Filobasidium spp. (22.00 vs. 36.13%) and Aureobasidium spp. (21.34 vs. 42.03%). However, the value of Vishniacozyma spp. was almost nine times higher in stigmas from the polluted area (33.16 vs. 3.73%). A similar amount was recorded for Mrakiella spp. at both sites (7.79 vs. 8.14%). For the rest of the OTUs, higher values of abundance were recorded from the polluted area for Cystofilobasidium spp. (5.45 vs. 1.66%), Bullera spp. (1.65 vs. 0.28%), Itersonilia spp. (1.86 vs. 0.34%), Dioszegia spp. (0.73 vs. 0.02%), and Starmerella spp. (0.95 vs. 0.00%). The abundance of Alternaria spp. (0.15 vs. 2.57%), Mycosphaerella spp. (0.85 vs. 1.07%) and Cladosporium spp. (0.41 vs. 0.80%) was higher in the unpolluted area (Table 4).
Changes in the ecology of tested microbial communities
For the NGS tested microbiome, based on the calculated statistical coefficients, noticeable alterations in the ecology of the tested microbial communities were observed. The Shannon diversity index achieved the highest value in mature stigmas from the polluted site (PO MA) for both bacteria (H ‘ = 1.21) and fungi (H ‘ = 1.82). In immature stigmas from both sites, the index reached very similar values for bacteria (H ‘ = 0.90 vs. 0.89) and fungi (H ‘ = 1.44 vs. 1.42), which cannot be stated in the case of mature stigmas. Interestingly, it was noticed that there was a greater difference in the value of this indicator between the stages of stigma development in the case of the polluted site (Tables 3, 4). The noticeable difference was observed based on the dominance index. For the polluted site, this index reached higher values in immature stigmas for both bacteria (λ = 0.51 vs. 0.47) and fungi (λ = 0.32 vs. 0.31). In turn, in the unpolluted habitat, the value of this index was higher in mature stigmas (λ = 0.34), although the differences are not that clear. For bacteria, the Pielou index reached higher values in samples from immature stigmas from both sites (J ‘ = 0.20 and 0.30), while when analyzing fungi, the value of this index was at a similar level with the highest value in the case of immature stigmas from the unpolluted area (J ‘ = 0.13) (Tables 3, 4).
Regarding the AHC analysis of bacteria, results were less ordered, where one of the two clades consisted of samples from different sites and stages of pistil stigma development (PO IM2 and UPO MA1). The second clade was represented by two groups. In the first group consisting of three subgroups, two from the unpolluted area were similar to each other, i.e. UPO IM1 and UPO MA2, next to PO MA2. The second group was represented by samples from one site (polluted area) but with different degrees of stigma development. These samples consisted of a group with a proportionally increasing dissimilarity consisting of immature stigmas (PO IM1, PO IM3) to mature stigmas (PO MA1, PO MA3) (Fig. 2). In contrast, in AHC analysis of fungi the results showed well-grouped samples, with three samples forming an individual clade corresponding to the unpolluted area (UPO). A separate group was formed by the samples UPO MA1 and UPO IM1 next to the single UPO MA2. In the case of the second clade, the samples were grouped into smaller subgroups according to the stage of pistil stigma development from the polluted site, i.e. PO IM1-IM3 and PO MA1-MA3 (Fig. 3).
Dendrogram of AHC (Agglomerative hierarchical clustering) results showing dissimilarity between analyzed bacterial samples of immature stigmas Orobanche lutea from closed flowers (IM1, IM2, IM3) and mature stigmas from opened flowers (MA1, MA2, MA3) from polluted (PO) and unpolluted areas (UPO) (on the left); Non-metric multidimensional scaling (nMDS) plot of bacterial community structure from each sample (on the right).
Dendrogram of AHC (Agglomerative hierarchical clustering) results showing dissimilarity between analyzed fungal samples of immature stigmas Orobanche lutea from closed flowers (IM1, IM2, IM3) and mature stigmas from opened flowers (MA1, MA2, MA3) from polluted (PO) and unpolluted areas (UPO) (on the left); Non-metric multidimensional scaling (nMDS) plot of fungal community structure from each sample (on the right).
Similarly, the results of nMDS analysis showed that fungal communities were clearly separated among polluted (PO) and unpolluted areas (UPO) groups, also taking into account the stage of development of stigmas. In the case of bacteria, no such division is visible (Figs. 2, 3). Moreover, a dissimilarity test using Adonis further confirmed significantly (P < 0.05) different structures of microbial communities (both for bacteria and fungi) with different location of samples (Tables 3, 4).
The results of a PCA biplot enabled the differentiation of microorganism compositions between immature and mature stigmas of both O. lutea samples from the unpolluted and polluted sites (Fig. 4). For bacteria, the first two components (PC1 (44.34%) and PC2 (16.08%)) accounted for a high proportion of the initial variability of the data, i.e., 60.42%. PC1 and PC2 represented up to 57.82% of the total variance of the observations (32.17% and 25.65%, respectively) for fungi. High values of Betaproteobacteria, Moraxellaceae, Pedobacter and Sphingomonas genera, with diversity indeces, were noted in mature stigmas of the polluted site (PO MA1). Moreover, in those samples we noted the genera Paenibacillus, as well as Pseudomonas, which were positively correlated. The higher abundance of Pseudomonadaceae were corelated with mature stigmas from the unpolluted (UPO MA2) and polluted sites (PO MA2). No clear relationships were observed in the case of immature stigmas at the polluted site (PO IM1-IM3). In turn, the domination and evenness indicators were positively correlated with immature stigmas from the unpolluted site (UPO IM1), and a higher value of Pantoea and Plesiomonas genera, as well as Enterobacteriaceae, were noted at this site in immature (UPO IM1) and mature stigmas (UPO MA1). The variant of O. lutea immature stigmas sample (PO IM1) was positively correlated with Bullera and Vishniacozyma spp.. The samples of mature stigmas from the polluted area (PO MA1-MA3) were positively correlated with the number of OTUs and with the Shannon diversity index, as well as with Cystofilobasidium, Dioszegia, Itersonilia, Starmerella genera. In turn, the higher abundance of Alternaria, Aureobasidium, Filobasidium genera, and the domination index were correlated with the mature stigma samples from the unpolluted area (UPO MA1-MA2). A negative correlation was observed between PO MA1-MA3 and UPO MA1 results (Fig. 4).
PCA showing overall relationships between microbial communities and diversity indices in analyzed bacterial (on the top) and fungal (at the bottom) samples of immature stigmas Orobanche lutea from closed flowers (IM1, IM2, IM3) (green) and mature stigmas from opened flowers (MA1, MA2, MA3) (orange) from polluted (PO) (squares) and unpolluted areas (UPO) (dots).
Discussion
Our research focused on the microbiological analysis of pistil stigmas of the Orobanche plant sampled from the polluted and unpolluted areas where O. lutea forms numerous populations41. Each tested sample of stigmas contained both bacterial strains exhibiting plant growth promoting biochemical qualities and the bacterial strains displaying no such activity. The biological role of the latter bacteria remains unknown. The bacteria that did not exhibit plant growth-promoting (PGP) traits in the microbiome of O. lutea stigmas may still play critical ecological roles, contributing to the functional diversity of the microbial community. Bacteria play various roles in the ecosystem, and their presence on a plant’s flower stigma can have both beneficial and detrimental effects. Some bacteria can aid in pollination by attracting pollinators to the flower. For instance, certain bacteria produce fragrances or volatile compounds that attract insects or other animals, thereby increasing the chances of pollination70. Certain bacteria living on the stigma can protect the plant from pathogenic microorganisms (Pseudomonas canadensis) by outcompeting them for space and resources or by producing antimicrobial compounds that inhibit the growth of harmful pathogens. This can help prevent diseases that could otherwise damage the reproductive structures of the plant71. Bacteria on the stigma can participate in nutrient cycling by breaking down complex organic compounds (Acinetobacter guillouiae), into simpler forms that the plant can absorb and utilize for growth and development, as takes place in the soil, where this nutrient cycling is essential for maintaining soil fertility and overall plant health72. However, some bacteria on the stigma can also have detrimental effects, such as causing diseases like bacterial blight or bacterial wilt, which can lead to the wilting or death of the flower or the entire plant (Erwinia persicina)73. Additionally, some bacteria are known to cause disease symptoms in other plants (Staphylococcus warneri). Overall, the role of bacteria on a plant’s flower stigma can vary depending on the specific species present and environmental conditions (Pantoea agglomerans), but they can have significant impacts on plant health, pollination, and ecosystem functioning. Furthermore, the coexistence of PGP and non-PGP bacteria could indicate a synergistic relationship, where non-PGP bacteria facilitate the activity of PGP strains through complementary metabolic functions or by modifying environmental stressors18,19. Not much is known about the microbiome of the stigma of Orobanchaceae flowers, however our study corresponds to the studies of the Cistanche seed microbiome, because we detected the presence of PGP endophytes of Pantoea and Bacillus genera (Tables 1, 2), just like it was done for the seed microbiome of Cistanche18. Also, we detected the presence of bacterial strains highly tolerant to saline (10% NaCl, OD600 > 3.0, Table 2). Salt tolerant bacteria were detected also in Cistanche seeds19. Based on these results, we may assume that there might be a connection between the stigma microbiome and seed microbiome of parasitic plants, however further tests are required to confirm this assumption. Also, it was showed that the urbanization gradient affects the microbiome of the nectar flower38. Moreover, the species composition of the nectar microbiome was reported to be strongly affected by environmental conditions and by the composition of bacterial species originally colonizing plants, the endophytes39,40. This is in congruence with our findings which were that the Shannon diversity index analysis showed that polluted site was characterized by the presence of greater diversity than unpolluted site among both groups of microorganisms (for bacteria 1.06 vs. 0.93 and for fungi 1.63 vs. 1.44) (Tables 2, 3). Additionally, greater diversity occurs in the case of mature stigmas compared to immature ones (for bacteria 1.21 vs. 0.9 and for fungi 1.82 vs. 1.44) (Tables 2, 3) from the polluted site. We also confirmed the presence of a greater diversity of fungi (1.44 and 1.63) than bacteria (0.93 and 1.06) (Tables 2, 3) for both the unpolluted and polluted sampling sites. In previous studies on microorganisms inhabiting the stigmas of Orobanche alsatica, O. bartlingii and Phelipanche arenaria23,24, we also observed a greater diversity of bacteria and fungi in mature stigmas and an overall greater diversity among fungi. Once again, in the microorganism profile we observe that bacteria reach higher abundance in samples, while fungi were a more diverse group comprising less common taxa (Tables 3, 4). The PCA revealed a distinct correlation between selected microorganisms and indicators, particularly evident in the fungi analysis, depending on the stage of pistil stigma development and location. The highest values of the diversity index were observed among microorganisms, both bacteria and fungi, colonizing mature stigmas from the polluted area, contrasting with the dominance index. Furthermore, the results of the PCA were largely corroborated by the agglomerative hierarchical clustering (AHC) (Figs, 2,3,4).
Next to fungi, bacteria are crucial for the proper functioning of plants. It has been proved that bacteria promote plant growth by interacting with plant host tissues. The first and obligatory stage of plant growth promotion is colonization of the plant`s tissue. The colonization is possible because bacteria display several biochemical traits that have been recorded as plant growth promoting. These features include motility74,75, polysaccharide production, dissolution of inorganic phosphates, and production of protease, lipase and chitinase enzymes facilitating rhizosphere colonization76, production of indole-acetic-acid (IAA)77, production of siderophores78,79 including the fluorescent siderophore, pyoverdine80, phosphate dissolution81, and indole acetic acid and ACC deaminase production79. Endophytic bacteria occur in specific tissues of the plant like the root cortex or xylem, where they exchange nutrients with plants, with the aid of the enzymes like lipase, catalase, oxidase, and functional agents like siderophores and biosurfactants, which they produce. However, nothing is known about the composition and abundance of both culturable and NGS-detected bacteria in the O. lutea plants, especially within the stigma, which can be crucial for the functioning of this parasitic plant. This plant’s life strategy involves the production of a large number of seeds per plant (over 600,000) and a long seed viability in the soil of up to 20 years82,83. Among the tested samples, immature stigmas from the unpolluted area (UPO IM) were the least abundant in culturable bacterial morphotypes involving only two strains (3A1D, 3B-1D) in contrast to the other three samples that contained from 7 to 9 morphotypes each (Table 2). None of the tested bacterial strains were capable of producing auxins (Table 2), regardless of heavy metal pollution level and maturity of stigmas. It is possible that the bacteria producing auxin are not required on stigmas of Orobanche. On the other hand, bacteria producing cellulose were detected only in mature stigmas from the polluted area (PO MA) (Table 2). A possible explanation for this is that the bacteria on the stigma can participate in nutrient cycling by breaking down organic matter into simpler forms that the plant can absorb and utilize for growth and development. This nutrient cycling is essential for maintaining soil fertility and overall plant health72. However, cellulase degradation can occur on the stigma as a potential element of defense of the plant against biofilms created by other, e.g. plant pathogenic bacteria. In contrast, of the tested plant growth promoting (PGP) biochemical qualities, only phosphate solubilization was exhibited by the bacterial strains in virtually every sample tested. The other PGP qualities like protease and lipase production, and motility were detected in every sample tested, excluding immature stigmas from the unpolluted area (UPO IM) (Table 2). In the case of the tested culturable bacteria, the specific relationship between environmental pollution of the sampling site and the composition of bacterial species was not so clear (Table 1). We also found no relationships between the frequency of occurrence of plant growth promoting biochemical traits in the tested bacteria, the pollution level of sampling site and the level of stigma maturity. In all cases, we observed bacteria exhibiting multiple plant growth promoting biochemical traits, as well as bacteria displaying no such activity (Table 2). This shows that the role of both groups of bacteria in the microbiome of O. lutea is still unknown.
Flowers have assumed the main role in terms of reproductive success and eventual expansion of these species under suitable circumstances through seed production. The abundant quantity, lightweight nature, effortless dissemination, and prolonged viability in soil of Orobanche seeds are the features that led to this84. So far, rare and endangered plants, including parasitic plants, have not been the subject of many microbiological studies concerning the composition of species colonizing the microbiome of this group of plants. The microbial populations inhabiting flowers (anthosphere) exhibit clear distinctions from those residing in leaves (phyllosphere) and roots (rhizosphere). Despite these differences, many members are shared among these various environments26. We are in the early stages of comprehending the extensive range and variety of microorganisms in the stigmas, but certain trends are becoming apparent. Research examining various plant types has identified consistent bacterial groups; for instance, bacteria belonging to the genus Sphingomonas and Pseudomonas genera are frequently found in flower samples85,86. In this study, we confirmed the occurrence of these bacteria as inhabiting the stigmas of O. lutea. Moreover, our earlier results confirm previous reports about the dominant abundance of bacterial groups from the Pseudomonadaceae (genus Pseudomonas) and Enterobacteriaceae (genus Pantoea) family noted in stigmas23,24,31,32. Furthermore, research on fungi inhabiting the stigmas of parasitic plants23,24 has shown that yeast-like fungi such as Aureobasidium spp. are the dominant group. Similarly, concerning this group of microorganisms in O. lutea for both unpolluted and polluted sites, the genera Filobasidium, Vishniacozyma and Aureobasidium were predominant. In a similar way, changes over time in the expression of floral traits can influence the dynamics of microbial populations. For example, the exudates from the stigmatic papillae of apple and pear flowers decrease as the flowers mature, eventually halting the support for the growth of the pathogen Erwinia amylovora87. Moreover, the extent of floral infection is influenced to some degree by the prevailing environmental conditions. Appropriate temperature (above 15 degrees) and high rainfall are excellent factors causing the multiplication of bacteria attacking the stigmas of apple flowers by E. amylovora88. Our research also confirmed the occurrence of two representatives of plant pathogens of the Erwinia genus, i.e. E. persicina and E. rhapontici (Table 1). It has been shown that E. persicina (seed-borne) affects the growth and physiology of alfalfa89. This fact is particularly intriguing because alfalfa serves as one of the hosts for O. lutea. We also confirmed the presence of Pseudomonas fluorescens (Table 1), which is used due to its high efficacy in controlling infections by E. amylovora90. The increased likelihood of fungal community occurrence, including pathogens, and may be attributed to flowers that remain constantly open as observed in O. lutea, rendering them more susceptible to infections from pollinators91. Consequently, this elevates the risk of spreading the infection to other parts of plants, as well as to plants in close proximity.
Orobanche and Phelipanche species exhibit an individual inflorescence with a dozen or even several dozen flowers, making them attractive to pollinators who can efficiently gather pollen without expending energy on visiting plants that produce only one flower. Furthermore, the flowers of Orobanche and Phelipanche genera are morphologically adapted as ‘nototribic flowers’, depositing pollen on the upper side of visitors during pollination92. Moreover, there has been a convergent evolution of morphological specializations in some pollinators, such as those with a specialized pollen-collecting apparatus on their faces93. Consequently, microbiological analysis of flower surface, including mature stigmas from opened flowers, can indirectly provide valuable information about microorganisms transmitted by pollinators. These microbes can serve as a ‘fingerprint’ of the insect species involved in pollination94. In mature stigmas of opened flowers, we found yeasts such as Starmerella sp. and Itersonilia sp. which were not recorded in immature stigmas or were present in lesser abundance. For instance, Starmerella bombicola has been isolated from nectar samples of wild flowers visited by bumblebees, bees, and other insects, suggesting its transport by flower visitors95. Not only bees, bumblebees and wasps can transmit microorganisms research; by de Vega and Herrera96 proved that ants feeding on nectar transfer yeast to flowers. Ants, frequently observed in the flowers of Orobanche and Phelipanche genera97, were also noted in our research.
The potential link between stigma and seed microbiomes in parasitic plants underscores intriguing ecological and evolutionary dynamics, particularly regarding the shared presence of certain bacterial genera. The stigma, as the primary site of pollen reception, is exposed to diverse microbial communities from pollinators and the environment. These interactions may influence the composition of the seed microbiome. Notably, previous research suggests that seed-fungal communities are predominantly transmitted horizontally via the environment and soil, whereas seed-bacterial communities are primarily passed on vertically98. This overlap suggests a possible vertical transmission route, where microbes from the stigma may be incorporated into developing seeds during fertilization and fruit maturation. The shared bacterial genera between stigma and seed microbiomes may point to a selective process in which beneficial microbes are retained during reproductive stages. In our earlier works23,24, we identified bacterial genera present in the stigma of Orobanche and Phelipanche, including Pseudomonas, Sphingomonas, Stenotrophomonas, Paenibacillus, and Pedobacter. These genera have also been widely documented in P. ramosa seeds15. Most of these bacteria, i.e., Pseudomonas, Paenibacillus, Pedobacter and Sphingomonas, were also identified in the stigmas of O. lutea (Table 3). In our previous works on the genus Cistanche, we also noted endophytic bacteria among Bacillus, Microbacterium, Paenibacillus, Pantoea, Pseudomonas, Serratia, and Stenotrophomonas in C. armena seeds18, and Bacillus, Microbacterium, Paenibacillus, Pseudomonas, Sphingomonas, and Stenotrophomonas in the seeds of C. phelypaea19, which were also recorded in stigmas. Furthermore, the environmental context, such as pollution or nutrient availability, may shape the stigma microbiome, influencing the initial microbial composition inherited by seeds. This hypothesis is supported by findings in this study showing that stigma microbiomes from the polluted site harbored bacteria with specific stress-tolerant traits, which might be transferred to seeds, potentially aiding their establishment in harsh environments. Overall, the shared presence of microbial genera between stigma and seed microbiomes highlights the importance of stigmas as a microbial reservoir with potential implications for seed health, germination success, and subsequent plant development. Future studies focusing on tracking microbial transmission from stigmas to seeds and analyzing functional roles of shared taxa will be essential to confirm these links and understand their ecological significance. Additionally, the vascular connection between the parasitic plant and their host plays a crucial role in shaping the final composition of the parasite’s seed microbiome. An in vitro study demonstrated extensive microbiome transfer between the parasitic plant Phelipanche aegyptiaca and its tomato host following parasitic attachment14. Similarly, an in situ study on Orobanche hederae and ivy revealed that the microbiota of the parasitic plant’s roots and shoots originated from the host plant’s microbiota16. Based on the above work, it is likely that the Medicago spp. host will also influence the formation of the O. lutea microbiome. This connection highlights the need to explore the extent to which host plant microbiomes contribute to microbial diversity in parasitic plants, particularly during their reproductive stages.
O. lutea has the ability to adapt to specific habitats, including polluted ones, which are often inaccessible to other plants which translocate toxic heavy metals from soil into reproductive floral organs, decreasing reproductive success. This impact may manifest directly through decreased pollen viability and seed mass, or indirectly through alterations in visitation behavior of flower pollinators99. As the concentration of heavy metals increases, a consistent decline is observed in the number, diversity and abundance of solitary wild bees100. Furthermore, pollinators collect less nectar from flowers affected by metals101. A reduced number of visits of pollinators affects the reproductive success and changes in the microbiome structure of pollinators compared to plants occupying sites not polluted with heavy metals. The presence of beneficial and niche-specific microorganisms in contaminated areas is regarded as a highly promising approach for enhancing stress tolerance, as these microbes play a pivotal role in diminishing the accumulation of metals in plants102,103. The observed differences in microbial diversity between the polluted and unpolluted sites provide valuable insights into microbial adaptation to environmental stressors, particularly heavy metal contamination. These pollutants can exert selective pressure on microbial populations, favoring the proliferation of metal-tolerant bacterial taxa (e.g., Pseudomonas and Paenibacillus) known for their metal resistance and bioremediation capabilities. Such bacteria may dominate the microbial community, reducing overall diversity but increasing the abundance of specific operational taxonomic units (OTUs) adapted to these stress conditions19. Among the bacteria identified, Pseudomonas helmanticensis was recorded in immature stigmas from the polluted site (Table 1), having the ability to produce fluorescent siderophores (Table 2). These genera are known for their plant-associated capabilities104. Siderophore-producing bacteria play a crucial role in supporting plant survival and growth in metal-contaminated soils105. Siderophores facilitate the detoxification of heavy metals, underscoring their utility in bioremediation. They also serve as potential biocontrol agents against phytopathogens such as Fusarium spp., offering an alternative to chemical pesticides106. In our study, several bacteria inhabiting stigmas in the polluted area, such as Pseudomonas trivialis (in immature stigmas) and Staphylococcus warneri and Acinetobacter septicus (in mature stigmas), exhibited the ability to produce lipases (Tables 1, 2). Interestingly, heavy metals have been found to both stimulate and inhibit lipase production, depending on the context. Lipases play a key role in regulating cellular metabolism and are increasingly recognized for their role as virulence factors in fungal plant pathogens107,108. Two Pseudomonas species, P. helmanticensis and P. trivialis, identified in immature stigmas from the polluted site, demonstrated hydrogen cyanide (HCN) production (Table 2). HCN production is closely associated with biocontrol processes, including plant protection against pathogenic fungi, as well as suppression of weeds, insects, termites, and nematodes109,110. Interestingly, one of the bacteria in contaminated areas, P. helmanticensis, has the ability to produce siderophores and HCN (Table 2). Additionally, biosurfactant sophorolipids produced by Starmerella bombicola (a genus recorded in mature stigmas) have demonstrated the ability to remove heavy metals from contaminated soils, highlighting their potential as bioremediation agents for heavy metal-polluted environments111. Studying these adaptive traits not only deepens our understanding of microbial resilience but also has practical implications, such as identifying bioindicator species for pollution monitoring and exploring bioremediation strategies using metal-tolerant microbes. Conversely, the unpolluted site, such as the xerothermic grasslands in Ostra Góra, offer relatively stable and nutrient-rich environments. Fungi, including Aureobasidium and Filobasidium (Table 4), which achieved greater abundance in the unpolluted site, often rely on organic matter decomposition and nutrient recycling, processes that may be inhibited in polluted soils due to toxicity. There are evidence that Aureobasidium is tolerance toward heavy metals as well as could be a promising source as a biosorbent for heavy metal removal112. Plants inoculated with this fungus that are exposed to heavy metals also show better plant growth and a higher content of photosynthetic pigments compared to uninoculated plants113.
Among Orobanche and Phelipanche genera, there are rare and endangered species as well as noxious crop pests (only a few species, like, Phelipanche ramosa and Orobanche cumana). O. lutea could potentially become a serious weed of lucerne, as it’s occurrence has already been noted114. Currently, it predominantly grows on wild and rarely cultivated Medicago species41. Further research on microorganisms may contribute to an understanding of these as a promising alternative to ward off these plants that cause significant damage to different crops in agroecosystems. There are a several microorganisms that damage the developmental stages of broomrape species, including in particular from the Fusarium genus, F. oxysporum115, F. solani116, and F. verticillioides117, as well as Azospirillum brasilense118, Pseudomonas fluorescens119, and P. ogarae120. However, the mode of action of microorganisms as well as the identification of the metabolites responsible for their inhibition effect remain often uncharted. Further research is warranted to unravel the functional significance of specific microbial taxa and their interactions with O. lutea and associated plant communities.
Conclusions
Microbial communities of Orobanche lutea stigmas are dominated by Proteobacteria, particularly represented by families Enterobacteriaceae and Pseudomonadaceae. Among fungi, Basidiomycota and Ascomycota are the predominant phyla, with specific taxa such as Filobasidium spp. and Aureobasidium spp. being highly abundant. There are distinct differences observed in the species composition of the microbiome of O. lutea stigmas between polluted and unpolluted sites, with variations in the abundance of specific bacterial and fungal taxa. Polluted site showed a greater diversity in bacterial and fungal communities compared to the unpolluted site. The microbial composition of stigmas varies across different stages of flower development, with notable shifts in the abundance of specific bacterial and fungal taxa. Immature and mature stigmas exhibit distinct microbial profiles, highlighting the dynamic nature of microbial colonization during plant development. The level of pollution and the stage of flower development influence the microbiological composition of stigmas of O. lutea. Microbial populations from polluted site show abundance of certain bacterial and fungal taxa compared to unpolluted site, with varying patterns across different stages of flower development. This suggests complex interactions between environmental factors and plant biology in shaping microbial communities associated with the stigmas of O. lutea. This study contributes to the understanding of microbial diversity and dynamics in O. lutea ecosystems, providing valuable insights into their ecological roles and potential applications in agriculture and environmental management. To our knowledge, this is the first study investigating the microbiome of stigmas of O. lutea.
Data availability
The raw Illumina sequencing data generated in this study are available in the NCBI’s Sequence Read Archive (SRA) under BioProject: PRJNA1092550.The GenBank accession numbers for the nucleotide sequence of 16S rRNA gene used for identification of culturable bacteria strains are from PP833009 to PP833026 and from PP907926 to PP907932.
References
Nickrent, D. L. Parasitic angiosperms: How often and how many?. Taxon 69(1), 5–27. https://doi.org/10.1002/tax.12195 (2020).
Press, M. C. & Phoenix, G. K. Impacts of parasitic plants on natural communities. New Phytol. 166, 737–751. https://doi.org/10.1111/j.1469-8137.2005.01358.x (2005).
Těšitel, J. et al. The bright side of parasitic plants: what are they good for?. Plant Physiol. 185, 1309–1324. https://doi.org/10.1093/plphys/kiaa069 (2021).
Watson, D. M., Mclellan, R. C. & Fonturbel, F. E. Functional roles of parasitic plants in a warming world. Annu. Rev. Ecol. Evol. Syst. 53, 25–45. https://doi.org/10.1146/annurev-ecolsys-102320-115331 (2022).
Bani, A. et al. Relationship between the Ni hyperaccumulator Alyssum murale and the parasitic plant Orobanche nowackiana from serpentines in Albania. Ecol. Res. 33, 549–559. https://doi.org/10.1007/s11284-018-1593-1 (2018).
Turnau, K., Jędrzejczyk, R., Domka, A., Anielska, T. & Piwowarczyk, R. Expansion of a holoparasitic plant, Orobanche lutea (Orobanchaceae), in post-industrial areas - a possible Zn effect. Sci. Total Environ. 639, 714–724. https://doi.org/10.1016/j.scitotenv.2018.05.189 (2018).
Dimitrakopoulos, P. G., Aloupi, M., Tetradis, G. & Adamidis, G. C. Broomrape species parasitizing Odontarrhena lesbiaca (Brassicaceae) individuals act as nickel hyperaccumulators. Plants 10, 816. https://doi.org/10.3390/plants10040816 (2021).
Piwowarczyk, R. et al. Correlational nutritional relationships and interactions between expansive holoparasite Orobanche laxissima and woody hosts on metal-rich soils. Phytochemistry https://doi.org/10.1016/j.phytochem.2021.112844 (2021).
Cartry, D., Steinberg, C. & Gibot-Leclerc, S. Main drivers of broomrape regulation. Rev. Agron. Sustain. Dev. 41, 17. https://doi.org/10.1007/s13593-021-00669-0 (2021).
Kreutz, C. A. J. Orobanche. The European broomrape species. Central and northern Europe. (Limburg: Natuurhistorisch Genootschap, 1995).
Tóth, P., Undas, A. K., Verstappen, F. & Bouwmeester, H. Floral volatiles in parasitic plants of the Orobanchaceae. Ecological and taxonomic implications. Front. Plant Sci. 7, 312. https://doi.org/10.3389/fpls.2016.00312 (2016).
Piwowarczyk, R. & Kasińska, J. Petal epidermal micromorphology in holoparasitic Orobanchaceae and its significance for systematics and pollination ecology. Aust. Syst. Bot. 30, 48–63. https://doi.org/10.1071/SB16028 (2017).
Ruraż, K. & Piwowarczyk, R. Morphological diversity of pistil stigmas and its taxonomic significance of representatives of holoparasitic Orobanchaceae from Central Europe. PhytoKeys 215, 1–25. https://doi.org/10.3897/PHYTOKEYS.215.96263 (2022).
Iasur Kruh, L. et al. Host-parasite-bacteria triangle: The microbiome of the parasitic weed Phelipanche aegyptiaca and tomato-Solanum lycopersicum (Mill.) as a host. Front. Plant Sci. 8, 269. https://doi.org/10.3389/fpls.2017.00269 (2017).
Huet, S. et al. Populations of the parasitic plant Phelipanche ramosa influence their seed microbiota. Front. Plant Sci. 11, 1075. https://doi.org/10.3389/fpls.2020.01075 (2020).
Fitzpatrick, C. R. & Schneider, A. C. Unique bacterial assembly, composition, and interactions in a parasitic plant and its host. J. Exp. Bot. 71, 2198–2209. https://doi.org/10.1093/jxb/erz572 (2020).
Durlik, K., Żarnowiec, P., Piwowarczyk, R. & Kaca, W. Culturable endophytic bacteria from Phelipanche ramosa (Orobanchaceae) seeds. Seed Sci. Res. 31, 69–75. https://doi.org/10.1017/S0960258520000343 (2021).
Petrosyan, K. et al. Characterization and diversity of seed endophytic bacteria of the endemic holoparasitic plant Cistanche armena (Orobanchaceae) from a semi-desert area in Armenia. Seed Sci. Res. 32, 264–273. https://doi.org/10.1017/S0960258522000204 (2022).
Petrosyan, K. et al. Diversity and potential plant growth promoting capacity of seed endophytic bacteria of the holoparasite Cistanche phelypaea (Orobanchaceae). Sci. Rep. 13, 11835. https://doi.org/10.1038/s41598-023-38899-9 (2023).
Xi, J. et al. Microbial community roles and chemical mechanisms in the parasitic development of Orobanche cumana. Imeta https://doi.org/10.1002/imt2.31 (2022).
Miao, Y., Zhang, X., Pei, J., Liu, C. & Huang, L. Adaptive bacterial and fungal matching between a parasitic plant and its host: A case of Cistanche deserticola and Haloxylon ammodendron. Ind. Crops. Prod. 191, 115932. https://doi.org/10.1016/j.indcrop.2022.115932 (2023).
Miao, Y. et al. From guest to host: parasite Cistanche deserticola shapes and dominates bacterial and fungal community structure and network complexity. Environ. Microbiomes 18, 11. https://doi.org/10.1186/s40793-023-00471-3 (2023).
Ruraż, K. et al. Stigmas of holoparasitic Phelipanche arenaria (Orobanchaceae) – a suitable ephemeric flower habitat for development unique microbiome. BMC Plant Biol. 23, 486. https://doi.org/10.1186/s12870-023-04488-1 (2023).
Ruraż, K., Przemieniecki, S. W. & Piwowarczyk, R. Interspecies and temporal dynamics of bacterial and fungal microbiomes of pistil stigmas in flowers in holoparasitic plants of the Orobanche series Alsaticae (Orobanchaceae). Sci. Rep. 13, 6749. https://doi.org/10.1038/s41598-023-33676-0 (2023).
Aleklett, K., Hart, M. & Shade, A. The microbial ecology of flowers: an emerging frontier in phyllosphere research. Botany 92, 253–266. https://doi.org/10.1139/cjb-2013-0166 (2014).
Rebolleda-Gómez, M. et al. Gazing into the anthosphere: considering how microbes influence floral evolution. New Phytol. 224, 1012–1020. https://doi.org/10.1111/nph.16137 (2019).
Vannette, R. L. The floral microbiome: Plant, pollinator, and microbial perspectives. Annu. Rev. Ecol. Evol. Syst. 51, 363–386. https://doi.org/10.1146/annurev-ecolsys-011720-013401 (2020).
Rejón, J. D. et al. Proteomics profiling reveals novel proteins and functions of the plant stigma exudate. J. Exp. Bot. 64, 5695–5705. https://doi.org/10.1093/jxb/ert345 (2013).
Pusey, P. L., Stockwell, V. O. & Mazzola, M. Epiphytic bacteria and yeasts on apple blossoms and their potential as antagonists of Erwinia amylovora. Phytopathology 99, 571–581. https://doi.org/10.1094/PHYTO-99-5-0571 (2009).
Shade, A., McManus, P. S. & Handelsman, J. Unexpected diversity during community succession in the apple flower microbiome. mBio 4, e00602–12 (2013). https://doi.org/10.1128/mBio.00602-12.
Steven, B., Huntley, R. B. & Zeng, Q. The influence of flower anatomy and apple cultivar on the apple flower phytobiome. Phytobiomes J. 2, 171–179. https://doi.org/10.1094/PBIOMES-03-18-0015-R (2018).
Cui, Z., Huntley, R. B., Zeng, Q. & Steven, B. Temporal and spatial dynamics in the apple flower microbiome in the presence of the phytopathogen Erwinia amylovora. ISME J. 15, 318–329. https://doi.org/10.1038/s41396-020-00784-y (2021).
Schaeffer, R. N. et al. Disease management during bloom affects the floral microbiome but not pollination in a mass-flowering crop. J. Appl. Ecol. 60, 64–76. https://doi.org/10.1111/1365-2664.14320 (2023).
Nelson, E. B. The seed microbiome: Origins, interactions, and impacts. Plant Soil 422, 7–34. https://doi.org/10.1007/s11104-017-3289-7 (2018).
Asiminicesei, D.-M., Fertu, D. I. & Gavrilescu, M. Impact of heavy metal pollution in the environment on the metabolic profile of medicinal plants and their therapeutic potential. Plants 13, 913. https://doi.org/10.3390/plants13060913 (2024).
Meindl, G. A. & Ashman, T. L. Nickel accumulation by Streptanthus polygaloides (Brassicaceae) reduces floral visitation rate. J. Chem. Ecol. 40, 128–135. https://doi.org/10.1007/s10886-014-0380-x (2014).
Scott, S. B., Lanno, R. & Gardiner, M. M. Acute toxicity and bioaccumulation of common urban metals in Bombus impatiens life stages. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2024.169997 (2024).
Bartlewicz, J., Lievens, B., Honnay, O. & Jacquemyn, H. Microbial diversity in the floral nectar of Linaria vulgaris along an urbanization gradient. BMC Ecol. 16, 18. https://doi.org/10.1186/s12898-016-0072-1 (2016).
Samuni-Blank, M. et al. The role of abiotic environmental conditions and herbivory in shaping bacterial community composition in floral nectar. PLoS One https://doi.org/10.1371/journal.pone.0099107 (2014).
Russell, K. A. & McFrederick, Q. S. Floral nectar microbial communities exhibit seasonal shifts associated with extreme heat: Potential implications for climate change and plant-pollinator interactions. Front. Microbiol. https://doi.org/10.3389/fmicb.2022.931291 (2022).
Piwowarczyk, R. & Krajewski, Ł. Orobanche lutea Baumg. (Orobanchaceae) in Poland: Revised distribution, taxonomy, phytocoenological and host relations. Biodivers. Res. Conserv. 34, 17–39 (2014). https://doi.org/10.2478/biorc-2014-0008.
Piwowarczyk, R., Pedraja, Ó. S., Khutsishvili, M. & Kharazishvili, D. Holoparasitic Orobanchaceae in Georgia (Caucasus): Taxonomic revision, diversity, distribution, habitats and host range. Phytotaxa 604, 1–103 (2023). https://doi.org/10.11646/phytotaxa.604.1.1.
Piwowarczyk, R., Chmielewski, P. & Cwener, A. The distribution and habitat requirements of the genus Orobanche L. (Orobanchaceae) in SE Poland. Acta Soc. Bot. Pol. 80, 37–48. https://doi.org/10.5586/asbp.2011.006 (2011).
Piwowarczyk, R. The genus Orobanche L. (Orobanchaceae) in the Małopolska Upland (S Poland): distribution, habitat, host preferences, and taxonomic problems. Biodiv. Res. Conserv. 26, 3–22. https://doi.org/10.2478/v10119-012-0009-2 (2012).
Dziubanek, G. et al. Long-term exposure to urban air pollution and the relationship with life expectancy in cohort of 3.5 million people in Silesia. Sci. Total Environ. 580, 1–8 (2017). https://doi.org/10.1016/j.scitotenv.2016.11.217.
Provincial Inspectorate for Environmental Protection in Katowice. The fifteenth annual assessment of air quality in the Silesian Voivodeship, covering 2016. Katowice (2017), pp. 1–80. (in Polish). https://powietrze.gios.gov.pl/pjp/rwms/publications/card/795.
Mielecka-Kubień Z. & Wójcik A. Air pollution and health condition of inhabitants of big cites in Śląskie Voivodeship. Wiadomości Statystyczne. The Polish Statistician 65, 39–51 (2020). (In Polish). https://doi.org/10.5604/01.3001.0014.2344.
Dobrzańska, J. Flora and ecological studies on calamine flora in the district of Bolesław and Olkusz. Acta Soc. Bot. Pol. 24, 357–417 (1995).
Grodzińska, K., Korzeniak, U., Szarek-Łukaszewska, G. & Godzik, B. Colonization of zinc mine spoils in southern Poland – preliminary studies on vegetation, seed rain and seed bank. Fragm. Flor. Geobot. 45, 123–145 (2000).
Kiczor, B. Soils. In: Tkaczuk, U. (Ed.), State of the environment in the Świętokrzyskie Voivodeship. Report 2017. Wojewódzki Inspektorat Ochrony Środowiska, Kielce, 135–146 (2017). (In Polish). https://powietrze.gios.gov.pl/pjp/rwms/publications/card/549.
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315. https://doi.org/10.1002/JOC.5086 (2017).
Harris, I., Osborn, T. J., Jones, P. & Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 7, 109. https://doi.org/10.1038/s41597-020-0453-3 (2020).
Thiers, B. [continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/ Accessed 14 Nov 2023.
The International Plant Names Index (IPNI). 2022. https://www.ipni.org/ Accessed 17 November 2023.
Ishii, Y., Matsuura, Y., Kakizawa, S., Nikoh, N. & Fukatsua, T. Diversity of bacterial endosymbionts associated with macrosteles leafhoppers vectoring phytopathogenic phytoplasmas. Appl. Environ. Microbiol. 79, 5013–5022. https://doi.org/10.1128/AEM.01527-13 (2013).
Ghodsalavi, B., Ahmadzadeh, M., Soleimani, M., Madloo, P. B. & Taghizad-Farid, R. Isolation and characterization of rhizobacteria and their effects on root extracts of Valeriana officinalis. Aust. J. Crop Sci. 7, 338–344 (2013).
Hankin, L. & Anagnostakis, S. L. Solid media containing carboxymethylcellulose to detect Cx cellulase activity of micro organisms. J. Gen. Microbiol. 98, 109–115. https://doi.org/10.1099/00221287-98-1-109 (1977).
Patten, C. L. & Glick, B. R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 42, 207–220. https://doi.org/10.1139/M96-032 (1996).
Schaad, N. W., Jones, J. B. & Chun, W. Laboratory guide for the identification of plant pathogenic bacteria. American Phytopathological Society Press (2001).
Sharma, S., Kumar, V. & Tripathi, R. B. Isolation of phosphate solubilizing microorganism (PSMs) from soil. J. Microbiol. Biotech. Res. 1, 90–95 (2011).
Maurhofer, M., Keel, C., Haas, D. & Défago, G. Influence of plant species on disease suppression by Pseudomonas fluorescens strain CHAO with enhanced antibiotic production. Plant Pathol. 44, 40–50. https://doi.org/10.1111/J.1365-3059.1995.TB02714.X (1995).
Sasirekha, B. & Srividya, S. Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chilli. Agric. Nat. Resour. 50, 250–256. https://doi.org/10.1016/j.anres.2016.02.003 (2016).
Rashid, S., Charles, T. C. & Glick, B. R. Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl. Soil Ecol. 61, 217–224. https://doi.org/10.1016/J.APSOIL.2011.09.011 (2012).
Marques, A. P. G. C., Pires, C., Moreira, H., Rangel, A. O. S. S. & Castro, P. M. L. Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol. Biochem. 42, 1229–1235. https://doi.org/10.1016/j.soilbio.2010.04.014 (2010).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. https://doi.org/10.1038/s41587-019-0209-9 (2019).
McLaren, M. R. Silva SSU taxonomic training data formatted for DADA2 (Silva version 138) Zenodo. 10.5281/zenodo.3986799 (2020).
Abarenkov, K. et al. UNITE general FASTA release for Fungi. UNITE Community (Version 04.02.2020). https://doi.org/10.15156/BIO/786368 (2020).
Lumivero, 2023. XLSTAT basic solutions. https://www.xlstat.com/en/solutions/basic. Accessed 7 Sept 2023.
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: Paleontological statistics software package for education and data analysis. Palaeont. Electr. 4, 9. (2021). http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
Junker, R. R. & Tholl, D. Volatile organic compound mediated interactions at the plant-microbe interface. J. Chem. Ecol. 39, 810–825. https://doi.org/10.1007/s10886-013-0325-9 (2013).
Berlec, A. Novel techniques and findings in the study of plant microbiota: Search for plant probiotics. Plant Sci. 193–194, 96–102. https://doi.org/10.1016/j.plantsci.2012.05.010 (2012).
Philippot, L., Raaijmakers, J. M., Lemanceau, P. & Van Der Putten, W. H. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, 789–799. https://doi.org/10.1038/nrmicro3109 (2013).
Mansfield, J. et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13, 614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x (2012).
Santoyo, G., Moreno-Hagelsieb, G., del Carmen Orozco-Mosqueda, M. & Glick, B. R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 183, 92–99. https://doi.org/10.1016/j.micres.2015.11.008 (2016).
Afzal, I., Shinwari, Z. K., Sikandar, S. & Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 221, 36–49. https://doi.org/10.1016/j.micres.2019.02.001 (2019).
Krawczyk, K., Zwolińska, A., Kamasa, J., Maćkowiak-Sochacka, A. & Przemieniecki, S. Identification and characterization of plant growth promoting endophytic bacteria. Prog. Plant Prot. 56, 100–109 (2016). https://doi.org/10.14199/ppp-2016-018.
Gravel, V., Antoun, H. & Tweddell, R. J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 39, 1968–1977. https://doi.org/10.1016/j.soilbio.2007.02.015 (2007).
Radzki, W. et al. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol. 104, 321–330 (2013). https://doi.org/10.1007/s10482-013-9954-9.
Abbamondi, G. R. et al. Plant growth-promoting effects of rhizospheric and endophytic bacteria associated with different tomato cultivars and new tomato hybrids. Chem. Biol. Technol. Agric. 3, 1. https://doi.org/10.1186/s40538-015-0051-3 (2016).
Nagata, T., Oobo, T. & Aozasa, O. Efficacy of a bacterial siderophore, pyoverdine, to supply iron to Solanum lycopersicum plants. J. Biosci. Bioeng. 115, 686–690. https://doi.org/10.1016/j.jbiosc.2012.12.018 (2013).
Abou El-Yazeid, A. & Abou-Aly, H. E. Enhancing growth, productivity and quality of tomato plants using phosphate solubilizing microorganisms. Aust. J. Basic. Appl. Sci. 5, 371–379 (2011).
Parker, C. Parasitic weeds: A world challenge. Weed Sci. 60, 269–276. https://doi.org/10.1614/WS-D-11-00068.1 (2012).
Piwowarczyk, R. & Jankowska-Błaszczuk, M. Intra-specific diversity of seed productivity and morphological features in parasitic species Orobanche bartlingii Griseb. (Orobanchaceae). Polish J. Ecol. 62, 723–738. https://doi.org/10.3161/104.062.0415. (2014).
Piwowarczyk, R., Ruraż, K., Krasylenko, Y., Kasińska, J. & Sánchez-Pedraja, Ó. Seed micromorphology of representatives of holoparasitic Orobanchaceae genera from the Caucasus region and its taxonomic significance. Phytotaxa 432, 223–251. https://doi.org/10.1164/phytotaxa.432.3.1. (2020).
Wei, N. & Ashman, T. L. The effects of host species and sexual dimorphism differ among root, leaf and flower microbiomes of wild strawberries in situ. Sci. Rep. 8, 5195. https://doi.org/10.1038/s41598-018-23518-9 (2018).
Rebolleda Gómez, M. & Ashman, T. L. Floral organs act as environmental filters and interact with pollinators to structure the yellow monkeyflower (Mimulus guttatus) floral microbiome. Mol. Ecol. 28, 5155–5171. https://doi.org/10.1111/mec.15280 (2019).
Thomson, S. V. & Gouk, S. C. Influence of age of apple flowers on growth of Erwinia amylovora and biological control agents. Plant Dis. 87, 502–509. https://doi.org/10.1094/PDIS.2003.87.5.502 (2003).
Taylor, R. K., Hale, C. N., Henshall, W. R., Armstrong, J. L. & Marshall, J. W. Effect of inoculum dose on infection of apple (Malus domestica) flowers by Erwinia amylovora. New Zeal. J. Crop Hortic. Sci. 31, 325–333. https://doi.org/10.1080/01140671.2003.9514268 (2003).
Yao, B., Huang, R., Zhang, Z. & Shi, S. Seed-borne Erwinia persicina affects the growth and physiology of alfalfa (Medicago sativa L.). Front. Microbiol. https://doi.org/10.3389/fmicb.2022.891188 (2022).
Cabrefiga, J., Bonaterra, A. & Montesinos, E. Mechanisms of antagonism of Pseudomonas fluorescens EPS62e against Erwinia amylovora, the causal agent of fire blight. Int. Microbiol. 10, 123–132 (2007).
Marshall, D. L., Avritt, J. J., Maliakal-Witt, S., Medeiros, J. S. & Shaner, M. G. M. The impact of plant and flower age on mating patterns. Ann. Bot. 105, 7–22. https://doi.org/10.1093/aob/mcp260 (2010).
Westerkamp, C. & Claßen-Bockhoff, R. Bilabiate flowers: The ultimate response to bees?. Ann. Bot. 100, 361–374. https://doi.org/10.1093/aob/mcm123 (2007).
Müller, A. Convergent evolution of morphological specializations in Central European bee and honey wasp species as an adaptation to the uptake of pollen from nototribic flowers (Hymenoptera, Apoidea and Masaridae). Biol. J. Linn. Soc. 57, 235–252. https://doi.org/10.1006/bijl.1996.0013 (1996).
Ushio, M. et al. Microbial communities on flower surfaces act as signatures of pollinator visitation. Sci. Rep. 5, 8695. https://doi.org/10.1038/srep08695 (2015).
De Clercq, V., Roelants, S. L. K. W., Castelein, M. G., De Maeseneire, S. L. & Soetaert, W. K. Elucidation of the natural function of sophorolipids produced by Starmerella bombicola. J. Fungi 7, 917. https://doi.org/10.3390/jof7110917 (2021).
De Vega, C. & Herrera, C. M. Microorganisms transported by ants induce changes in floral nectar composition of an ant-pollinated plant. Am. J. Bot. 100, 792–800. https://doi.org/10.3732/ajb.1200626 (2013).
Jones, M. Studies on the pollination of Orobanche species in the British Isles. Conference paper. Progress in Orobanche research. Proceedings of the international workshop on Orobanche research. Obermarchtal, Germany, 19–22 August 1989, Eberhard-Karls-Universität, Tübingen, Germany, 6–17. (1991).
Klaedtke, S. et al. Terroir is a key driver of seed-associated microbial assemblages. Environ. Microbiol. 18, 1792–1804. https://doi.org/10.1111/1462-2920.12977 (2016).
Xun, E., Zhang, Y., Zhao, J. & Guo, J. Translocation of heavy metals from soils into floral organs and rewards of Cucurbita pepo: Implications for plant reproductive fitness. Ecotoxicol. Environ. Saf. 145, 235–243. https://doi.org/10.1016/j.ecoenv.2017.07.045 (2017).
Moroń, D. et al. Abundance and diversity of wild bees along gradients of heavy metal pollution. J. Appl. Ecol. 49, 118–125. https://doi.org/10.1111/j.1365-2664.2011.02079.x (2012).
Xun, E., Zhang, Y., Zhao, J. & Guo, J. Heavy metals in nectar modify behaviors of pollinators and nectar robbers: Consequences for plant fitness. Environ. Pollut. 242, 1166–1175. https://doi.org/10.1016/j.envpol.2018.07.128 (2018).
Gladkov, E. A., Tereshonok, D. V., Stepanova, A. Y. & Gladkova, O. V. Plant–microbe interactions under the action of heavy metals and under the conditions of flooding. Diversity 15, 175. https://doi.org/10.3390/d15020175 (2023).
Islam, M. & Sandhi, A. Heavy metal and drought stress in plants: The role of microbes—A review. Gesunde Pflanz. 75, 695–708. https://doi.org/10.1007/s10343-022-00762-8 (2023).
Lozano-González, J. M. et al. evaluation of siderophores generated by Pseudomonas bacteria and their possible application as Fe biofertilizers. Plants. 12, 4054. https://doi.org/10.3390/plants12234054 (2023).
Rajkumar, M., Ae, N., Prasad, M. N. & Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 28, 142–149. https://doi.org/10.1016/j.tibtech.2009.12.002 (2010).
Saha, M. et al. Microbial siderophores and their potential applications: a review. Environ. Sci. Pollut. Res. 23, 3984–3999. https://doi.org/10.1007/s11356-015-4294-0 (2016).
Gaillardin, C. Lipases as Pathogenicity Factors of Fungi. In: Timmis, K. N. (eds) Handbook of Hydrocarbon and Lipid Microbiology. Springer, Berlin, Heidelberg (2010). https://doi.org/10.1007/978-3-540-77587-4_247.
Saha, J., Chaki, M. G., Karmakar, S., Chatterjee, A. & Pal, A. Effect of different heavy metals on lipase production by a multiple heavy metal-resistant Pseudomonas aeruginosa strain isolated from arable land. Biologia 78, 2975–2985. https://doi.org/10.1007/s11756-023-01465-9 (2023).
Blumer, C. & Haas, D. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch. Microbiol. 173, 170–177. https://doi.org/10.1007/s002039900127 (2000).
Sehrawat, A., Sindhu, S. S. & Glick, B. R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 32, 15–38. https://doi.org/10.1016/S1002-0160(21)60058-9 (2022).
Qi, X. et al. Removal of cadmium and lead from contaminated soils using sophorolipids from fermentation culture of Starmerella bombicola CGMCC 1576 fermentation. Int. J. Environ. Res. Public Health 15, 2334. https://doi.org/10.3390/ijerph15112334 (2018).
Nakkeeran, E., Rathna, R. & Viveka, R. Mechanism and action of Aureobasidium pullulans on biosorption of metals. In Waste Bioremediation. Energy, Environment, and Sustainability (ed. Varjani, S.) (Springer, 2018).
Ali, A. et al. Endophytic Aureobasidium pullulans BSS6 assisted developments in phytoremediation potentials of Cucumis sativus under Cd and Pb stress. J. Plant Interact. 14, 303–313. https://doi.org/10.1080/17429145.2019.1633428 (2019).
Teryokhin, E. S. Weed Broomrapes, Systematics, Ontogenesis, Biology, Evolution (Aufstieg-Verlag, 1997).
Saremi, H. & Okhovvat, S. M. Biological control of Orobanche aegyptiaca by Fusarium oxysporum F. sp. Orobanche in northwest Iran. Commun. Agric. Appl. Biol. Sci. 73, 931–938 (2008).
Dor, E. & Hershenhorn, J. Evaluation of the pathogenicity of microorganisms isolated from Egyptian broomrape (Orobanche aegyptiaca) in Israel. Weed Biol. Manag. 9, 200–208. https://doi.org/10.1111/j.1445-6664.2009.00340.x (2009).
Dor, E., Hershenhorn, J., Andolfi, A., Cimmino, A. & Evidente, A. Fusarium verticillioides as a new pathogen of the parasitic weed Orobanche spp. Phytoparasitica 37, 361–370. https://doi.org/10.1007/s12600-009-0049-0 (2009).
Dadon, T., Nun, N. B. & Mayer, A. M. A factor from Azospirillum brasilense inhibits germination and radicle growth of Orobanche aegyptiaca. Isr. J. Plant Sci. 52, 83–86 (2004).
Zermane, N., Souissi, T., Kroschel, J. & Sikora, R. Biocontrol of broomrape (Orobanche crenata Forsk and Orobanche foetida Poir) by Pseudomonas fluorescens isolate Bf7–9 from the faba bean rhizosphere. Biocontrol Sci. Technol. 17, 483–497. https://doi.org/10.1080/09583150701309535 (2007).
Lurthy, T. et al. Inhibition of broomrape germination by 2,4-diacetylphloroglucinol produced by environmental Pseudomonas. Microb. Biotechnol. 16, 2313–2325. https://doi.org/10.1111/1751-7915.14336 (2023).
Acknowledgements
We would like to thank Karol Zubek (Jan Kochanowski University) for his help in setting up the microscope for taking stereomicroscope photographs.
Funding
This study was funded in part by [National Science Centre, Poland] [no. 2021/05/X/NZ8/01154]. For the purpose of Open Access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript (AAM) version arising from this submission. This research was also supported by grants from Jan Kochanowski University (no. SUPB.RN.23.244, SUPB.RN.24.207 (2023–2024) (to R.P., K.W.). This research was partially done within the WB-KK statutory project of the Plant Protection Institute—National Research Institute, Poznań, Poland, funded by the Ministry of Science and Higher Education (to K.K.) The results presented in this paper were obtained as a part of comprehensive study financed by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry (no. 30.610.011–110) (to S.W.P.). This work was co-financed by the Minister of Science (Poland) under the "Regional Excellence Initiative" program (project no.: RID/SP/0015/2024/01).
Author information
Authors and Affiliations
Contributions
R.P. generated the idea of the study. R.P. and K.W. designed the study, collected and identified plant material. K.W. prepared plant material for the laboratory experiments. S.W.P., K.K. and A.H. performed the laboratory experiments, analysed the data. S.W.P., K.K. and A.H. performed statistical calculations, bioinformatic analysis. K.W. and K.K. prepared analysis of the results. K.W., S.W.P., K.K., A.H. and R.P. wrote a draft of the manuscript. All the authors took part in conceptualization of the research and editing the manuscript and consent to its publication. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This experiment does not involve human and animal experiments. Experimental research and field studies on plants, including the collection of plant material was complied with relevant institutional, national, and international guidelines and legislation, and necessary permissions were obtained.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wiśniewska, K., Przemieniecki, S.W., Krawczyk, K. et al. Impact of pollution on microbiological dynamics in the pistil stigmas of Orobanche lutea flowers (Orobanchaceae). Sci Rep 15, 3382 (2025). https://doi.org/10.1038/s41598-024-84717-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84717-1